Introduction
Adenoid cystic carcinoma (AdCC) is a rare type of
cancer, evolving from salivary glands, with a so far unknown
aetiology (1-5).
It accounts for around 10% of all cancers in the major salivary
glands and 30% of all cancers in the minor salivary glands in the
head and neck region, and is therefore regarded as one of the most
common malignant salivary tumours (2). AdCC can, however, also arise in other
locations within the head and neck region and in salivary glands
outside of this region, as well as throughout the body (2). It is more common in females as
compared to males, and prevalent between 50-70 years of age,
although it can occur at all ages (3,5). Its
symptoms are unspecific, and its diagnosis is challenging, due to
its slow and indolent growth. Moreover, there is a lack of reliable
specific diagnostic and prognostic markers (3,5).
However, genetic alterations, such as MYB-NFIB and MYBL1-NFIB
fusions, and mutations in the Notch-1 signalling and DNA damage
repair pathways, have recently been disclosed (6,7).
Currently, patients are initially treated with surgery, often
followed by adjuvant radiotherapy. However, despite the intensive
treatment, late relapses are frequent (3). Clearly, AdCC therefore presents both
a diagnostic and clinical challenge. In other cancers, e.g., head
and neck squamous cell carcinomas, it has been shown that a high
presence of CD8+ infiltrating lymphocytes or a high
CD8+/FOXP3 coefficient, or HLA class I staining could be
of prognostic benefit, as a normal/high expression of HLA class I
staining (8-12).
In this exploratory study, we therefore attempted to
evaluate whether staining by immunohistochemistry (IHC) of HLA
class I, CD4, CD8, and FOXP3, markers of immunologically prognostic
interest for other cancers, could be of prognostic value.
Patients and methods
Patients
Of patients diagnosed with AdCC between 2000-2014 in
the Stockholm region of Sweden, and included in prior studies
(13,14), all were regarded as eligible to be
included in the present investigation. Of these, 72 had
paraffin-embedded diagnostic material available for further
analysis in this study. The characteristics of these patients and
their tumours are presented in Table
I.
 | Table ICharacteristics of patients with
adenoid cystic cancer and their tumours. |
Table I
Characteristics of patients with
adenoid cystic cancer and their tumours.
| Characteristic | Value |
|---|
| All patients, n
(%) | 72 (100.0) |
| Age at diagnosis,
years | |
|
Mean
(SD) | 55 (14.5) |
| Sex, n (%) | |
|
Male | 27 (37.5) |
|
Female | 45 (62.5) |
| Subsite, n (%) | |
|
Parotid
gland | 19 (26.4) |
|
Oral
cavity | 16 (22.2) |
|
Submandibular
gland | 23 (31.9) |
|
Nasal cavity
& paranasal sinuses | 7 (9.7) |
|
Lip | 1 (1.4) |
|
Oropharynx | 5 (6.9) |
|
Nasopharynx | 1 (1.4) |
| Stage, n (%) | |
|
I | 23 (31.9) |
|
II | 20 (27.8) |
|
III | 11 (15.3) |
|
IV | 18 (25.0) |
| Smoking, n (%) | |
|
Ever | 38 (52.8) |
|
Never | 34 (47.2) |
| Treatment, n
(%) | |
|
Surgery | 66 (91.7) |
|
Radical
surgery | 9 (12.5) |
|
RT | 51 (70.8) |
|
CRT | 21 (29.2) |
|
Induction
ChT | 3 (4.2) |
| Recurrence, n
(%) | |
|
Locoregional | 16 (22.2) |
|
Distant
metastasis | 31 (43.1) |
|
None | 38 (52.8) |
IHC
IHC was done by manual processing, with a standard
avidin-streptavidin method on three tissue microarrays (TMAs)
(9,10,15).
Each TMA comprised two 1-mm cores per case, including one tumour
core and one matched adjacent normal tissue core. TMA sections were
deparaffinized and rehydrated, followed by antigen retrieval
(citrate buffer pH 6.0), and the endogenous peroxidase was blocked.
The slides were then incubated in horse serum, followed by
incubation at 4˚C overnight with the primary antibodies. Three
primary antibodies were from Abcam, Cambridge, UK and diluted 1:200
and these were the anti-CD4 antibody (clone EPR6855), an IgG,
rabbit, monoclonal; the anti-FOXP3 antibody (clone 236A/E7) an IgG,
mouse monoclonal; and the anti-HLA Class I ABC antibody (clone
EMR8-5), recognizing human HLA class I A, B, and C, an IgG, mouse
monoclonal. The anti-CD8 antibody was obtained from Leica
Biosystems, Mölndal, Sweden (Novocastra Laboratories clone 4B11),
diluted 1:40, and was an IgG2b mouse monoclonal.
