Introduction
Nasopharyngeal carcinoma (NPC) poses a global health
burden with the highest incidence rates in Southeast Asia, South
China and North Africa. Based on the 2020 global cancer statistics,
there were ~133,000 new cases of NPC worldwide, accounting for 0.7%
of all newly diagnosed cancer cases, with 80,000 deaths,
representing 0.8% of global cancer deaths (1). Both incidence and mortality rates
have increased compared with 2018 statistics (129,079 new cases and
72,987 deaths), suggesting a potential upward trend (2). In 2022, China is estimated to have
~64,165 new cases of NPC and 36,315 deaths (3), despite advancements in diagnostic and
therapeutic technologies, NPC incidence and mortality rates have
not substantially declined. Common clinical manifestations of NPC
include nasal congestion, epistaxis, otalgia or ear fullness,
hearing loss, diplopia and headaches. Currently, a combination of
chemotherapy and radiotherapy is a key strategy for treating
advanced-stage NPC. Due to improvements in diagnostic imaging,
early detection through large-scale screening, and the emergence of
Intensity Modulated Radiation Therapy (IMRT), the survival rate of
locally advanced NPC has increased (4,5).
However, even after treatment, ~5-15% of patients with advanced NPC
experience local recurrence, and clinical manifestations of distant
metastasis are observed in 15-30% of patients (4). Distant metastasis is the most common
post-treatment failure pattern (6). For locally advanced NPC, clinical
trials have demonstrated that adjuvant chemotherapy after
radiotherapy significantly improves the failure-free survival rate
in patients with advanced NPC. It is not only manageable in terms
of safety but also does not affect the quality of life (7).
Previous studies have explored the impact of
clinical, histological and biochemical factors on patients with
NPC. Among them, disease staging and plasma Epstein-Barr virus
(EBV) DNA concentration have been considered routine prognostic
factors in clinical practice (8,9).
Baseline serum lactate dehydrogenase (LDH) levels are also
associated with prognosis in patients with NPC undergoing radical
IMRT treatment (10). However,
even among patients with identical disease stages and pretreatment
EBV DNA levels, NPC exhibits significant biological heterogeneity,
making prognosis prediction challenging. Exploring new prognostic
factors to guide clinical decisions and provide favorable and
accurate treatment for NPC patients is urgently needed.
Serum uric acid (SUA) is the final product of purine
metabolism, possessing dual functions of antioxidation and
pro-oxidation (11). As a systemic
antioxidant, it plays a crucial role in protecting cells from
damage induced by free radicals. Previous studies on elevated SUA
indicate a risk of poor survival in patients with advanced HCC
(12). Furthermore, research has
demonstrated a positive correlation between uric acid levels and
the incidence of kidney cancer, especially in female subjects
(13). Meanwhile, the latest
studies suggest the practicality of UA as a clinical target for
breast cancer prevention. The public and clinical significance of
reducing SUA may help reduce the incidence of breast cancer in
overweight postmenopausal women (14). Most previous studies have focused
on pre-treatment SUA, observing its predictive role in diseases,
with little exploration of post-chemotherapy SUA. The predictive
value of SUA levels after the end of the treatment cycle in
patients with advanced NPC has not been determined. The present
study aimed to confirm whether post-chemotherapy SUA levels have
prognostic significance for patients with advanced NPC, potentially
informing optimal treatment selection.
Materials and methods
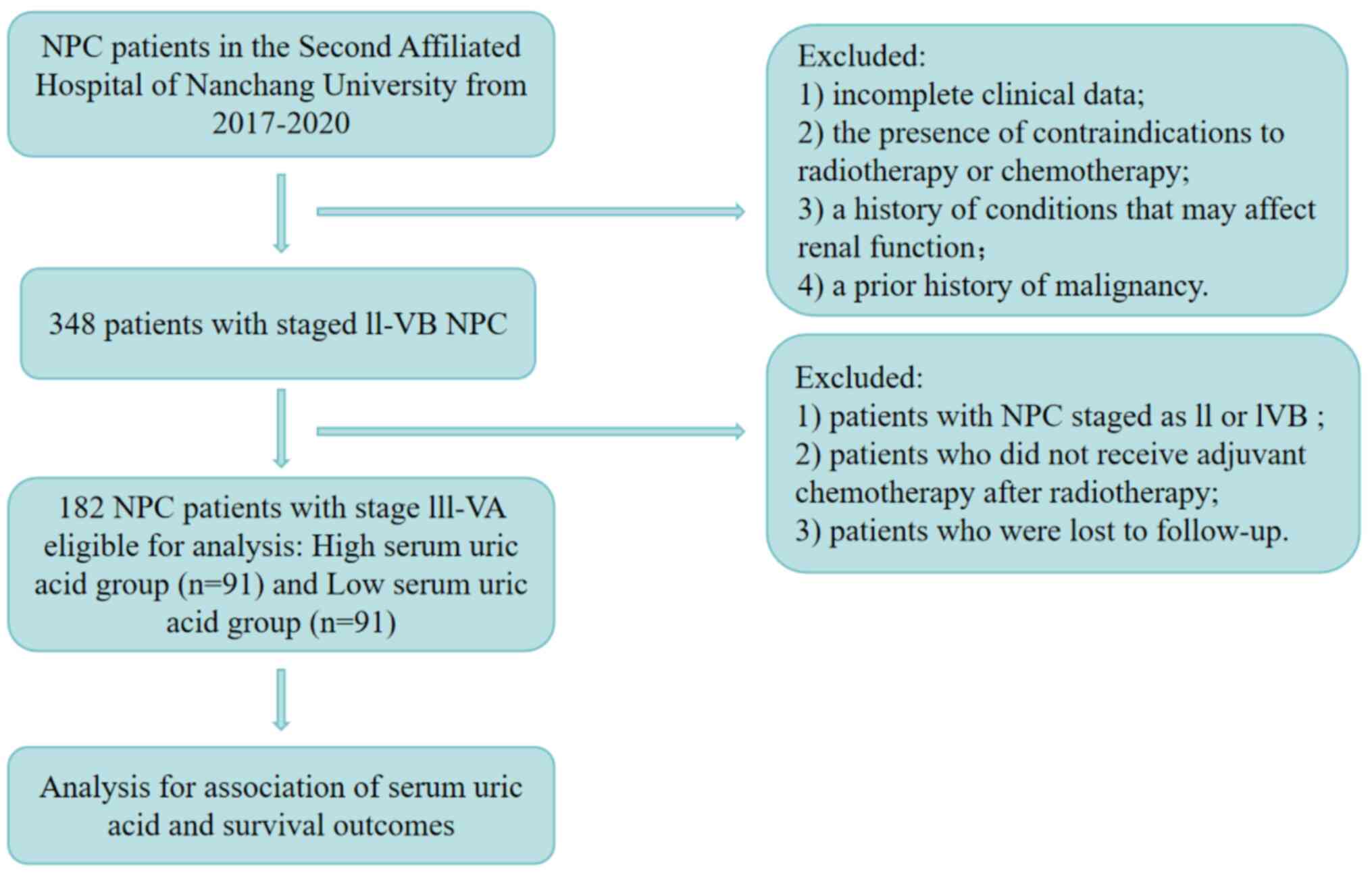
The present study included 182 patients with locally
advanced NPC who were admitted in January 2020, confirmed by
pathological diagnosis and without distant metastasis at
presentation. The cohort consisted of 119 male (65.4%) and 63
female (34.6%) patients. The median age was 50 years (range: 18-78
years). Inclusion criteria (all required): i) Patients initially
diagnosed with NPC by the Department of Pathology of The Second
Affiliated Hospital of Nanchang University (Nanchang, China); ii)
exclusion of hyperuricemia or gout before treatment; iii) patients
staged from III to IVb according to the 7th edition of AJCC staging
criteria in 2010; iv) patients who have not undergone antitumor
treatments such as radiotherapy and chemotherapy in other
hospitals; and v) availability of complete clinical data. Exclusion
criteria (any of the following): i) Patients with incomplete
clinical data; ii) patients with contraindications to radiotherapy
and chemotherapy; iii) patients with a history of diabetes,
hypertension, rheumatic autoimmune diseases, or other conditions
that may affect kidney function; iv) patients with a history of
hyperuricemia, gout, renal insufficiency, or other conditions
affecting SUA levels; v) patients with a history of malignant
tumors; vi) patients concurrently suffering from malignant tumors
in other organs; and vii) patients who did not complete
intensity-modulated radiation therapy within the specified time.
