Introduction
Trifluridine/tipiracil (TAS-102) is an oral
antitumour agent comprising a thymidine-based nucleic acid
analogue, trifluridine, and the thymidine phosphorylase inhibitor,
tipiracil hydrochloride, at a molar ratio of 1:0.5. In a phase 3
clinical trial, TAS-102 demonstrated a significant survival benefit
over placebo in terms of overall survival (OS) in individuals with
metastatic colorectal cancer (mCRC) who were unresponsive to
standard therapies, including fluoropyrimidines, irinotecan, and
oxaliplatin (1,2). Additionally, a randomised phase 2
trial revealed that TAS-102 in combination with bevacizumab (Bmab)
extended progression-free survival (PFS) compared to that of
TAS-102 monotherapy (3).
Neutropenia is the most frequently observed adverse
event (AE) associated with TAS-102 monotherapy. In the RECOURSE
trial, 37.9% of individuals receiving TAS-102 monotherapy developed
grade 3 or higher neutropenia (1).
Furthermore, the incidence of grade 3 or higher neutropenia was
higher in individuals receiving TAS-102 plus Bmab than in those
receiving TAS-102 monotherapy (67% vs. 38%) (3). Several retrospective cohort studies
have suggested that the development of neutropenia during TAS-102
monotherapy or TAS-102 plus Bmab therapy is associated with
improved long-term survival (4-7).
In cases where TAS-102 cannot be combined with Bmab,
TAS-102 monotherapy is administered, often as a late-line treatment
following regimens known to cause bone marrow suppression such as
FOLFOX (fluorouracil/leucovorin and oxaliplatin) therapy. To
mitigate severe neutropenia, dose reduction of TAS-102 may be
considered from the initial administration, particularly in
individuals with a history of severe neutropenia from prior
chemotherapy. However, given that severe neutropenia is a
prognostic factor, there is concern that reducing the dose could
not only lower the risk of neutropenia but also potentially
compromise OS.
Understanding the impact of dose reduction at the
initiation of TAS-102 monotherapy in mCRC management is crucial for
optimising treatment strategies. Therefore, this study aimed to
investigate the effect of dose reduction during the initial
administration of TAS-102 monotherapy on the occurrence of severe
neutropenia and OS.
Patients and methods
Patients and evaluations
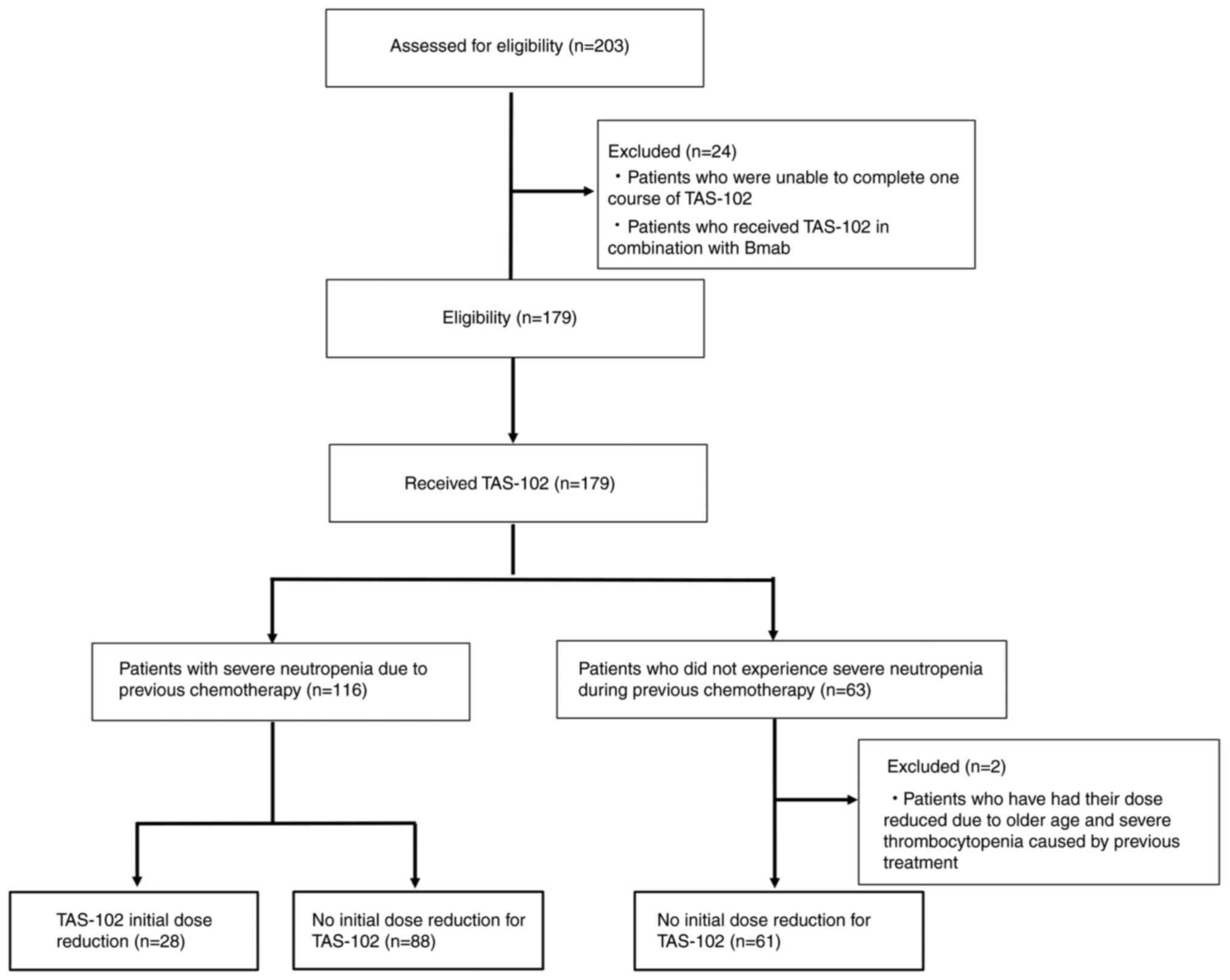
A total of 203 patients treated with TAS-102 among
patients with advanced or recurrent colorectal cancer (CRC) at
Ogaki Municipal Hospital (Ogaki, Japan) between January 2015 and
December 2024 were retrospectively evaluated. Patients who were
unable to complete one course of TAS-102 and who received TAS-102