The sections were incubated with a biotinylated
secondary anti-mouse IgG or anti-rabbit IgG followed by incubation
with an avidin-streptavidin complex (VECTASTAIN Elite ABC-kit,
Vector Laboratories, Burlingame, CA, USA) for detection and DAB for
visualization. The slides were counterstained with
haematoxylin.
Staining evaluation
Tumour sections and normal adjacent tissue were
examined independently by two researchers (MZ and MB) blinded for
clinical outcome. CD4, CD8 and FOXP3 were quantified as number of
positive cells in epithelial tumour and tumour stroma. HLA-I
staining was quantified as percentage of stained tumour tissue, as
well as intensity of staining in the tumour tissue compared to that
in the stroma (equal intensity or lower). Any disagreements were
resolved by consensus through consultation with AN (specialist in
clinical pathology).
Statistical analysis
The χ2 or Fisher's exact test was used
for categorical data, while comparison of mean values was done with
paired or unpaired Student's t-tests, when appropriate.
Clinical outcome data were obtained from medical records. Overall
survival (OS) was calculated in years from the date of diagnosis
until the date of death or the date of OS assessment (11-12 May
2022). Disease-free survival (DFS) was calculated in years from the
date of diagnosis until the first documented recurrence or until
the date of DFS assessment (11-12 May 2022). Patients without a
disease-free period were censored at day 0, and patients who died
without a documented recurrence were censored at their date of
death. For HLA class I data, the Kaplan-Meier estimator was used to
visualize OS and DFS, and the log-rank test was applied to assess
differences in survival. P<0.05 was considered to indicated a
statistically significant difference. All analyses were performed
using IBM SPSS Statistics for Mac, version 31 (IBM Corp.).
Results
Patients, their clinical
characteristics and treatment, as well as tumour staging in
association with presence or absence of recurrence
Patient and tumour characteristics of the 72 AdCC
patients are depicted in Table I.
More specifically, there were 45 female and 27 male patients, with
a mean age of 55 years (Table I).
Most patients had surgery, which was not radical for most, and this
was followed by radiotherapy in all patients, irrespective of
whether surgery was radical or not.
According to the Union of International Cancer
Control (UICC) staging, 23 patients had stage I tumours and of
these 5/23 (21.7%) had a recurrence; 20 patients had stage II
tumours and of these 5/20 (25%) had a recurrence; 11 patients had
stage III tumours and 9/11 (81.1%) had recurrences; and 18 patients
had stage IV tumours and 15/18 (83.3%) had a recurrence (Table I). Altogether, 34/72 (47.2%) of the
patients encountered a recurrence, however, of note, patients with
stage I and II tumours 10/43 (23.3%) had significantly fewer
recurrences than those with stage III and IV tumours 24/29 (82.8%),
P<0.0001 (χ2).
We also examined whether sex, smoking or age had any
prognostic impact regarding presenting a recurrence, but as shown
previously (8), this was not the
case. Regarding sex, 21/45 (46.7%) of women and 13/27 (48.1%) of
the men had a recurrence (P=0.903, χ2). Additionally,
there was no difference regarding smoking status among the patients
who had a recurrence, with 16/34 (47.1%) never smokers and 18/38
(47.4%) ever smokers presenting with recurrence (P=0.973,
χ2). Lastly, there was no difference in mean age between
the 38 patients with and the 34 patients without recurrence (55.3
vs. 54.6 years, P=0.848, unpaired t-test).