Pretreatment evaluation included blood biochemistry, fiberoptic
nasopharyngoscopy, nasopharyngeal and neck magnetic resonance
imaging (MRI), chest computed tomography (CT), abdominal
ultrasonography and whole-body bone scan. All SUA measurements were
performed in the same central laboratory of the Second Affiliated
Hospital of Nanchang University following standardized clinical
procedures. The flowchart for patient selection is shown in
Fig. 1.
Treatment methods
The present study was approved (approval no.
IIT-O-2023-169; Nanchang, China) by the Ethics Committee of the
Second Affiliated Hospital of Nanchang University. All 182 patients
received comprehensive treatment.
Regarding chemotherapy, all patients were treated
under a comprehensive model for advanced NPC. This model combines
induction chemotherapy with adjuvant chemotherapy based on radical
IMRT. Regardless of concurrent or sequential chemotherapy, all
patients completed the full course of induction chemotherapy and
adjuvant chemotherapy. The chemotherapy regimen included drugs such
as gemcitabine, docetaxel, or paclitaxel in combination with
platinum-based agents. The platinum-based chemotherapy drugs used
were cisplatin, carboplatin, or nedaplatin. Of the included
patients with NPC, 100 (54.9%) received concurrent chemotherapy.
Monotherapy (single-agent gemcitabine or single-agent
platinum-based synchronous chemotherapy) and combination
chemotherapy with two drugs were used as synchronous chemotherapy
regimens. The two-drug chemotherapy was based on platinum
agents.
Radiotherapy was planned according to the 2010 NPC
Intensity-Modulated Radiation Therapy Target Area and Dose Design
Expert Consensus, and the 2017 NPC Clinical Target Volume
International Guidelines, and other relevant literature.
Radiotherapy planning followed the 2010 NPC Intensity-Modulated
Radiation Therapy Target Area and Dose Design Expert Consensus and
the 2017 NPC Clinical Target Volume International Guidelines. The
prescribed doses for each planned target area are as follows: the
primary tumor (GTVnx) is 70-74 Gy, positive lymph nodes (GTVnd) is
66-70 Gy, high-risk clinical target volume (CTV1) is 60-66 Gy,
ow-risk clinical target volume (CTV2) is 56-60 Gy, and bilateral
neck lymph drainage area (CTVLn) is 50-54 Gy. Patients included in
the study were treated with conventional radiotherapy, once a day,
5 times a week. According to the standards of the Radiation Therapy
Oncology Group 0255 trial, the irradiation doses to organs at risk
were kept within the specified tolerable dose range (6). Patients with MRI-detected residual
lesions after intensified radiotherapy received an additional 2-3
fractions of 4-6 Gy to improve local control. For well-responding
small primary lesions, a slight reduction in the total dose can be
considered (for example, 66-68 Gy). Overall, out of 182 patients,
82 received only IMRT, and 100 patients received concurrent
chemotherapy on the basis of IMRT. Treatment modifications were
made for patients with age-related limitations or organ dysfunction
that might indicate intolerance to standard chemotherapy
regimens.
Follow-up and statistical
analysis
Follow-up commenced from the date of NPC treatment
and continued until patient death or the last follow-up. Survival
endpoints include overall survival (OS), progression-free survival
(PFS), distant metastasis-free survival (DMFS) and locoregional
relapse-free survival (LRFS). OS was defined as the time from the
initiation of treatment to death from any cause or the last
follow-up if no death occurred; PFS was defined as the time from
the initiation of treatment to disease progression, death from any
cause, or the last follow-up, whichever occurred first. DMFS was
defined as the time from the initiation of treatment to the
occurrence of distant metastasis or the last follow-up if no
distant metastasis occurred. LRFS was defined as the time from the
initiation of treatment to the first local primary lesion or neck
lymph node treatment failure (including recurrence) or the last
follow-up if no treatment failure occurred. Patients were assessed
every 3 months for the first 3 years, and then every 6 months
thereafter until death. The last follow-up time was July 15, 2023,
with a median follow-up time of 50 months (range 30.0-71.0
months).
All statistical analyses were performed using SPSS
26.0 software (IBM Corp.). Survival analysis employed the
Kaplan-Meier method, with log-rank tests for comparing two groups.
Continuous variables were compared using independent samples
t-tests, while categorical variables were analyzed using chi-square
tests. Multivariate analysis was performed using the Cox
proportional hazards model, with independent significance tested by
backward elimination of non-significant explanatory variables. All
trials include host factors (including age and sex) and clinical
factors (including T classification, N classification and the
presence of synchronous radio-chemotherapy) as covariates. The
statistical significance criterion was set at P<0.05, with
P-values using two-sided tests. A repeated measures analysis of
variance was employed to compare pretreatment SUA, post-induction
chemotherapy SUA, post-radiotherapy SUA and post-adjuvant
chemotherapy SUA for all patients.
