Introduction
The vertebral canal is the tissue formed by the
vertebral foramen of the vertebral body and the sacral canal of the
sacrum, which is connected to the foramen magnum and reaches down
to the sacral hiatus. It contains the spinal cord, spinal cord
peritoneum, nerve roots, blood vessels and a small amount of
connective tissue. There are multiple types of lesions in the
spinal canal, which can originate from the aforementioned different
tissues. According to their positional association with the spinal
cord and dura mater, they can be classified into intramedullary,
extramedullary subdural and epidural lesions. Astrocytoma and
ependymoma are the most common intramedullary lesions, while
neurilemmoma and meningioma are the most frequent extramedullary
subdural lesions. Metastases and lymphomas are common in epidural
space lesions, which are comprised mainly of congenital tumors,
such as teratomas and dermoid cysts (1).
Digital radiography (DR) and computer tomography
(CT) are not suitable for intravertebral lesions and have certain
difficulties as regards their diagnosis, while magnetic resonance
imaging (MRI) can display the morphological information of the
spinal vertebrae and spinal cord by its high soft-tissue
resolution. Based on the accurate localization of the lesion,
coupled with the MRI signal characteristics and enhancement mode,
the accurate diagnosis of the majority of lesions can be achieved.
This technique is widely used in clinical applications and is
usually the preferred examination technique for intravertebral
lesions (2).
Positron emission tomography (PET)/CT is a
non-invasive imaging technique that provides crucial information on
tissue function and metabolism, and accurately displays anatomical
structures and localizes lesions. It is often used to assess the
malignancy of tumors, the post-treatment response, or to identify
tumor recurrence and necrotic tissue. It also has the advantages of
an enhanced sensitivity, accuracy and safety (3,4).
Fluorine-18 (18F)-fluorodeoxyglucose (FDG) is a commonly
used radiotracer to identify tumors.
The two aforementioned examination techniques have
unique advantages in the clinical diagnosis of benign and malignant
intraspinal lesions. However, to date, to the best of our
knowledge, comparative studies on PET/CT and MRI are limited
(2,5). Thus, the aim of the present study was
to retrospectively analyze the imaging data of 58 cases with
lesions examined using sequential MRI and 18F-FDG PET/CT
scans in order to compare their value in the diagnosis and
differentiation of intravertebral lesions.
Patients and methods
Patients
The present retrospective study was approved by
Review Committee of the 4th People's Hospital of Shenyang and all
patients enrolled gave informed consent. The inclusion criteria
were as follows: i) Patients who had undergone MRI (or enhanced
MRI) and 18F-FDG PET/CT examinations successively from
January, 2017 to December, 2020 (the interval between two
examinations did not exceed 10 days); ii) final diagnosis was
obtained by performing a post-operative pathological examination or
following a tissue biopsy; and iii) clinical, imaging and
pathological data were complete. The exclusion criteria were the
following: i) No consent provided for surgery; or ii) patients with
incomplete clinical or imaging data. Finally, a total of 58 cases
were enrolled. The patients were aged 14-82 years of age, of which
9 patients had a history of malignancy. All of them were
post-operative reviews, which included four cases of lung cancer,
two cases of breast cancer, and one case of gastric, liver and
esophageal cancer, respectively. The majority of patients presented
with varying degrees of limb pain and sensory disorders, walking
disorders, and even paralysis in severe cases.
MRI and PET/CT acquisition
MRI (GE Signa HDxt 3.0T machine) routine scanning
sequences were as follows: Sagittal T1WI fast spin echo (FSE)
repetition time (TR)/echo time (TE), 300/8.04 msec; T2WI FSE TR/TE,
2,760/127.12 msec; Field-of-view (FOV), 360/340 mm; transverse T2WI
SE TR/TE, 3,500/109.62 msec; FOV, 160/140 mm; matrix, 512x512;
fractional anisotropy (FA), 90; layer thickness, 3 mm; layer
spacing, 3.5 mm. The contrast agent (Gd-DTPA-BMA, OmniScan, GE
Healthcare Ireland) 0.2 mmol/kg was injected through the elbow vein
at a flow rate of 2 ml/sec, for T1 FSE + C transverse-axis (TR/TE,
380/13.96 msec), coronal (TR/TE, 380/8.02 msec) and sagittal
(TR/TE, 700/9.46 msec) with layer thickness of 3 mm and a layer
spacing of 3.3/3.5 mm.