HLA class I staining by IHC in the
tumour and the stroma in patients with and without recurrence
Most patients 64/72 (88.9%), had HLA class I
staining in their tumours, but the difference in intensity of the
staining between tumour and stroma varied. 44/72 (59.7%) samples
had equal intensity staining in the tumour compared to that in the
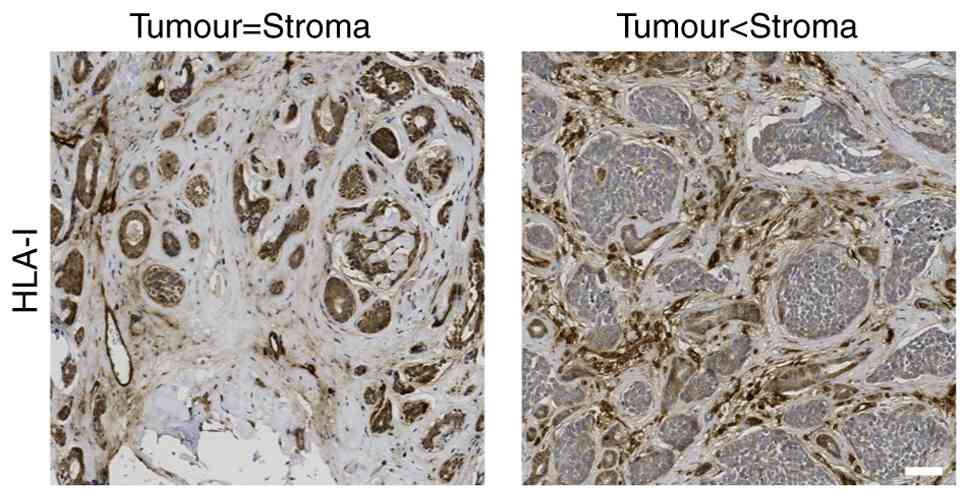
stroma. Fig. 1 demonstrates
normal/equal intensity HLA class I staining in the tumour compared
to that in the stroma and weaker/lower intensity HLA class I
staining in the tumour compared to the stroma.
A weaker HLA class I staining, as compared to the
surrounding stroma, was observed significantly more often in
patients with recurrence compared to those without [18/34 (52.9%)
vs. 10/38 (26.3%), P=0.021, χ2]. There was no
significant difference in fraction of positive cells between
patients with and without recurrence [28/34 (82.4%) vs. 36/38
(94.7%), P=0.138, Fisher's exact test].
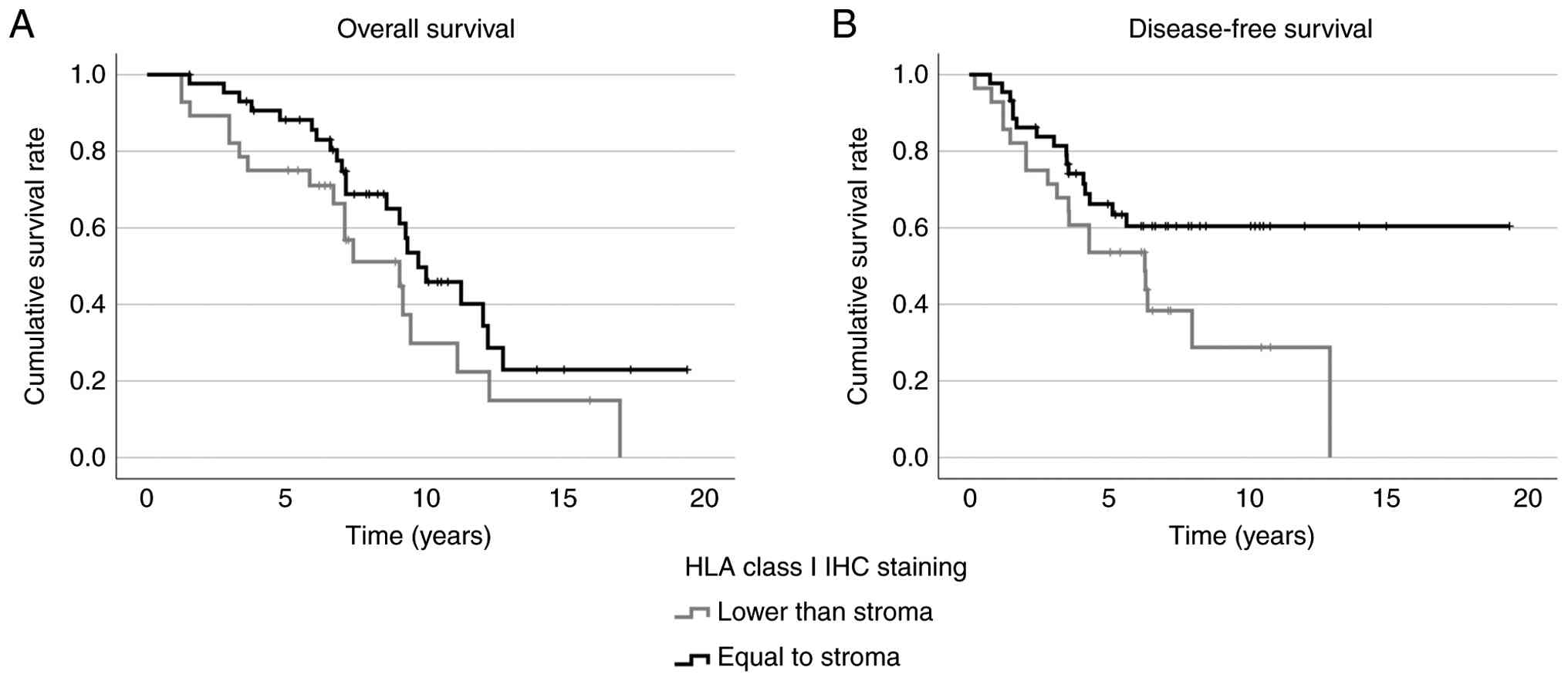
Kaplan Meier curves for DFS and OS and HLA class I
staining are presented in Fig. 2.