Results
Patient characteristics and
prognosis
Baseline characteristics of 182 patients with NPC
were analyzed to evaluate correlations between SUA levels and
clinical features. Patients were stratified into high (SUA >350
µmol/l) and low (SUA ≤350 µmol/l) groups based on the median
post-adjuvant chemotherapy SUA level (350.48 µmol/l) of the entire
cohort. This median value was close to the upper limit of the
normal reference range for SUA in numerous clinical laboratories,
thus providing both a statistical and clinically relevant cutoff
point for analysis. Demographically, the mean ages of the two
groups were 51.77 years and 49.11 years, respectively, showing a
slight but statistically significant difference (P=0.026). In terms
of sex distribution, a higher proportion of men were observed in
the higher SUA group (69.2 vs. 61.5%, P=0.027), while women were
slightly more prevalent in the lower SUA group. WHO Type III was
the predominant histopathological subtype in both groups (70.4 and
76.9%), with no statistically significant difference (P=0.313).
There was also no statistically significant difference in the
distribution of EBV-DNA status between the two groups (P=0.182).
Smoking history and family history did not show statistically
significant differences between the groups (P=0.553). Regarding TNM
staging, the proportions of T3-T4 and N2-N3 stages were slightly
higher in the high SUA group; only N staging approached statistical
significance (P=0.097), while T staging showed no statistically
significant difference (P=0.344). Treatment modalities
(radiotherapy alone vs. chemoradiotherapy) were similarly
distributed between the two groups (P=0.766). These baseline data
(Table I) provide important
context for further analysis of the impact of SUA levels on
treatment response and prognosis in patients with NPC.
 | Table IDemographical characteristics and
clinical data of the patients. |
Table I
Demographical characteristics and
clinical data of the patients.
| Characteristics | SUA after treatment
of IC ≤350 µmol/l (%) | SUA after treatment
of IC >350 µmol/l (%) | P-value |
|---|
| Total | 91 (50.0%) | 91 (50.0%) | |
| Age (years, mean ±
SD) | 51.77±8.95 | 49.11±11.87 | 0.026 |
| Sex | | | 0.027 |
|
Male | 56 (61.5%) | 63 (69.2%) | |
|
Female | 35 (38.5%) | 28 (30.8%) | |
| Pathological
type | | | 0.313 |
|
WhO II | 27 (29.6%) | 21 (23.1%) | |
|
Who III | 64 (70.4%) | 70 (76.9%) | |
| EBV-DNA status | | | 0.182 |
|
EBV-DNA
negative | 52 (57.1%) | 43 (47.3%) | |
|
EBV-DNA
positive | 39 (42.9%) | 48 (52.7%) | |
| Smoking
history | | | 0.553 |
|
No | 47 (51.6%) | 38 (41.8%) | |
|
Yes | 44 (48.4%) | 53 (58.2%) | |
| Family history of
nasopharyngeal carcinoma | | | 0.553 |
|
No | 84 (92.3%) | 86 (94.5%) | |
|
Yes | 7 (7.7%) | 5 (5.5%) | |
| T stage | | | 0.344 |
|
T1-T2 | 33 (36.3%) | 27 (29.7%) | |
|
T3-T4 | 58 (63.7%) | 64 (70.3%) | |
| N stage | | | 0.097 |
|
N0-N1 | 30 (33.3%) | 20 (22.0%) | |
|
N2-N3 | 61 (67.0%) | 71 (78.0%) | |
| Chemotherapy | | | 0.766 |
|
Radiotherapy
alone | 40 (44.0%) | 42 (46.2%) | |
|
Chemoradiotherapy | 51 (56.0%) | 49 (53.8%) | |
Correlation of post-treatment uric
acid levels with prognosis
At the end of follow-up, 6 cases (3.2%) had local or
regional recurrence, and 15 cases (8.2%) had distant metastasis,
including 5 cases of lung metastasis, 2 cases of bone metastasis, 3
cases of liver metastasis, 1 case of lymph node metastasis, 3 cases
of multiple-site metastasis, and 1 case of metastasis to other
sites (cardia). In total, 22 patients (12.2%) succumbed, including
20 due to tumor progression, 1 due to cerebral infarction, and 1
due to other causes.
After completing the entire treatment cycle, the
median SUA level for the entire cohort was 350.48 µmol/l.
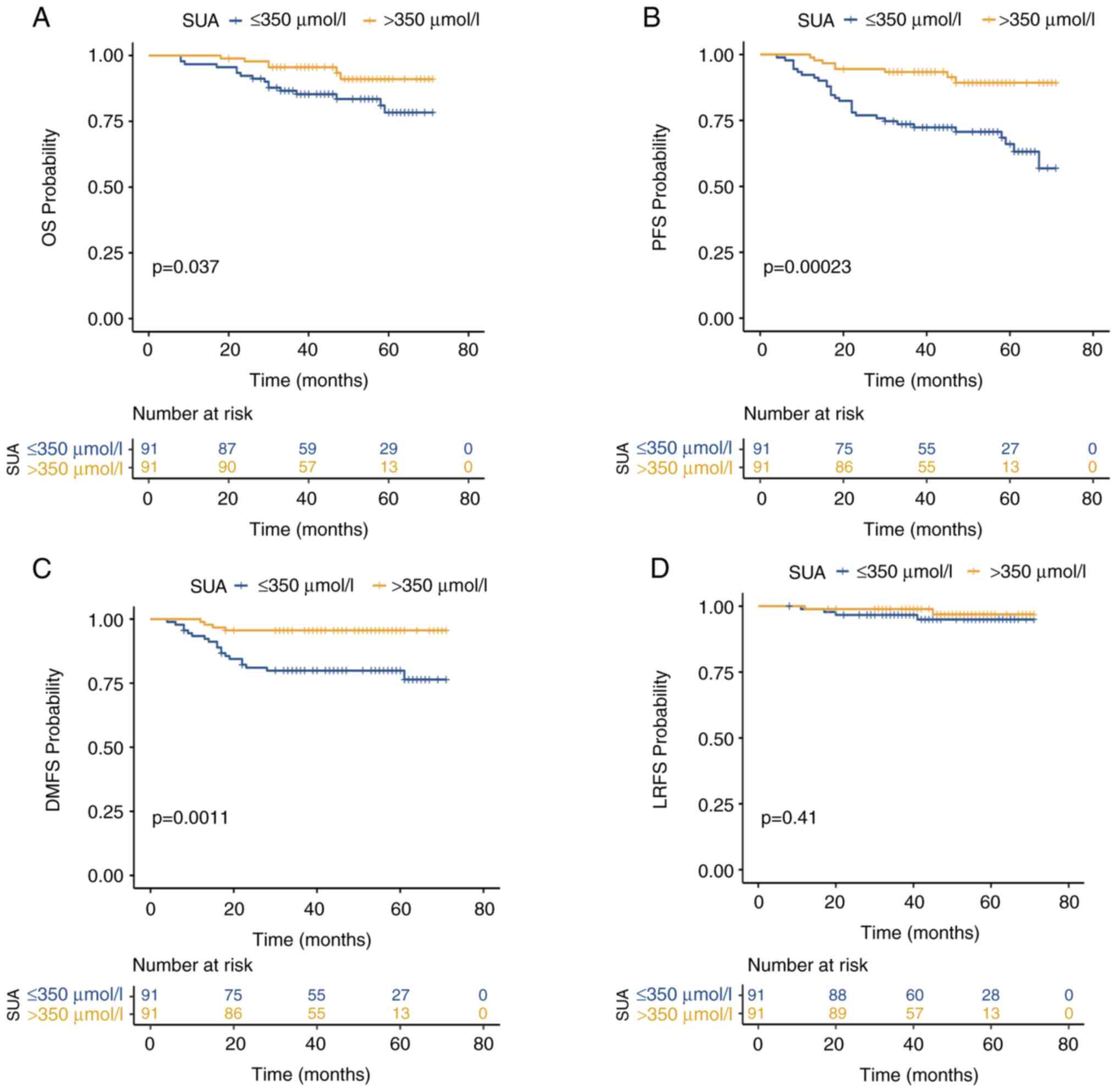
Kaplan-Meier analysis revealed significantly superior 3-year OS,
PFS and DMFS in the high SUA group compared with the low SUA group:
3-year OS, 95.6% [95% confidence interval (CI):8 5.54-90.30%] vs.