in combination with Bmab were excluded from the study, as this
regimen had not yet been routinely adopted at our institution
during the study period. Furthermore, patients who underwent an
initial dose reduction of TAS-102 for reasons other than
neutropenia were excluded from the study. Thus, 177 patients were
considered eligible for this study. Participants were categorised
into those who did not experience severe neutropenia during
previous chemotherapy and did not undergo dose reduction (A group,
n=61), those who experienced severe neutropenia during previous
chemotherapy and received an initial dose reduction of TAS-102 (B1
group, n=28), and those who had severe neutropenia but did not
receive a dose reduction (B2 group, n=88). The flow diagram is
presented in Fig. 1. We analysed
the patients' characteristics, OS, PFS, and neutropenia grade with
TAS-102 treatment in patients with CRC using data collected from
electronic charts and pharmacy service records. AEs were evaluated
according to the Common Terminology Criteria for Adverse Events,
version 5.0(8), and the most
severe grades during chemotherapy were reported. Personal
information was protected in aggregated data. The study protocol
was approved by the Institutional Review Board of Ogaki Municipal
Hospital, Ogaki, Japan (approval no. 202520227-22). The requirement
for informed consent was waived owing to the retrospective study
design. Consent to participate was waived by the Institutional
Review Board of Ogaki Municipal Hospital owing to the retrospective
study design. Consent to publish was also waived by the
Institutional Review Board for the same reason.
Treatment protocol
Trifluridine/tipiracil combination tablet: TAS-102
(at a dose of 35 mg per square metre) was administered twice daily
after morning and evening meals for 5 days, followed by a 2-day
rest period. This regimen was repeated for 2 weeks, followed by a
14-day rest period, constituting one treatment cycle. The cycle was
repeated every 4 weeks.
The dose of TAS-102 was generally reduced by one
level in individuals with severe neutropenia due to prior
chemotherapy.
Statistical analysis
Kruskal-Wallis tests or Fisher's exact probability
tests were used for comparisons of patient characteristics, AEs,
and reasons for treatment discontinuation. Steel-Dwass test was
used for multiple comparisons of creatinine clearance among the 3
groups. The Kaplan-Meier method and log-rank tests were used to
compare treatment duration. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using EZR software (v1.30; Saitama Medical Centre, Jichi
Medical University, Saitama, Japan) (9).