For DFS, the 5-year survival was 63% in patients with normal/equal
to stroma staining vs. 54% in those with weaker tumour staining.
The corresponding 10-year figures were 60% vs. 29% (log rank test:
P=0.048). For OS, the 5-year survival was 88% vs. 75%, and the
10-year survival was 50% vs. 30%; differences that did not reach
significance (log rank test: P=0.10).
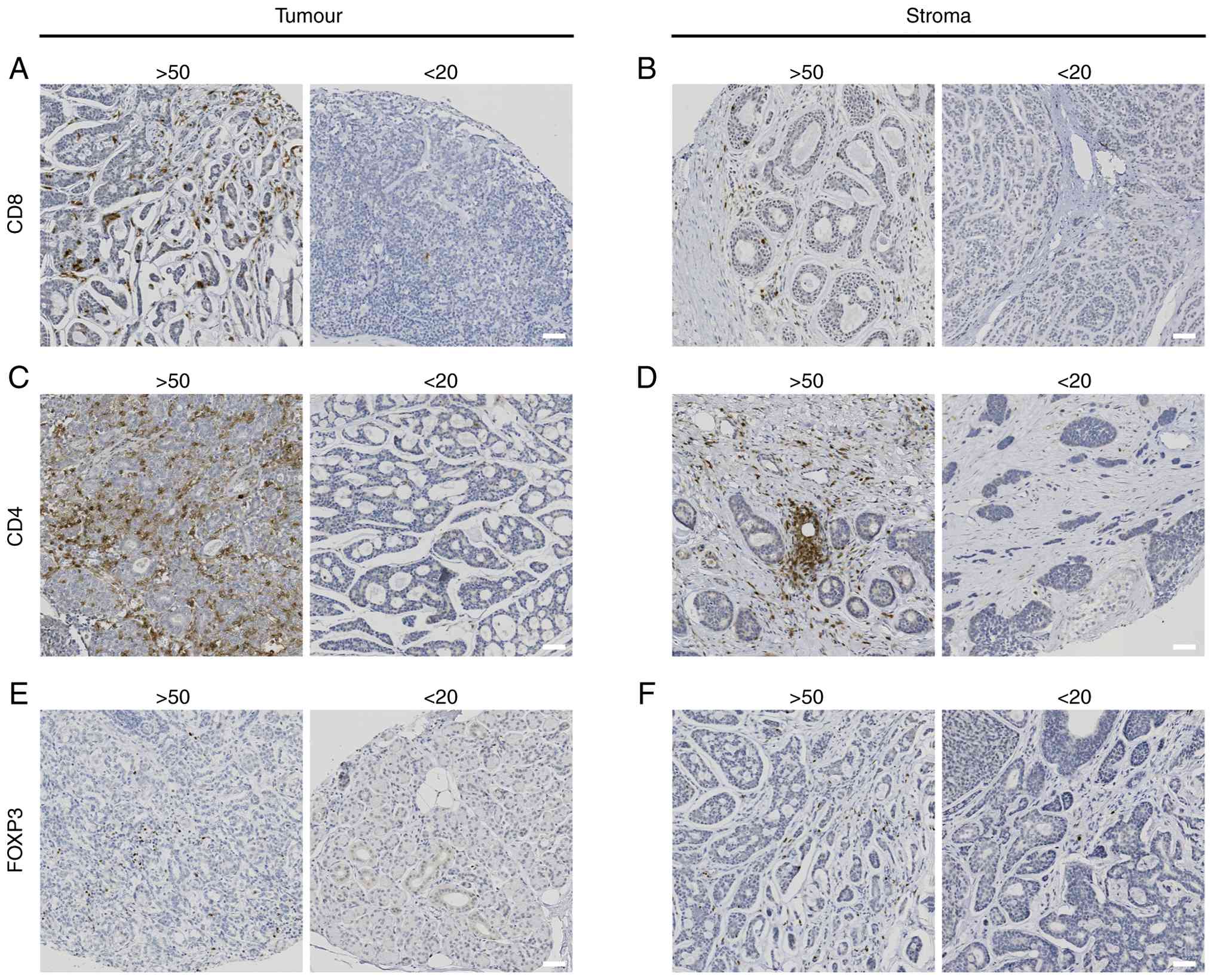
CD8, CD4 and FOXP3 staining by IHC in
the tumour and stroma in patients with and without recurrence
CD8, CD4 and FOXP3 staining in the tumour and/or
stroma varied from 0 to >300 positive cells per mm2.