85.3% (95% CI: 79.14-88.57%; P=0.037) (Fig. 2A); 3-year PFS, 93.4% (95% CI:
88.44-95.48%) vs. 72.4% (95% CI: 64.44-78.48%; P<0.001)
(Fig. 2B). In the 3-year DMFS
analysis, the survival rate was 95.6% (95% CI: 86.14-98.90%c)
compared with 79.9% (95% CI: 71.72-86.10%; P=0.0011) (Fig. 2C). However, there was no
statistically significant difference in the 3-year LRFS between the
two groups: 98.9% (95% CI: 94.12-99.44%) vs. 94.9% (95% CI:
88.39-97.91%; P=0.41) (Fig. 2D).
The Kaplan-Meier survival curves for these four subgroups are
shown. Post-treatment SUA levels showed statistically significant
associations with OS, PFS and DMFS (P=0.037, P<0.001 and
P<0.001, respectively). Patients with higher uric acid levels
have improved survival rates. However, these increases do not
indicate a significant correlation between uric acid levels and
LRFS rates (P=0.41).
Prognostic factors for survival
outcomes in NPC: Univariate and multivariate analysis
The present retrospective study aimed to evaluate
the prognostic factors influencing survival outcomes in patients
with NPC by assessing the association between various clinical
variables and patient outcomes, particularly OS, PFS, DMFS and
LRFS. The univariate analysis indicated that age (P=0.007) and
EBV-DNA status (P=0.012) were significantly associated with OS. In
the multivariate analysis, age [hazard ratio (HR): 1.059, 95% CI:
1.012-1.108; P=0.013] and EBV-DNA status (HR: 4.043; 95% CI:
1.576-10.44; P=0.004) remained independent prognostic factors of
OS. An increase in age was associated with a higher HR for
decreased OS, and EBV-DNA positivity was linked to a worse
prognosis. The univariate analysis showed that age (P=0.22), sex
(P=0.04), EBV-DNA status (P=0.003) and N stage (P=0.013) were
associated with PFS. In the multivariate analysis, sex (HR: 2.530;
95% CI: 1.098-5.826; P=0.029), EBV-DNA status (HR: 3.631, 95% CI:
1.816-7.258; P<0.001) and N stage (HR: 5.004, 95% CI:
1.516-15.52; P=0.008) were confirmed as independent predictors of
PFS. Male sex, EBV-DNA positivity and advanced N stage were all
associated with a higher risk of disease progression. The
univariate analysis identified sex (P=0.039), EBV-DNA status
(P=0.024) and N stage (P=0.169) as factors associated with DMFS. In
the multivariate analysis, sex (HR: 5.023; 95% CI: 1.475-17.10;
P=0.01) and EBV-DNA status (HR 3.851; 95% CI: 1.562-9.490; P=0.003)
emerged as independent predictors of DMFS. Male sex and EBV-DNA
positivity were associated with a higher risk of distant
metastasis. The univariate analysis revealed that pathological type
(P=0.054) and T stage (P=0.033) were associated with LRFS. However,
in the multivariate analysis, only SUA level (HR: 0.149; 95% CI:
0.050-0.442; P=0.001) was confirmed as an independent predictor of
DMFS. Lower SUA levels were associated with a higher risk of LRFS.
SUA level was found to be a significant prognostic factor for OS
(multivariate HR: 0.265; 95% CI: 0.101-0.690; P=0.007), PFS
(multivariate HR: 0.168; 95% CI: 0.076-0.372; P<0.001) and DMFS
(multivariate HR: 0.149; 95% CI: 0.050-0.442; P=0.001). Higher SUA
levels were consistently associated with better outcomes across
these endpoints (Table II).