Prior to PET/CT (GE Discovery PET/CT Elite, GE
Healthcare; Cytiva) scan, the patients were injected intravenously
with 18F-FDG (prepared by GE MINItrace™ II, GE
Healthcare; Cytiva; with a chemical purity of >96%) through the
elbow according to the body mass of 3.7 MBq/kg. The patients then
lay flat for 60 min, and images were collected from the upper
femoral trunk to the cranial apex, 3-5 min/bed. PET scanning used a
3D model and point spread function acquisition and a matrix of
192x192. The spiral CT scanning parameters were as follows: 120-140
kV, 30-210 mA and a layer thickness of 3.25 mm. Following CT
attenuation correction and iterative image reconstruction, the PET,
CT and PET/CT fusion images of the whole body were obtained.
The region of interest (ROI) on the PET/CT images
was automatically outlined using the post-processing workstation
PET VCAR AW4.5 (GE Healthcare; Cytiva) to obtain the maximum
standardized uptake value (SUVmax), peak standardized uptake value
(SUVpeak), mean standardized uptake value (SUVmean), metabolic
tumor volume (MTV) and total lesion glycolysis (TLG). The TLG was
calculated as follows: TLG=SUVmean x MTV (cm3) (6), with SUVmax >2.5 as the reference
threshold for determining the radio concentration (7). A tissue biopsy, post-operative
pathology, or follow-up results were used as the gold standard. Two
radiologists (WS and WT), each with >10 years experience and
holding dual licenses in radiology and nuclear medicine, analyzed
the MRI and PET/CT images and negotiated in the case of any
disagreement. The case was then examined and verified by a senior
doctor who made the final judgment.
Statistical analysis
SPSS (version 26.0, IBM Corp.) software was used for
data analysis. The sensitivity, specificity, accuracy, positive
predictive value and negative predictive value of MRI and
18F-FDG PET/CT were calculated, respectively. Count
variables were compared using the χ2 test. The normality
of quantitative data were evaluated using Shapiro-Wilk tests, and
are expressed as mean ± SD or as the median (P50), 25th
percentile (P25) and 75th percentile (P75). The values
of PET metabolic parameters SUVmax, SUVpeak, SUVmean, MTV and TLG
were calculated for the benign and malignant groups, respectively.
Normally distributed data were compared using the t-test or
Fisher's test, while the Mann-Whitney U test was used for
non-normally distributed data. The diagnostic performance was
assessed by receiver operating characteristic curve (ROC) analysis,
and the cut-off value of maximum performance evaluated using the
Youden index. P<0.05 was considered to indicate a statistically
significant difference.
Results
A total of 58 patients who met the inclusion
criteria were included in the present study, including 33 males and
25 females with an average age of 56.71±16.16 years (Table I). The samples included 30 cases with
malignant intraspinal lesions, 19 cases of metastasis (MRI and
PET/CT images of one such case are presented in Fig. 1), nine cases of lymphoma, one case of
glioblastoma multiforme and one case of melanoma. There were 28
cases of benign intraspinal lesions, including six cases of
schwannoma (MRI and PET/CT images of one such case are presented in
Fig. 2), five cases of meningioma,
four cases of myelitis, two cases each of neurofibroma, abscess,
chronic inflammation with fibromatous hyperplasia and spinal cord
edema, and one case of diffuse astrocytoma, pilocytic astrocytoma,
teratoma, sacral cyst and tuberculosis (Table I).