Examples of CD8, CD4 and FOXP3 staining are illustrated in Fig. 3.
For CD8+ staining, the overall mean cell
infiltration was higher in stroma than in tumour tissue (59.8 vs.
40.5 CD8+ cells), but this difference was not
statistically significant (paired t-test: P=0.15). Patients without
recurrence showed higher CD8+ levels compared to those
with recurrence, both in tumour (52.7 vs. 27.1) and stroma (78.1
vs. 39.9). However, these differences were not significant
(independent t-test: P=0.3 and 0.2, respectively).
For CD4+ staining, the overall mean
infiltration was similar between tumour and stroma (144.7 vs. 162.8
CD4+ cells, paired t-test: P=0.40). There was no notable
difference between patients without and with recurrence in tumour
(140.7 vs. 148.9, independent t-test: P=0.96) or stroma (166.5 vs.
158.8, independent t-test: P=0.82).
For FOXP3, the overall mean infiltration was low in
both compartments (13.3 in stroma and 6.8 in tumour, paired t-test:
P=0.22). There were no significant differences in FOXP3
infiltration between patients with or without recurrence
(independent t-test: stroma: P=1.0 and tumour: P=0.4).
Discussion
In this exploratory study, we evaluated the
prognostic relevance of the immune markers HLA class I, CD8, CD4,
and FOXP3, markers known to be of prognostic value in several
carcinomas. In addition, clinical parameters, including stage, age,
and sex were assessed. Here, of the assessed markers, only low
intensity HLA class I tumour staining was significantly associated
with an increased risk of recurrence. Among the clinical factors,
low stage (I-II) disease was, as expected, also significantly
associated with fewer recurrences compared to high stage (III-IV)
disease.
That normal expression of HLA class I staining in
the tumour was associated with having less recurrences, was
immunologically not unexpected, but of interest since data on HLA
class I expression in AdCC are limited. Moreover, HLA class I
molecules are well-known to be critical to adapt immune responses
to tumours and viral infections by conveying proteosome-generated
tumour and viral antigen peptides to CD8+ T-cells
(16-22).
Here, almost 40% of the patients had lower HLA class
I staining in the tumour compared to that in the stroma, which
concords with a report that roughly half of the samples from 15
AdCC patients had downregulated HLA-antigens in their tumours upon
transcriptomic analysis (23).
Moreover, in another report, studying the immune landscape of AdCC,
a near-complete absence of beta-2-microglobulin (B2M) expression
and decreased HLA class I expression was disclosed in 20/24
examined AdCC patient samples (24). However, it was also shown that B2M
and HLA class I could experimentally be partially restored using
interferon-γ, or a stimulator of the interferon genes (STING)
agonist in vitro (24). The
authors concluded that this could potentially be of benefit for
patient treatment and they also described that treatment of a
patient with a metastatic breast AdCC with a STING agonist and
pembrolizumab led to a partial response (24). The latter could be of potential
future therapeutic interest and pinpoints the importance of HLA
class I expression for an adequate immune response in AdCC.
That high numbers of CD8+ and
CD4+ cells, or a low number of FOXP3+ cells
in tumour and stroma, did not correlate with a reduced risk of
recurrence was disappointing, as these markers have shown
prognostic relevance in other cancers (8,9,18,22,24).
Moreover, our findings partly contradict previous reports
indicating a high complexity of the microenvironment (25). However, our findings may be
explained by the fact that, although high HLA class I expression is
important for an effective immune response, the generally low HLA
class I expression in AdCC could abrogate potential effects of
immune cells, as also suggested by others (20-24).
This phenomenon is not unusual in relation to viral infections,
where many viruses downregulate HLA expression, e.g. where the E5
protein of Human papillomavirus and the adenovirus type 12 E1A that
target and downregulate HLA class I expression (26,27).