 | Table IIUnivariate and multivariate analysis
of prognostic factors for patients with nasopharyngeal
carcinoma. |
Table II
Univariate and multivariate analysis
of prognostic factors for patients with nasopharyngeal
carcinoma.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Endpoint | Variable | P-value | HR (95% CI) | P-value | HR (95% CI) |
|---|
| OS | Age | 0.007 | 1.072
(1.019-1.028) | 0.013 | 1.059
(1.012-1.108) |
| | Sex | 0.284 | 1.726
(0.636-4.686) | | |
| | Pathological
type | 0.651 | 1.230
(0.501-3.020) | | |
| | EBV-DNA status | 0.012 | 3.255
(1.297-8.170) | 0.004 | 4.043
(1.576-10.44) |
| | Smoking
history | 0.869 | 1.078
(0.467-2.489) | | |
| | Family history of
NPC | 0.185 | 0.438
(0.129-0.483) | | |
| | T stage | 0.346 | 1.552
(0.622-3.872) | | |
| | N stage | 0.034 | 9.038
(1.185-68.96) | | |
| | Chemotherapy | 0.377 | 1.477
(0.622-3.511) | | |
| | SUA level | 0.028 | 0.344
(0.133-0.890) | 0.007 | 0.265
(0.101-0.690) |
| PFS | Age | 0.220 | 1.022
(0.987-1.058) | | |
| | Sex | 0.040 | 2.361
(1.038-5.368) | 0.029 | 2.530
(1.098-5.826) |
| | Pathological
type | 0.068 | 1.866
(0.973-3.577) | | |
| | EBV-DNA status | 0.003 | 2.806
(1.411-5.580) | 0.000 | 3.631
(1.816-7.258) |
| | Smoking
history | 0.587 | 1.194
(0.629-2.264) | | |
| | Family history of
NPC | 0.264 | 1.807
(0.640-5.105) | | |
| | T stage | 0.776 | 1.102
(0.563-2.160) | | |
| | N stage | 0.013 | 3.865
(1.323-11.29) | 0.008 | 5.004
(1.516-15.52) |
| | Chemotherapy | 0.350 | 1.368
(0.709-2.640) | | |
| | SUA level | 0.000 | 0.238
(0.108-0.526) | 0.0001 | 0.168
(0.076-0.372) |
| DMFS | Age | 0.635 | 0.990
(0.950-1.032) | | |
| | Sex | 0.039 | 3.602
(1.069-12.13) | 0.010 | 5.023
(1.475-17.10) |
| | Pathological
type | 0.153 | 1.841
(0.797-4.253) | | |
| | EBV-DNA status | 0.024 | 2.809
(1.144-6.897) | 0.003 | 3.851
(1.562-9.490) |
| | Smoking
history | 0.606 | 1.242
(0.545-2.833) | | |
| | Family history of
NPC | 0.716 | 1.451
(0.195-10.77) | | |
| | T stage | 0.831 | 0.910
(0.381-2.173) | | |
| | N stage | 0.169 | 2.225
(0.712-6.953) | | |
| | Chemotherapy | 0.377 | 1.481
(0.619-3.541) | | |
| | SUA level | 0.002 | 0.180
(0.060-0.539) | 0.001 | 0.149
(0.050-0.442) |
| LRFS | Age | 0.237 | 1.061
(0.962-1.170) | | |
| | Sex | 0.426 | 0.522
(0.105-2.589) | | |
| | Pathological
type | 0.054 | 5.315
(0.972-29.06) | | |
| | EBV-DNA status | 0.731 | 1.328
(0.264-6.686) | | |
| | Smoking
history | 0.136 | 5.115
(0.597-43.78) | | |
| | Family history of
NPC | 0.662 | 2.251
(0.575-8.527) | | |
| | T stage | 0.033 | 0.073
(0.007-0.815) | | |
| | N stage | 0.579 | 0.498
(0.043-5.821) | | |
| | Chemotherapy | 0.439 | 1.969
(0.354-10.96) | | |
| | SUA level | 0.745 | 0.748
(0.130-4.292) | | |
Repeated measures analysis of SUA
levels at different time points in patients with NPC
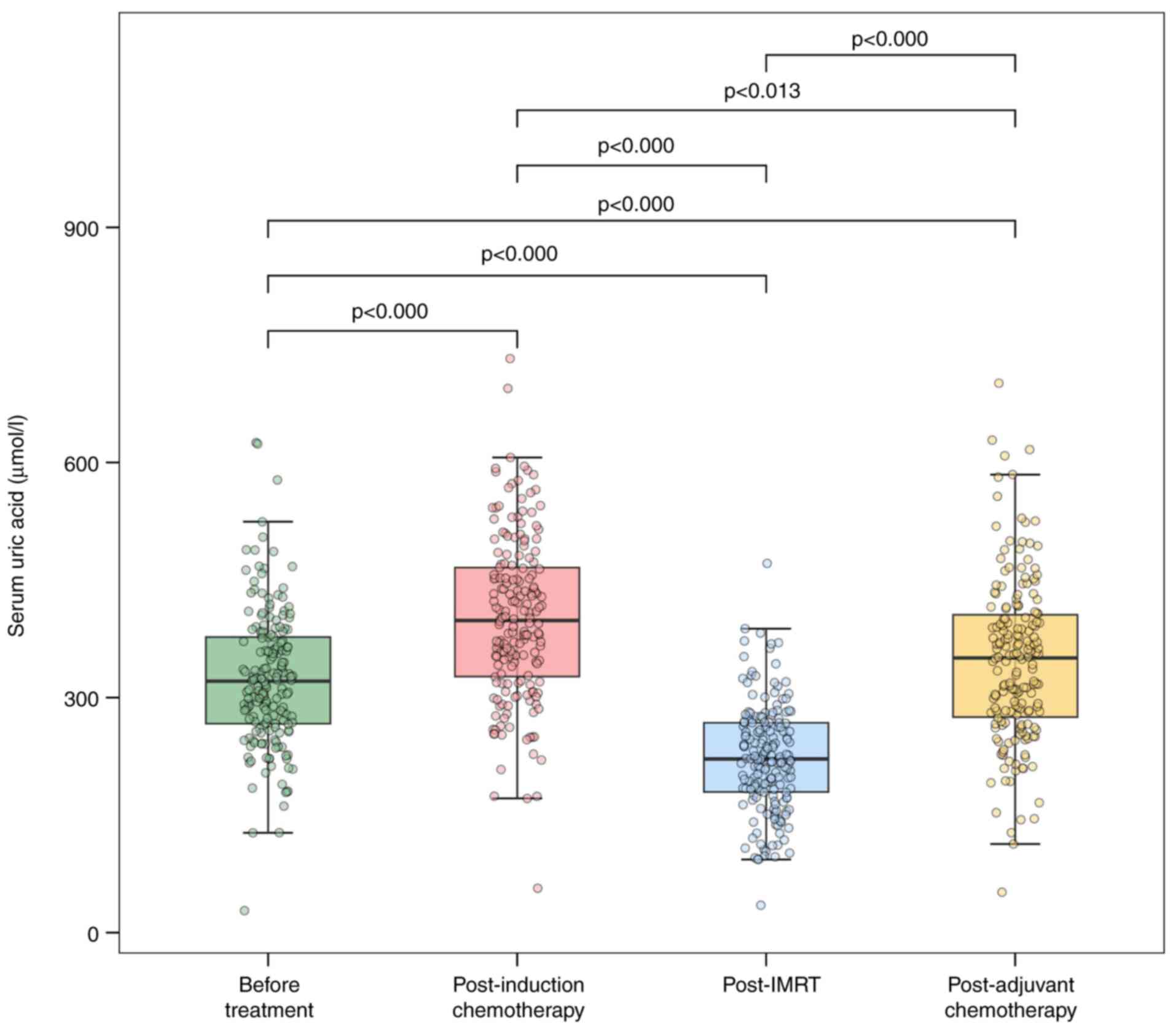
A total of 182 patients with NPC who completed
induction chemotherapy, radiotherapy and adjuvant chemotherapy were
included in this study. SUA levels were measured at four time
points: Pretreatment [mean=323.69 µmol/l, standard deviation
(SD)=87.56 µmol/l), post-induction chemotherapy (mean=400.40
µmol/l; SD=104.45 µmol/l), post-radiotherapy (mean=222.84 µmol/l;
SD=68.47 µmol/l) and post-adjuvant chemotherapy (mean=346.24
µmol/l; SD=104.05 µmol/l). The Greenhouse-Geisser correction for
sphericity was applied, with an estimated ε=0.894, indicating a
significant difference in SUA levels across the four measurement
points [F (2.68, 485.25)=282.76; P<0.001, partial η²=0.61)].