Results
Patient characteristics
The patient characteristics are summarised in
Table I. Differences in creatinine
clearance were observed among the 3 groups. Moreover, differences
were observed in certain metastatic sites. No significant
differences were observed in other variables.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variable | A group (n=61) | B1 group (n=28) | B2 group (n=88) | P-value |
|---|
| Age, years | 67 (41-83) | 72 (49-79) | 70 (36-83) | 0.299 |
| Sex, Male/Female | 37/24 | 16/12 | 49/39 | 0.838 |
| Height, cm | 164.0
(139.0-184.0) | 161.0
(145.0-178.7) | 159.3
(141.0-181.7) | 0.240 |
| Body weight, kg | 59.8 (35.9-95.0) | 54 (36.4-85.0) | 52 (30.9-85.0) | 0.133 |
| Body surface area,
m2 | 1.62 (1.15-2.07) | 1.60 (1.23-2.03) | 1.54 (1.14-1.96) | 0.151 |
| Ccr,
ml/mina | 83.4
(19.0-142.0) | 58.4
(37.6-150.1) | 67.0
(32.9-171.1) | 0.007b |
| Number of prior
chemotherapy regimens | 2 (1-4) | 2 (1-4) | 2 (1-5) | 0.961 |
| Pre-chemotherapy
regimens | | | | |
|
FOLFOX ±
BV | 37 | 15 | 58 | 0.546 |
|
FOLFOX +
Pan | 1 | 3 | 8 | 0.096 |
|
FOLFOX +
Cet/AFL/Ram | 7 | 2 | 7 | 0.785 |
|
XELOX ±
BV | 13 | 7 | 9 | 0.122 |
|
SOX ±
BV | 1 | 2 | 2 | 0.311 |
|
FOLFIRI ±
BV | 32 | 20 | 58 | 0.253 |
|
FOLFIRI +
Cet/Pan/Ram/AFL | 23 | 12 | 28 | 0.690 |
|
CPT-11 ±
Cet | 4 | 1 | 2 | 0.467 |
|
Xeloda ±
BV | 4 | 1 | 4 | 0.902 |
|
S-1 | 6 | 1 | 5 | 0.621 |
|
Others | 24 | 5 | 26 | 0.216 |
| Performance
status | 0 (0-2) | 0 (0-1) | 0 (0-2) | 0.936 |
| RAS mutation | 32 | 19 | 56 | 0.282 |
| Adjuvant
chemotherapy, yes | 21 | 6 | 27 | 0.491 |
|
Progression/recurrence | 33/28 | 15/13 | 49/39 | 0.979 |
| Metastatic
sites | | | | |
|
Liver | 30 | 20 | 60 | 0.470 |
|
Lung | 29 | 13 | 49 | 0.879 |
|
Lymph
nodes | 18 | 10 | 40 | 0.477 |
|
Peritoneal
dissemination | 21 | 7 | 25 | 0.414 |
|
Bone | 7 | 0 | 5 | 0.094 |
|
Ovaries | 1 | 2 | 0 | 0.029b |
|
Brain | 1 | 0 | 1 | 1 |
|
Skin | 1 | 0 | 0 | 0.479 |
|
Others | 3 | 5 | 3 | 0.043b |
| Neutrophil count,
/mm3 | 3,580
(1,880-13,040) | 2,905
(1,560-8,490) | 2,910
(1,300-1,2440) | 0.064 |
| Treatment after
TAS-102 | | | | |
|
No | 50 | 20 | 64 | 0.368 |
|
Regorafenib | 11 | 7 | 20 | 0.682 |
|
Others | 0 | 1 | 4 | 0.239 |
A significant difference in creatinine clearance was
observed between groups A and B2 (P=0.006). No difference was
observed between groups A and B1 or between groups B1 and B2
(P=0.059, 0.892, respectively).
No significant differences were observed in prior
treatment regimens among the three groups (Table I).