Unfortunately, as noted there are limited studies on
the immune microenvironment in AdCC for comparison. However, in one
report, the combination of having a high PD1 expression score
(>5%) together with high CD8+ TIL abundance in the
peritumoral microenvironment was associated with worse prognosis
(28). In another study, in 50
AdCC patients, sections were stained for CD3, CD4, CD8 and CD20 and
evaluated regarding their distribution of TILs (29). Patterns were determined as
infiltrated-excluded, infiltrated-inflamed and presence of tertiary
lymphoid structures. In the inflamed phenotype CD3+
cells were the most abundant lymphocyte subtype, and within this
compartment, CD8+ T cells dominated. However, there was
no influence on survival by any of the TIL patterns. The authors
indicated that this was due to the very low immunogenicity of AdCC
and that even abundance of lymphocytes did not seem to improve
prognosis for this disease (29).
The latter is in concordance with our findings.
Likewise, there are limited numbers of
investigations on the potential association of FOXP3 staining and
recurrence rates of AdCC, and in this report none were indicated.
This could correlate very well with AdCC being an indolently
growing tumour with low immunogenicity. In the report of Li et
al (24), above like ours,
FOXP3 expression was low, suggesting a limited number of Tregs in
AdCC.
Of note, in this study stage I and II AdCC presented
fewer recurrences than stage III and IV disease, which was in
concurrence with our previous studies and that of others (14,30,31).
Likewise, in this smaller cohort, like the previously examined
whole group, we did not disclose age, sex or smoking as prognostic
factors (14). Nevertheless,
others have reported that older age is correlated with more
advanced disease stages and worse prognosis (31,32).
Therefore, the association of age to increased recurrence rate
should likely still be pursued further.
This study has some limitations. First, the cohort
was small, however, it was quite comparable to other studies
(32). Furthermore, subsite,
histomorphology markers, and perineural growth were not included in
the analysis, and despite perineural growth not being considered a
prognostic factor by us previously per se, it can be shown as such
when combining several studies e.g. in a systematic review
(14,33). Finally, assaying markers in TMAs
has its limitations, since core samples do not represent the
tumour's entire immune landscape or HLA class I representation, and
this in turn could lead to an underestimation or the opposite of
the markers true prognostic value.
To conclude, we were able to show that analogous HLA
class I staining in the tumour as compared to the stroma was
associated with a significant decrease in recurrence as compared to
that in patients with low HLA class I staining in the tumour. Our
finding is in concordance with others, and with the suggestion by
others that ways to increase HLA class I expression in AdCC may be
beneficial for AdCC patients; however, the latter needs to be
further investigated.
Acknowledgements
Not applicable.
Funding
Funding: The Swedish Cancer Foundation (grant no. 23700PJ), the
Stockholm Cancer Society (grant nos. 221082 and 241131), the
Stockholm City Council, the Swedish Cancer and Allergy Foundation
(grant no. 10662), Swedish Society of Medicine (grant no.
SLS-999914), the Karolinska Institutet, the Sigurd and Elsa Golges
Memory Foundation (grant no. LA2023-0042), the Magnus Bergvalls
Stiftelse (grant no. 2024-1334), the Tornspiran Foundation 2024 and
the Serafimerlasarettet Foundation 2024 are greatly acknowledged
for supporting this research.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AN, MZ, MB and TD designed the study. MZ, MB, TD and
AN contributed to data acquisition, formal analysis, and
validation. SF contributed to data acquisition and formal analysis.
MB and MZ confirm the authenticity of all the raw data. MZ, MB, TD
and AN contributed to funding of the project. TD initiated the
writing of the original draft together with MB. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted according to ethical
permissions 99-237, from the Ethics Committee at Karolinska
Institutet (Stockholm, Sweden); 2005/431-31/4, 2009/1278-31/4,
2012/83-31/2, 2017/1035-31/2, 2019-05211 from the Stockholm
Regional Ethical Review Board (Karolinska Institutet, Stockholm,
Sweden) and 2022-05287-02 from the Swedish Ethical Review Authority
(Uppsala, Sweden). Written informed consent was obtained from all
patients to participate in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Young A and Okuyemi OT: Malignant salivary
gland tumors. In: StatPearls. StatPearls Publishing, Treasure
Island, FL, 2025.
|
|
2
|
Ouyang DQ, Liang LZ, Zheng GS, Ke ZF, Weng
DS, Yang WF, Su YX and Liao GQ: Risk factors and prognosis for
salivary gland adenoid cystic carcinoma in southern china: A
25-year retrospective study. Medicine (Baltimore).