Post-hoc multiple comparisons using the Bonferroni
adjustment revealed significantly lower SUA levels
post-radiotherapy compared with pretreatment [t(543)=-19.10;
P<0.001; Cohen s d=-1.47], post-induction chemotherapy
[t(543)=-29.15; P<0.001; Cohen s d=-2.59] and post-adjuvant
chemotherapy [t(543)=-18.50; P<0.001; Cohen s d=-1.80].
Repeated-measures ANOVA confirmed that SUA levels were lowest
post-radiotherapy compared with all other time points (Fig. 3).
Discussion
SUA, the end product of purine metabolism excreted
by the kidneys and intestines, is a potent antioxidant that
scavenges singlet oxygen and free radicals (15). Its role in tumor biology is complex
and not fully understood, with elevated levels potentially
inhibiting or promoting tumorigenesis. Numerous clinical studies
have investigated the association between SUA and the prognosis of
various cancers, reporting correlations with tumor incidence,
mortality and metastatic potential. Theoretically, elevated SUA
levels may prevent tumor occurrence by enhancing antioxidant
effects. However, extensive research results suggest that elevated
SUA levels are associated with increased tumor incidence, mortality
rates (12-14,16-22)
and promotion of tumor metastasis (23). Previously, an increasing body of
research has confirmed that elevated SUA levels can have a
protective effect, contributing to improved patient survival rates
(24-27).
Therefore, it was investigated whether SUA levels correlate with
OS, PFS, DMFS and LRFS in patients with NPC following induction
chemotherapy, IMRT and adjuvant chemotherapy.
In the present study, based on the median uric acid
level in the plasma after adjuvant chemotherapy being 350.45
µmol/l, 182 patients with NPC who completed the full treatment
course were divided into two groups, the high SUA group (SUA
>350 µmol/l) and the low SUA group (SUA ≤350 µmol/l). The
results showed that post-adjuvant chemotherapy SUA levels could
serve as prognostic biomarkers for OS, PFS and DMFS. The OS, PFS
and DMFS were significantly higher in the high SUA group than in
the low SUA group (P=0.037, P<0.001 and P=0.001, respectively).
However, the LRFS difference showed no statistical significance
(P=0.424). Multifactorial analysis has revealed that the SUA level
after adjuvant chemotherapy is an independent prognostic factor for
patients with NPC. The high SUA group has an improved prognosis
than the low SUA group, which is consistent with the conclusions of
the aforementioned study. Consistent with the present findings,
previous research reported increased SUA levels in patients with
metastatic colorectal cancer (CRC) who responded favorably to
bevacizumab chemotherapy (28).
This is similar to the results of the present study, suggesting
that chemotherapy drugs may be effective in preventing the
formation of new microvascular beds in existing tumor tissues. This
relative difference between tumor tissues and supplying vascular
tissues leads to local ischemia and the subsequent hyperuricemia.
Thus, hyperuricemia appears to serve as an alternative biomarker
for the efficacy of bevacizumab in treating patients with
metastatic CRC.
In the present longitudinal observational study, SUA
levels were repeatedly measured at four time points (pretreatment,
post-induction chemotherapy, post-radiotherapy and post-adjuvant
chemotherapy) in these patients with NPC. Through in-depth
analysis, a significant decrease was observed in SUA levels
following radiotherapy. Patients were measured for uric acid
pretreatment (Mean, 323.69; SD, 87.56), after induction
chemotherapy (Mean, 400.40; SD, 104.45), after radiotherapy (Mean,
222.84; SD, 68.47), and after adjuvant chemotherapy (M, 346.24; SD,
104.05). According to repeated measures analysis of variance, uric
acid after radiotherapy was significantly lower than uric acid
pretreatment [t(543)=-19.10; P<0.001; d=-1.47], uric acid after
induction chemotherapy [t(543)=-29.15; P<0.001; d=-2.59] and
uric acid after adjuvant chemotherapy [t(543)=-18.5; P<0.001;
d=-1.80]. While the present study was not designed to elucidate the
precise mechanisms behind this decrease, several potential
contributing factors could be hypothesize based on clinical
observations. The observed decline may be attributed to a
combination of factors, including the systemic side effects of IMRT
(for example, damage to taste buds and salivary glands), which
often lead to decreased appetite and swallowing difficulties,
potentially resulting in reduced nutritional intake and lower
purine consumption. Furthermore, the catabolic state associated
with advanced cancer and gastrointestinal side effects from
platinum-based chemotherapy may exacerbate nutritional
deterioration. However, it is crucial to note that these
explanations remain speculative, and the present study lacks direct
biochemical or clinical data to confirm these specific mechanisms.
This represents an important direction for future research.
The observed correlation between elevated
post-adjuvant chemotherapy SUA and improved survival outcomes
warrants mechanistic discussion: SUA exhibits potent antioxidant
activity and is regarded as a major antioxidant in humans, which is
considered to scavenge free radicals and contribute to the total
antioxidant capacity of plasma. Studies have indicated that uric
acid, by providing antioxidant defense, plays a protective role in
red blood cells and nerve cells (29,30).
In the context of antitumor therapy, Ames et al (31) initially hypothesized that uric
acid, through its role as a scavenger of singlet oxygen and
hydroxyl radicals (products of singlet oxygen conversion), provides
a major defense against human cancer and inhibits lipid
peroxidation in red blood cells. Another study supporting the
antioxidant ability of uric acid is related to the link between
extensive oxidative stress parameters and colon cancer survival.
Their conclusion is that only higher levels of uric acid in plasma
are associated with longer survival in patients with colon cancer.