Overall survival according to the
highest neutropenia grade
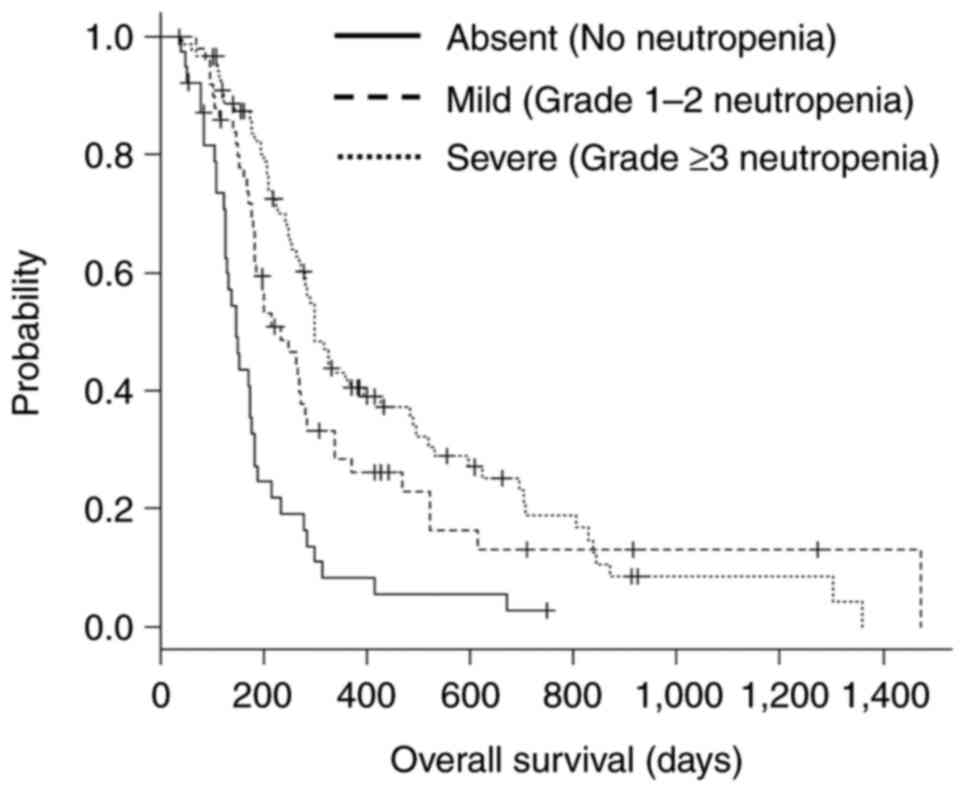
Kaplan-Meier survival curves stratified by
neutropenia severity are presented in Fig. 2. The median OS in the absent
(n=40), mild (n=49), and severe (n=88) neutropenia groups was 147
days (95% CI, 126-176), 234 days (95% CI, 183-281), and 300 days
(95% CI, 269-393), respectively (log-rank test, P<0.001).
Neutropenia grade during TAS-102
administration
Neutropenia grades among the 3 groups during TAS-102
administration are presented in Table
II. There was no significant difference in the incidence of
grade 3 or higher neutropenia among the 3 groups during TAS-102
administration (P=0.958). Differences in the incidence of grade 0
neutropenia were observed among the 3 groups (P=0.033). The dose
reductions of TAS-102 based on severe neutropenia due to prior
chemotherapy were as follows: 10 cases of a one-step dose
reduction; 16 cases of a two-step dose reduction; 2 cases of a
three-step dose reduction.
 | Table IINeutropenia grade during TAS-102
administration. |
Table II
Neutropenia grade during TAS-102
administration.
| Neutropenia
grade | A Group (n=61) | B1 Group
(n=28) | B2 Group