96(e5964)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dillon PM, Chakraborty S, Moskaluk CA,
Joshi PJ and Thomas CY: Adenoid cystic carcinoma: A review of
recent advances, molecular targets, and clinical trials. Head Neck.
38:620–627. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu Y, Han T, Guo D, Chen D and Li Y:
Exploring potential treatment opportunities in a head and neck
tumor patient with AdCC: A novel germline ERCC2 mutation case
report. Medicine (Baltimore). 104(e41233)2025.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zupancic M, Näsman A, Friesland S and
Dalianis T: Adenoid cystic carcinoma, clinical presentation,
current treatment and approaches towards novel therapies.
Anticancer Res. 44:1325–1334. 2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ferrarotto R, Mitani Y, Diao L, Guijarro
I, Wang J, Zweidler-McKay P, Bell D, William WN Jr, Glisson BS,
Wick MJ, et al: Activating NOTCH1 mutations define a distinct
subgroup of patients with adenoid cystic carcinoma who have poor
prognosis, propensity to bone and liver metastasis, and potential
responsiveness to notch1 inhibitors. J Clin Oncol. 35:352–360.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wagner VP, Bingle CD and Bingle L:
Myb-nfib fusion transcript in adenoid cystic carcinoma: Current
state of knowledge and future directions. Crit Rev Oncol Hematol.
176(103745)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nordfors C, Grün N, Tertipis N,
Ährlund-Richter A, Haeggblom L, Sivars L, Du J, Nyberg T, Marklund
L, Munck-Wikland E, et al: CD8+ and CD4+ tumour infiltrating
lymphocytes in relation to human papillomavirus status and clinical
outcome in tonsillar and base of tongue squamous cell carcinoma.
Eur J Cancer. 49:2522–2530. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Näsman A, Romanitan M, Nordfors C, Grün N,
Johansson H, Hammarstedt L, Marklund L, Marklund L, Munck-Wikland
E, Dalianis T and Ramqvist T: Tumor infiltrating CD8+ and Foxp3+
lymphocytes correlate to clinical outcome and human papillomavirus
(HPV) status in tonsillar cancer. PLoS One.
7(e38711)2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Näsman A, Andersson E, Nordfors C, Grün N,
Johansson H, Munck-Wikland E, Massucci G, Dalianis T and Ramqvist
T: MHC class I expression in HPV positive and negative tonsillar
squamous cell carcinoma in correlation to clinical outcome. Int J
Cancer. 132:72–81. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Koike K, Dehari H, Shimizu S, Nishiyama K,
Sonoda T, Ogi K, Kobayashi J, Sasaki T, Sasaya T, Tsuchihashi K, et
al: Prognostic value of HLA class I expression in patients with
oral squamous cell carcinoma. Cancer Sci. 111:1491–1499.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Caballero-Borrego M, Grau JJ, Basté N,
Castillo PC, Teixido C, Valduvieco I and Vilaseca I: Cancer stem
cell biomarkers in locally advanced head and neck squamous cell
carcinoma. Braz J Otorhinolaryngol. 91(101689)2025.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zupancic M, Holzhauser S, Cheng L,
Ramqvist T, Du J, Friesland S, Näsman A and Dalianis T: Analysis of
human papillomavirus (HPV) and polyomaviruses (HPYVS) in adenoid
cystic carcinoma (ADCC) of the head and neck region reveals three
HPV-positive cases with adenoid cystic-like features. Viruses.
14(1040)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zupancic M, Nasman A, Berglund A, Dalianis
T and Friesland S: Adenoid cystic carcinoma (ADCC): A clinical
survey of a large patient cohort. Cancers (Basel).
15(1499)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
de Flon CH, Haeggblom L, Holzhauser S,
Kostopoulou ON, Zupancic M, Dalianis T, Munck-Wikland E, Marklund L
and Näsman A: High levels of FGF11 correlate with poor survival in
patients with human papillomavirus (HPV)-positive oropharyngeal
squamous cell carcinoma. Cancers (Basel). 15(1954)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rötzschke O, Falk K, Deres K, Schild H,
Norda M, Metzger J, Jung G and Rammensee HG: Isolation and analysis
of naturally processed viral peptides as recognized by cytotoxic T
cells. Nature. 348:252–254. 1990.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Schulz M, Aichele P, Schneider R, Hansen
TH, Zinkernagel RM and Hengartner H: Major histocompatibility
complex binding and T cell recognition of a viral nonapeptide
containing a minimal tetrapeptide. Eur J Immunol. 21:1181–1185.