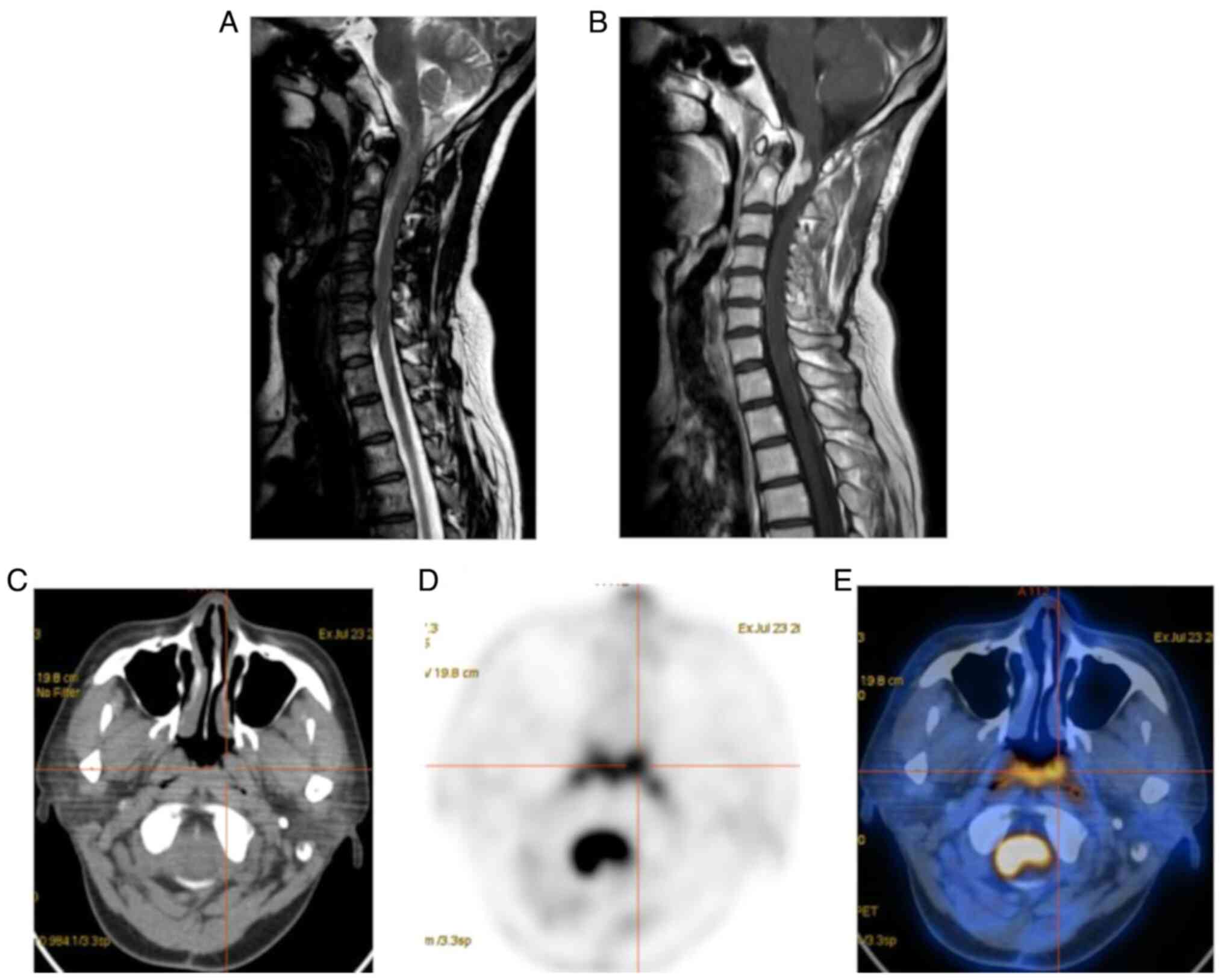 | Figure 1Female patient, 48 years of age,
reviewed after breast cancer surgery. (A) Sagittal T2WI; (B)
sagittal T1WI + C showing an irregular T1WI, low T2WI, slightly
high signal mass in the medulla oblongata and anterior to the
spinal cord at the level of cervical 1-2. The enhanced scan reveals
a significant enhancement, spinal cord compression, poorly
demarcated from the lesion, and localized signal elevation. (C)
Transverse CT; (D) transverse PET; (E) PET-CT fusion image showing
a slightly hyperdense occupancy with a CT value of ~55 HU and a
significantly increased FDG metabolism at the level of cervical 1-2
spinal canal with maximum standardized uptake value, 17.35; peak
standardized uptake value, 15.8; mean standardized uptake value,
10.63; metabolic tumor volume, 23.13 and total lesion glycolysis,
245.872. Metastasis was considered in the MRI and PET/CT of this
case, which was confirmed by a post-operative pathological
examination combined with immunohistochemistry. PET, positron
emission tomography; CT, computed tomography. |