(n=88) | P-value |
|---|
| Grade 3-4, n
(%) | 30 (49.2) | 15 (53.6) | 44 (50.0) | 0.958 |
| Grade 1-2, n
(%) | 12 (19.7) | 11 (39.3) | 26 (29.5) | 0.126 |
| Grade 0, n (%) | 19 (31.1) | 2 (7.1) | 18 (20.5) | 0.033a |
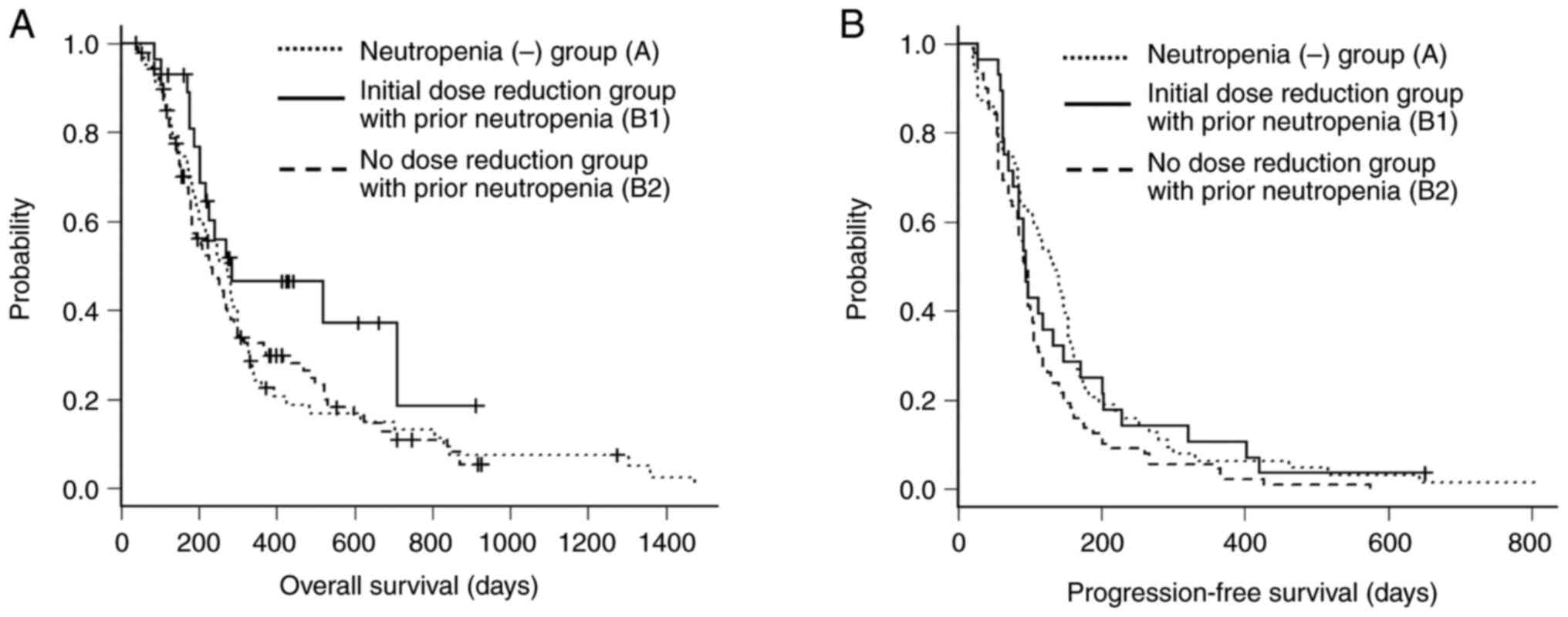
OS and PFS
OS and PFS among the 3 groups are presented in
Fig. 3. The median OS in the A
group (n=61), B1 group (n=28), and B2 group (n=88) were 273 days
(95% CI, 199-299), 285 days (95% CI, 202-NA), and 233 days (95% CI,
182-272), respectively (log-rank test, P=0.165). The median PFS
among the 3 groups were 133.0 days (95% CI, 98-153), 94.5 days (95%
CI, 77-133) and 93.5 days (95% CI, 83-100), respectively (log-rank
test, P=0.053).
Renal function and treatment
outcomes
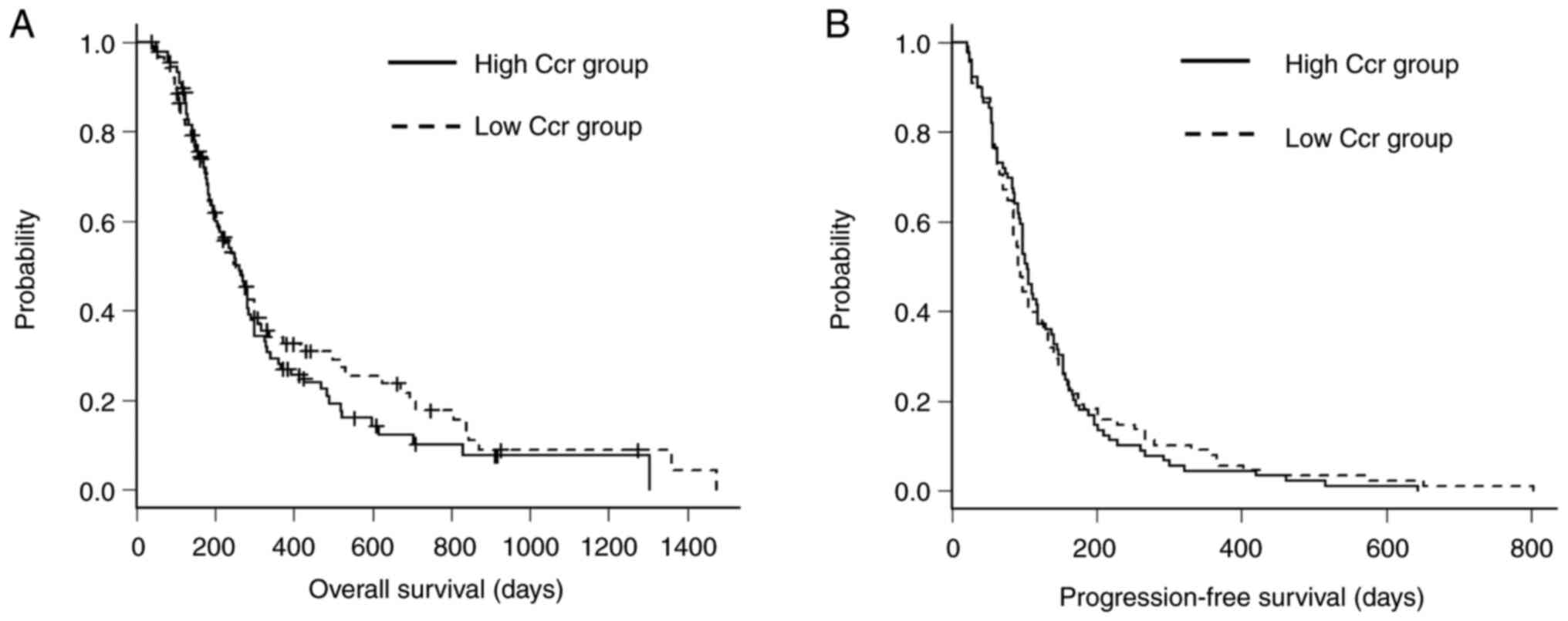
OS and PFS between the high and low Ccr groups are
presented in Fig. 4. To evaluate
whether renal function affected the incidence of severe neutropenia
or survival outcomes, a subgroup analysis was performed by
stratifying patients according to creatinine clearance (Ccr), using
the median value of 70.6 ml/min as the cutoff. Of the 177 patients,
89 were in the high Ccr group (≥70.6 ml/min), and 88 were in the
low Ccr group (<70.6 ml/min).