1991.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dersh D, Hollý J and Yewdell JW: A few
good peptides: MHC class I-based cancer immunosurveillance and
immunoevasion. Nat Rev Immunol. 21:116–128. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang K, Halima A and Chan TA: Antigen
presentation in cancer-mechanisms and clinical implications for
immunotherapy. Nat Rev Clin Oncol. 20:604–623. 2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kong S, Zhang J, Wang L, Li W, Guo H, Weng
Q, He Q, Lou H, Ding L and Yang B: Mechanisms of low MHC I
expression and strategies for targeting MHC I with small molecules
in cancer immunotherapy. Cancer Lett. 611(217432)2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Darabi A, Thuring C and Paulsson KM: HLA-I
antigen presentation and tapasin influence immune responses against
malignant brain tumors-considerations for successful immunotherapy.
Anticancer Agents Med Chem. 14:1094–1100. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Emtiazi N, Zolfi E, Mehr FK and Moradi Y:
The role of the major histocompatibility complex (MHC) class I in
prostate cancer therapy: A review. Crit Rev Oncol Hematol.
215(104897)2025.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tang YF, An PG, Gu BX, Yi S, Hu X, Wu WJ
and Zhang J: Transcriptomic insights into adenoid cystic carcinoma
via RNA sequencing. Front Genet. 14(1144945)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li A, Gonda BL, Codd EM, von Paternos A,
Mitchell DR, Herrmann MD, Kalyan P, Flynn SE, Dzu TQ, Gao C, et al:
Reversible downregulation of HLA class I in adenoid cystic
carcinoma. J Immunother Cancer. 13(e011380)2025.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mosconi C, de Arruda JAA, de Farias ACR,
Oliveira GAQ, de Paula HM, Fonseca FP, Mesquita RA, Silva TA,
Mendonça EF and Batista AC: Immune microenvironment and evasion
mechanisms in adenoid cystic carcinomas of salivary glands. Oral
Oncol. 88:95–101. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Campo MS, Graham SV, Cortese MS, Ashrafi
GH, Araibi EH, Dornan ES, Miners K, Nunes C and Man S: HPV-16 E5
down-regulates expression of surface HLA class I and reduces
recognition by CD8 T cells. Virology. 407:137–142. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Georgopoulos NT, Proffitt JL and Blair GE:
Transcriptional regulation of the major histocompatibility complex
(MHC) class I heavy chain, TAP1 and LMP2 genes by the human
papillomavirus (HPV) type 6b, 16 and 18 E7 oncoproteins. Oncogene.
19:4930–4935. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kesar N, Winkelmann R, Oppermann J,
Ghanaati S, Martin D, Neumayer T, Balster S, Rödel C, Rödel F, von
der Grün J and Balermpas P: Prognostic impact of CD8-positive
tumour-infiltrating lymphocytes and PD-L1 expression in salivary
gland cancer. Oral Oncol. 111(104931)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Doescher J, Meyer M, Arolt C, Quaas A,
Klußmann JP, Wolber P, Bankfalvi A, Schildhaus HU, Bastian T, Lang
S, et al: Patterns of tumor infiltrating lymphocytes in adenoid
cystic carcinoma of the head and neck. Cancers (Basel).
14(1383)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
de Morais EF, da Silva LP, Moreira DGL,
Mafra RP, Rolim LSA, de Moura Santos E, de Souza LB and de Almeida
Freitas R: Prognostic factors and survival in adenoid cystic
carcinoma of the head and neck: A retrospective clinical and
histopathological analysis of patients seen at a cancer center.
Head Neck Pathol. 15:416–424. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Spiro RH and Huvos AG: Stage means more
than grade in adenoid cystic carcinoma. Am J Surg. 164:623–628.
1992.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ko JJ, Siever JE, Hao D, Simpson R and Lau
HY: Adenoid cystic carcinoma of head and neck: Clinical predictors
of outcome from a canadian centre. Curr Oncol. 23:26–33.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ju J, Li Y, Chai J, Ma C, Ni Q, Shen Z,
Wei J and Sun M: The role of perineural invasion on head and neck
adenoid cystic carcinoma prognosis: A systematic review and
meta-analysis. Oral Surg Oral Med Oral. 122:691–701.
2016.PubMed/NCBI View Article : Google Scholar
|