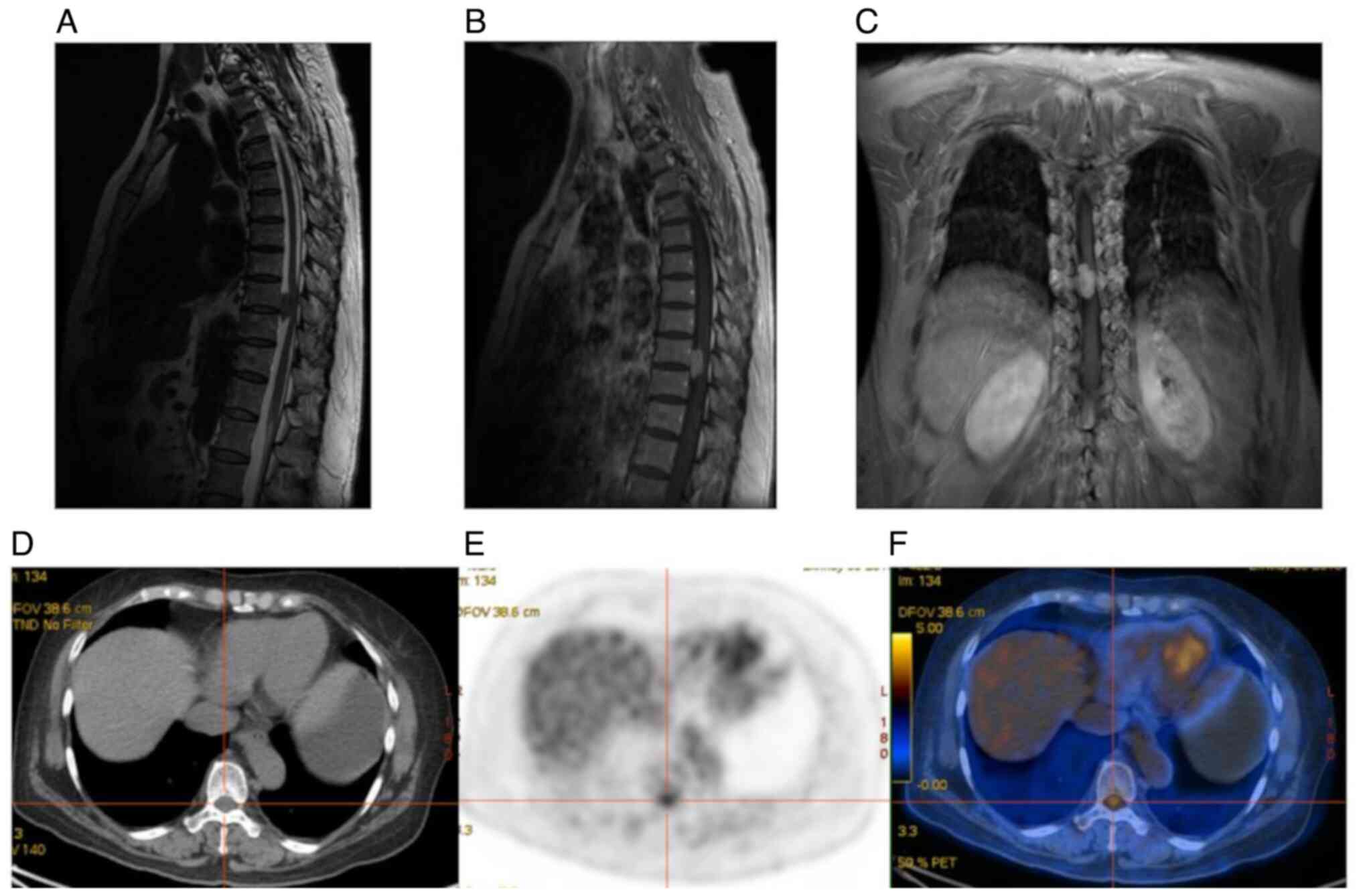 | Figure 2Female patient, 60 years of age, with
numbness in both lower limbs and paresthesia. (A) Sagittal T2WI;
(B) sagittal T1WI + C; (C) coronal T1WI + C showing a nodular T1
low T2 low signal shadow with clear borders in the extramedullary
dura at the level of thoracic 9-10 vertebrae, with adjacent spinal
cord compression, and significant enhancement with more uniform
enhancement. (D) Transverse CT; (E) transverse PET; and (F) PET-CT
fusion images showing slightly heterogeneous density within the
thoracic 9-10 spinal canal, no significant enlargement, no
destruction of adjacent bone, mildly increased FDG metabolism;
maximum standardized uptake value, 3.71; peak standardized uptake
value, 3.57; mean standardized uptake value, 2.46; metabolic tumor
volume, 4.39; and total lesion glycolysis, 10.799. Meningioma was
considered in the MRI and PET/CT, and the post-operative
pathological diagnosis was schwannoma. PET, positron emission
tomography; CT, computed tomography. |
 | Table ICharacteristics of the patients in the
present study. |
Table I
Characteristics of the patients in the
present study.