The incidence of grade ≥3 neutropenia was similar
between the two groups: 49/89 (55.1%) in the high Ccr group and
49/88 (55.7%) in the low Ccr group. There was no statistically
significant difference in the rate of neutropenia.
Median progression-free survival (PFS) was 104 days
(95% CI: 92-119) in the high Ccr group and 92.5 days (95% CI:
84-112) in the low Ccr group (P=0.789). Median overall survival
(OS) was 262 days (95% CI: 199-285) in the high Ccr group and 253
days (95% CI: 201-299) in the low Ccr group (P=0.411).
Discussion
This study is the first to clarify the impact of
initial dose reduction of TAS-102 monotherapy for colorectal cancer
(CRC) on the incidence of severe neutropenia and overall survival
(OS). Our results showed that patients who developed severe
neutropenia had longer OS, consistent with previous reports linking
chemotherapy-induced neutropenia to better prognosis (7,10-14).
Despite a history of severe neutropenia from prior chemotherapy,
initial dose reduction of TAS-102 did not reduce the incidence of
severe neutropenia, nor did it improve progression-free survival
(PFS) or OS compared to standard dosing.
Neutropenia may serve as a surrogate marker of
adequate drug exposure and antitumor activity (15-19).
However, factors such as prior treatment regimens, baseline
comorbidities, renal function, and actual dose intensity may also
influence outcomes. We considered performing multivariate analysis
including creatinine clearance (Ccr); however, due to the small
sample size and limited number of events in the B1 subgroup (n=28),
it was challenging to ensure the reliability of a multivariate
model. Nonetheless, we acknowledge the importance of further
investigation, and future large-scale prospective studies
incorporating multivariate analyses including Ccr are warranted to
better elucidate its potential confounding effect. We therefore
performed subgroup analyses stratified by Ccr, which showed no
significant differences in neutropenia incidence or survival,
suggesting renal function was unlikely to confound our main
findings. In addition, prior treatment regimens were comparable
across groups, as shown in Table
I, indicating that this factor was unlikely to bias the
results. By contrast, detailed comorbidity profiles and relative
dose intensity were not systematically evaluated in this study,
which should be acknowledged as limitations.
Consistent with this, no differences in severe
neutropenia or survival outcomes were observed between
dose-reduction and non-dose-reduction groups. A borderline trend in
PFS (P=0.053) may reflect the association between neutropenia and
improved OS but did not translate into significant group
differences.
Baseline absolute neutrophil count (ANC) has been
reported as a prognostic factor, with higher ANC correlating with
poorer outcomes and lower ANC predicting increased neutropenia risk
and better survival (20-22).
In our cohort, baseline ANC did not differ significantly between
dose groups, even among patients with prior severe neutropenia,
supporting the notion that initial dose reduction was not guided by
baseline ANC.
While TAS-102 pharmacokinetics can be affected by
renal function due to the renal excretion of tipiracil, 3 our
analyses did not detect a significant impact of renal function on
toxicity or efficacy, aligning with previous studies (23,24).
Traditional chemotherapy dosing based on body
surface area (BSA) may not fully account for interindividual
variability; lean body mass (LBM) and body composition analyses are
emerging tools for dose individualization (25-27).
However, these factors were not directly assessed in our study and
should be explored in future research.
Limitations of this study include its single-center
retrospective design, which may introduce selection bias and limit
generalizability. Additionally, patients receiving TAS-102 combined
with bevacizumab, a common regimen that may increase neutropenia
incidence, were excluded. The relatively small sample size in
certain subgroups and absence of multivariate analysis also limit
definitive conclusions. Therefore, future studies with larger
sample sizes are needed to confirm these findings.