It was considered that uric acid acts as a major antioxidant by
clearing free radicals and stabilizing ascorbic acid in human serum
(32). This suggests that high SUA
concentration may have a protective effect on patients with NPC
after adjuvant chemotherapy, and elevated uric acid levels may be a
result of tumor lysis syndrome. Furthermore, by scavenging reactive
oxygen species (ROS) generated during radiotherapy and
chemotherapy, SUA might help in preserving immune cell function,
particularly that of T lymphocytes, thereby indirectly sustaining
an effective anti-tumor immune response. It is crucial to note that
these potential mechanisms-tumor lysis, immune activation and ROS
scavenging-are not mutually exclusive and may operate concurrently.
Future studies integrating serial immune monitoring and metabolic
profiling are essential to validate these hypotheses and decipher
the precise role of SUA in the context of NPC treatment.
As a head and neck malignancy, NPC is primarily
treated with IMRT due to its unique biological characteristics
(4). However, IMRT inevitably
induces side effects, as the oral cavity, pharynx, larynx and
adjacent esophagus are often exposed to substantial radiation
doses. Early side effects include decreased appetite, taste
disturbance and salivary gland dysfunction, and swallowing
difficulties, often leading to impaired nutritional intake
(33). It is advisable to adopt a
low purine diet. Moreover, malignant tumors themselves are a
wasting disease. As the tumor progresses, the patient s
physical function declines, nutritional status deteriorates, and
the body s consumption exceeds intake. Combined with the
gastrointestinal reactions caused by the platinum-based
chemotherapy drug cisplatin, the nutritional status of patients
rapidly deteriorates after radiotherapy (34). This may be a major reason for the
post-radiotherapy SUA levels being lower than those of
pretreatment.
However, the present study has several limitations.
First, this is a single-center, retrospective study, which may
limit the generalizability of the present findings to other patient
populations and clinical settings. Second, despite the
authors efforts to control for confounding variables via
strict inclusion and exclusion criteria, selection bias and
unmeasured confounders inherent in retrospective designs may still
exist. Factors such as detailed nutritional status, precise renal
function metrics (for example, estimated glomerular filtration
rate) and the use of medications affecting uric acid levels (for
example, diuretics and allopurinol) were not systematically
accounted for and could influence the results. Third, the median
follow-up duration is only 50 months, therefore extending the
follow-up period to evaluate the long-term prognosis of patients
with intermediate to locally advanced NPC is essential. Therefore,
these findings need confirmation through larger-scale prospective
studies, However, the cutoff value of 350 µmol/l for SUA, while
based on the median of the current cohort and aligned with common
clinical reference standards, was determined post-hoc. Future
studies are needed to validate this threshold prospectively or to
explore more granular risk stratification using SUA as a continuous
variable. Furthermore, as all participants were recruited from a
single center in Southern China, an endemic region for NPC, the
generalizability of the present findings to other populations with
different genetic backgrounds and NPC incidence rates requires
careful consideration. Ethnic and genetic variations, such as
differences in purine metabolism enzymes or the strong association
between NPC and EBV in endemic populations, might influence both
baseline SUA levels and the host-tumor interaction.
In this context, the current comparative analysis
demonstrated that the prognostic value of post-adjuvant
chemotherapy SUA is independent of and complementary to established
markers such as EBV-DNA and baseline LDH. This suggests that SUA
may reflect distinct pathophysiological processes, such as systemic
oxidative stress or metabolic alterations during treatment, which
are not fully captured by EBV-DNA or LDH. Therefore, incorporating
SUA into existing prognostic models could enhance risk
stratification and personalized management for patients with NPC.
The findings of the present study have a guiding role in the
personalized treatment and health management of patients with
cancer. The role of hyperuricemia as an independent risk factor for
the occurrence and progression of cancer remains controversial and
may vary by sex. Elevated SUA levels may be a valuable long-term
surrogate marker rather than an independent risk factor. However,
it is worth noting that although our research reveals the impact of
radiotherapy and chemotherapy on uric acid levels, which may be
used to assess the prognosis of patients with NPC after adjuvant
chemotherapy, the specific mechanism of uric acid on the efficacy
of radiotherapy and chemotherapy needs further study and
large-sample validation. Further biochemical experiments and
molecular biology studies will help elucidate the molecular
mechanisms behind these biochemical changes, providing a
theoretical basis for the development of new drugs and treatment
strategies. Incorporating detailed nutritional assessments, purine
metabolic profiling and renal function monitoring are warranted to
validate these hypotheses. In addition, as biomarker research
advances, exploring more correlations between biochemical
indicators and treatment outcomes is anticipated to achieve more
personalized and precise treatment approaches.
In conclusion, the present study identified
post-adjuvant chemotherapy SUA level as an independent prognostic
factor for OS, PFS and DMFS in patients with locally advanced NPC.
Future studies should perform mechanism-based preclinical
investigations to enable targeted regulation of SUA levels without
promoting tumor development and/or metastasis. This can predict the
risk of disease recurrence in NPC patients and guide the
development of effective treatment plans.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported in part, by the Jiangxi
natural Science Foundation of China (grant no. 20202BAB206057), the
Applied Research Cultivation Program of Jiangxi (grant no.
20212BAG70047), the Beijing Xisike Clinical Oncology Research
Foundation (grant no. Y-XD202001/zb-0002) and the Second Affiliated
Hospital of Nanchang University Funding Program (grant no.
2022efyB05).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors contributions
XH, QL and YD conceived and designed the study. ZH,
XC, JD, SL and RH were responsible for the acquisition of data. JLH
and ML performed the data analysis. LT, JW, SD and JZ interpreted
the results. LZ drafted the manuscript. All authors critically
revised the manuscript for important intellectual content, read and
approved the final version of the manuscript. LZ is the guarantor
of this work and takes responsibility for the integrity of the data
and the accuracy of the analysis. XH, ML and LZ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second Affiliated Hospital of Nanchang University
(approval no. IIT-O-2023-169; Nanchang, China). The research was
carried out according to the guidelines of Declaration of Helsinki.
Written informed consent was obtained from all individual
participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer Statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen YP, Chan ATC, Le QT, Blanchard P, Sun
Y and Ma J: Nasopharyngeal carcinoma. Lancet. 394:64–80.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tang LL, Chen WQ, Xue WQ, He YQ, Zheng RS,
Zeng YX and Jia WH: Global trends in incidence and mortality of
nasopharyngeal carcinoma. Cancer Lett. 374:22–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ribassin-Majed L, Marguet S, Lee AWM, Ng
WT, Ma J, Chan ATC, Huang PY, Zhu G, Chua DTT, Chen Y, et al: What
is the best treatment of locally advanced nasopharyngeal carcinoma?
An individual patient data network Meta-analysis. J Clin Oncol.