Current NCCN and ESMO guidelines recommend
initiating TAS-102 at full approved doses, with dose modifications
guided by observed toxicities rather than preemptive reductions
(28,29). Our findings support this approach,
suggesting that initial dose reduction based solely on prior severe
neutropenia may not be necessary. Standard dosing with close
monitoring remains the preferred clinical strategy.
In conclusion, although grade ≥3 neutropenia
frequently leads to treatment interruptions during TAS-102
monotherapy, preemptive initial dose reduction does not reduce
neutropenia incidence or improve survival outcomes. Careful
monitoring and appropriate dose adjustments during treatment are
essential to optimize efficacy and safety.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CS and MK contributed to the study design, collected
and provided the data, served as the principal authors of the
report, and are the guarantors of the article and all associated
data. MG, YI and EU contributed to the study design, reviewed the
manuscript, and supervised the drafting of the report and the
submission process. CS and MK confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board of Ogaki Municipal Hospital (Ogaki, Japan; approval
no. 202520227-22). Consent to participate was waived by the
Institutional Review Board of Ogaki Municipal Hospital owing to the
retrospective study design.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: Randomized trial of TAS-102 for refractory
metastatic colorectal cancer. N Engl J Med. 372:1909–1919.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xu J, Kim TW, Shen L, Sriuranpong V, Pan
H, Xu R, Guo W, Han SW, Liu T, Park YS, et al: Results of a
randomized, double-blind, placebo-controlled, phase III trial of
trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with
previously treated metastatic colorectal cancer: The TERRA study. J
Clin Oncol. 36:350–358. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pfeiffer P, Yilmaz M, Möller S, Zitnjak D,
Krogh M, Petersen LN, Poulsen LØ, Winther SB, Thomsen KG and
Qvortrup C: TAS-102 with or without bevacizumab in patients with
chemorefractory metastatic colorectal cancer: An
investigator-initiated, open-label, randomised, phase 2 trial.
Lancet Oncol. 21:412–420. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kimura M, Usami E, Iwai M, Teramachi H and
Yoshimura T: Severe neutropenia: A prognosticator in patients with
advanced/recurrent colorectal cancer under oral
trifluridine-tipiracil (TAS-102) chemotherapy. Pharmazie. 72:49–52.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nose Y, Kagawa Y, Hata T, Mori R, Kawai K,
Naito A, Sakamoto T, Murakami K, Katsura Y, Ohmura Y, et al:
Neutropenia is an indicator of outcomes in metastatic colorectal
cancer patients treated with FTD/TPI plus bevacizumab: A
retrospective study. Cancer Chemother Pharmacol. 86:427–433.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kasi PM, Kotani D, Cecchini M, Shitara K,
Ohtsu A, Ramanathan RK, Hochster HS, Grothey A and Yoshino T:
Chemotherapy induced neutropenia at 1-month mark is a predictor of
overall survival in patients receiving TAS-102 for refractory
metastatic colorectal cancer: A cohort study. BMC Cancer.
16(467)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kamiimabeppu D, Osumi H, Shinozaki E, Ooki
A, Wakatsuki T, Yoshino K, Sato T, Nakayama I, Ogura M, Takahari D,
et al: Effect of neutropenia on survival outcomes of patients with
metastatic colorectal cancer receiving trifluridine/tipiracil plus
bevacizumab. Oncol Lett. 22(783)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
US Department Of Health And Human
Services: Common terminology criteria for adverse events (CTCAE)
version 5.0. United States, National Cancer Institute, 2017.
(https://ctep.Cancer.Gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf).
|
|
9
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ikagawa M, Kimura M, Iwai M, Usami E,
Yoshimura T and Yasuda K: Neutropenia as a prognostic factor and
safety of second-line therapy with S-1 for advanced or recurrent
pancreatic cancer. Mol Clin Oncol. 5:283–288. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kimura K, Usami E and Yoshimura T:
Association between severe neutropenia and progression-free
survival in patients with advanced or recurrent breast cancer
treated with Palbociclib. Pharmazie. 75:662–665. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Watanabe D, Fujii H, Ohata K, Iihara H,
Makiyama A, Kobayashi R, Hirose C, Hishida S, Matsuoka S, Tajima
JY, et al: Prognostic impact of severe neutropenia in colorectal
cancer patients treated with TAS-102 and bevacizumab, addressing
immortal-time bias. BMC Cancer. 23(1078)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Domínguez Senín L, Rodriguez Garcés MY,
Aviñó Tarazona V, Amor Urbano M, Santos-Rubio MD and Bayo Calero J:
Analysis of neutropenia as a predictive factor of the efficacy of
trifluridine-tipiracil treatment. Int J Clin Pharmacol Ther.