35:498–505. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen YP, Liu X, Zhou Q, Yang KY, Jin F,
Zhu XD, Shi M, Hu GQ, Hu WH, Sun Y, et al: Metronomic capecitabine
as adjuvant therapy in locoregionally advanced nasopharyngeal
carcinoma: A multicentre, open-label, parallel-group, randomised,
controlled, phase 3 trial. Lancet. 398:303–313. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lin JC, Wang WY, Chen KY, Wei YH, Liang
WM, Jan JS and Jiang RS: Quantification of plasma Epstein-Barr
virus DNA in patients with advanced nasopharyngeal carcinoma. N
Engl J Med. 350:2461–2470. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Leung SF, Zee B, Ma BB, Hui EP, Mo F, Lai
M, Chan KC, Chan LY, Kwan WH, Lo YM and Chan AT: Plasma
Epstein-Barr viral deoxyribonucleic acid quantitation complements
tumor-node-metastasis staging prognostication in nasopharyngeal
carcinoma. J Clin Oncol. 24:5414–5418. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou GQ, Tang LL, Mao YP, Chen L, Li WF,
Sun Y, Liu LZ, Li L, Lin AH and Ma J: Baseline serum lactate
dehydrogenase levels for patients treated with Intensity-modulated
radiotherapy for nasopharyngeal carcinoma: A predictor of poor
prognosis and subsequent liver metastasis. Int J Radiat.
82:e359–e365. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mi S, Gong L and Sui Z: Friend or Foe? An
unrecognized role of uric acid in cancer development and the
potential anticancer effects of uric Acid-Lowering drugs. J Cancer.
11:5236–5244. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu L, Yang W, Zhang Y, Du X, Jin N, Chen
W, Li H, Zhang S and Xie B: Elevated serum uric acid is associated
with poor survival in advanced HCC patients and febuxostat improves
prognosis in HCC Rats. Front Pharmacol. 12(778890)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dai XY, He QS, Jing Z and Yuan JQ: Serum
uric acid levels and risk of kidney cancer incidence and mortality:
A prospective cohort study. Cancer Med. 9:5655–5661.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Feng Y, Fu M, Guan X, Wang C, Yuan F, Bai
Y, Meng H, Li G, Wei W, Li H, et al: Uric acid mediated the
association between BMI and postmenopausal breast cancer incidence:
A bidirectional mendelian randomization analysis and prospective
cohort study. Front Endocrinol (Lausanne).
12(742411)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Becker BF: Towards the physiological
function of uric acid. Free Radic Biol Med. 14:615–631.
1993.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mi N, Huang J, Huang C, Lin Y, He Q, Wang
H, Yang M, Lu Y, Lawer AL, Yue P, et al: High serum uric acid may
associate with the increased risk of colorectal cancer in females:
A prospective cohort study. Int J Cancer. 150:263–272.
2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yiu A, Van Hemelrijck M, Garmo H, Holmberg
L, Malmström H, Lambe M, Hammar N, Walldius G, Jungner I,
Wulaningsih W, et al: Circulating uric acid levels and subsequent
development of cancer in 493,281 individuals: Findings from the
AMORIS Study. Oncotarget. 8:42332–42342. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Taghizadeh N, Vonk JM and Boezen HM: Serum
uric acid levels and cancer mortality risk among males in a large
general Population-based cohort study. Cancer Causes Control.
25:1075–1080. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Deng Z, Gu Y, Hou X, Zhang L, Bao Y, Hu C
and Jia W: Association between uric acid, cancer incidence and
mortality in patients with type 2 diabetes: Shanghai diabetes
registry study. Diabetes Metab Res Rev. 32:325–332. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Juraschek SP, Tunstall-Pedoe H and
Woodward M: Serum uric acid and the risk of mortality during 23
years follow-up in the Scottish Heart Health Extended Cohort Study.
Atherosclerosis. 233:623–629. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kobylecki CJ, Afzal S and Nordestgaard BG:
Plasma urate, cancer incidence, and All-cause mortality: A
mendelian randomization study. Clin Chem. 63:1151–1160.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kuo CF, Luo SF, See LC, Chou IJ, Fang YF
and Yu KH: Increased risk of cancer among gout patients: A
nationwide population study. J Bone Spine. 79:375–378.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Du XJ, Chen L, Li WF, Tang LL, Mao YP, Guo
R, Sun Y, Lin AH and Ma J: Use of pretreatment serum uric acid
level to predict metastasis in locally advanced nasopharyngeal
carcinoma. Head Neck. 39:492–497. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hsueh CY, Shao M, Cao W, Li S and Zhou L:
Pretreatment serum uric acid as an efficient predictor of prognosis
in men with laryngeal squamous cell cancer: A retrospective cohort
study. Oxid Med Cell Longev. 2019(1821969)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fini MA, Elias A, Johnson RJ and Wright
RM: Contribution of uric acid to cancer risk, recurrence, and
mortality. Clin Transl Med. 1(16)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Toyokuni S: Oxidative stress as an iceberg
in carcinogenesis and cancer biology. Arch Biochem Biophys.
595:46–49. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim AW, Batus M, Myint R, Fidler MJ, Basu
S, Bonomi P, Faber LP, Wightman SC, Warren WH, McIntire M, et al:
Prognostic value of xanthine oxidoreductase expression in patients
with non-small cell lung cancer. Lung Cancer. 71:186–190.
2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Selcukbiricik F, Kanbay M, Solak Y, Bilici
A, Kanıtez M, Balık E and Mandel NM: Serum uric acid as a surrogate
marker of favorable response to bevacizumab treatment in patients
with metastatic colon cancer. Clin Transl Oncol. 18:1082–1087.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Song Y, Tang L, Han J, Gao Y, Tang B, Shao
M, Yuan W, Ge W, Huang X, Yao T, et al: Uric acid provides
protective role in red blood cells by antioxidant defense: A
hypothetical analysis. Oxid Med Cell Longev.
2019(3435174)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Black CN, Bot M, Scheffer PG, Snieder H
and Penninx B: Uric acid in major depressive and anxiety disorders.
J Affect Disord. 225:684–690. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ames BN, Cathcart R, Schwiers E and
Hochstein P: Uric acid provides an antioxidant defense in humans
against oxidant- and radical-caused aging and cancer: A hypothesis.
Proc Natl Acad Sci USA. 78:6858–6862. 1981.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dziaman T, Banaszkiewicz Z, Roszkowski K,
Gackowski D, Wisniewska E, Rozalski R, Foksinski M, Siomek A,
Speina E, Winczura A, et al: 8-Oxo-7,8-dihydroguanine and uric acid
as efficient predictors of survival in colon cancer patients. Int J
Cancer. 134:376–383. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Brook I: Early side effects of radiation
treatment for head and neck cancer. Cancer Radiother. 25:507–513.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014.PubMed/NCBI View Article : Google Scholar
|