61:346–353. 2023.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Giuliani J and Bonetti A: The onset of
grade ≥3 neutropenia is associated with longer overall survival in
metastatic colorectal cancer patients treated with
trifluridine/tipiracil. Anticancer Res. 39:3967–3969.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shitara K, Matsuo K, Takahari D, Yokota T,
Inaba Y, Yamaura H, Sato Y, Najima M, Ura T and Muro K:
Neutropaenia as a prognostic factor in metastatic colorectal cancer
patients undergoing chemotherapy with first-line FOLFOX. Eur J
Cancer. 45:1757–1763. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shitara K, Matsuo K, Takahari D, Yokota T,
Shibata T, Ura T, Ito S, Sawaki A, Tajika M, Kawai H and Muro K:
Neutropenia as a prognostic factor in advanced gastric cancer
patients undergoing second-line chemotherapy with weekly
paclitaxel. Ann Oncol. 21:2403–2409. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gurney H: Dose calculation of anticancer
drugs: A review of the current practice and introduction of an
alternative. J Clin Oncol. 14:2590–2611. 1996.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gamelin E, Delva R, Jacob J, Merrouche Y,
Raoul JL, Pezet D, Dorval E, Piot G, Morel A and Boisdron-Celle M:
Individual fluorouracil dose adjustment based on pharmacokinetic
follow-up compared with conventional dosage: Results of a
multicenter randomized trial of patients with metastatic colorectal
cancer. J Clin Oncol. 26:2099–2105. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yoshino T, Cleary JM, Van Cutsem E, Mayer
RJ, Ohtsu A, Shinozaki E, Falcone A, Yamazaki K, Nishina T,
Garcia-Carbonero R, et al: Neutropenia and survival outcomes in
metastatic colorectal cancer patients treated with
trifluridine/tipiracil in the RECOURSE and J003 trials. Ann Oncol.
31:88–95. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yoshino T and Lenz HJ: Reply to the letter
to the editor ‘Neutropenia in metastatic colorectal cancer
receiving trifluridine/tipiracil’ by Colloca et al. Ann
Oncol. 31:1085–1087. 2020.
|
|
21
|
Grothey A, Yoshino T, Bodoky G, Ciuleanu
T, Garcia-Carbonero R, García-Alfonso P, Van Cutsem E, Muro K,
Mytelka DS, Li L, et al: Association of baseline absolute
neutrophil counts and survival in patients with metastatic
colorectal cancer treated with second-line antiangiogenic
therapies: Exploratory analyses of the RAISE trial and validation
in an electronic medical record data set. ESMO Open.
3(e000347)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Carus A, Gurney H, Gebski V, Harnett P,
Hui R, Kefford R, Wilcken N, Ladekarl M, von der Maase H and
Donskov F: Impact of baseline and nadir neutrophil index in
non-small cell lung cancer and ovarian cancer patients: Assessment
of chemotherapy for resolution of unfavourable neutrophilia. J
Transl Med. 11(189)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yasue F, Kimura M, Usami E, Iwai M,
Kawachi S, Mitsuoka M, Ikeda Y and Yoshimura T: Risk factors
contributing to the development of neutropenia in patients
receiving oral trifluridine-tipiracil (TAS-102) chemotherapy for
advanced/recurrent colorectal cancer. Pharmazie. 73:178–181.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Saito Y, Takekuma Y, Komatsu Y and
Sugawara M: Impact of renal impairment on early development of
severe neutropenia with trifluridine/tipiracil treatment for
metastatic colorectal cancer. Sci Rep. 14(26990)2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gurney H: How to calculate the dose of
chemotherapy. Br J Cancer. 86:1297–1302. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Newell DR: Getting the right dose in
cancer chemotherapy-time to stop using surface area? Br J Cancer.
86:1207–1208. 2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Drami I, Pring ET, Gould L, Malietzis G,
Naghibi M, Athanasiou T, Glynne-Jones R and Jenkins JT: Body
composition and dose-limiting toxicity in colorectal cancer
chemotherapy treatment; a systematic review of the literature.
Could muscle mass be the new body surface area in chemotherapy
dosing? Clin Oncol (R Coll Radiol). 33:e540–e552. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Benson AB, Venook AP, Adam M, Chang G,
Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming D, Garrido-Laguna
I, et al: Colon Cancer, version 3.2024, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 22 (2
D)(e240029)2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kiss I: New ESMO guidelines for clinical
practice in metastatic colorectal cancer-commentary on changes in
systemic therapy. Klin Onkol. 36:473–476. 2023.PubMed/NCBI View Article : Google Scholar
|