| Characteristic | Value (n=58) |
|---|
| Sex | |
|
Female | 25 |
|
Male | 33 |
| Age in years, mean
(range) | 56.71±16.16
(14-82) |
| Tumor volume
(metabolic tumor volume), mean (range) | 9.95±9.06
(2.42-42.51) |
| Histological type, n
(%) | |
|
Metastasis | 19 (32.8%) |
|
Lymphoma | 9 (15.5%) |
|
Glioblastoma
multiforme | 1 (1.7%) |
|
Melanoma | 1 (1.7%) |
|
Schwannoma | 6 (10.3%) |
|
Meningioma | 5 (8.6%) |
|
Myelitis | 4 (6.9%) |
|
Neurofibroma | 2 (3.4%) |
|
Abscess | 2 (3.4%) |
|
Chronic
inflammation with | 2 (3.4%) |
|
fibromatous
hyperplasia | |
|
Spinal cord
edema | 2 (3.4%) |
|
Diffuse
astrocytoma | 1 (1.7%) |
|
Pilocytic
astrocytoma | 1 (1.7%) |
|
Teratoma | 1 (1.7%) |
|
Sacral
cyst | 1 (1.7%) |
|
Tuberculosis | 1 (1.7%) |
The comparison between PET/CT and MRI as regards the
detection rate and diagnostic efficacy of benign and malignant
intravertebral lesions is detailed in Table II. The specificity of MRI was higher
than that of PET/CT, with a significant difference (P<0.05). The
accuracy and positive predictive value of MRI in the diagnosis were
slightly higher than those of PET/CT; in addition, the sensitivity
and negative predictive value of PET/CT were higher than those of
MRI (Table II), although these
differences were not statistically significant (P>0.05).
 | Table IIComparison of the diagnostic efficacy
of PET/CT and MRI for benign and malignant intravertebral
lesions. |
Table II
Comparison of the diagnostic efficacy
of PET/CT and MRI for benign and malignant intravertebral
lesions.
| | Gold standard | |
|---|
| Technique | Group | Malignant (n=30) | Benign (n=28) | Total (n=58) | Sensitivity | Specificity | Accuracy | Positive predictive
value | Negative predictive
value |
|---|
| MRI | Malignant | 25 | 5 | 30 | 83.3% | 82.1% | 82.8% | 83.3% | 82.1% |
| | Benign | 5 | 23 | 28 | | | | | |
| PET/CT | Malignant | 27 | 10 | 37 | 90.0% | 64.3% | 77.6% | 72.9% | 85.7% |
| | Benign | 3 | 18 | 21 | | | | | |
| P-value | | | | | 0.062 | 0.002 | 0.485 | 0.312 | 0.527 |
The mean ± SD values of the PET metabolic
parameters, SUVmax, SUVpeak, SUVmean, MTV and TLG, in the benign
and malignant groups were 4.27±1.25, 3.49±1.07, 2.49±0.84,
6.58±5.36 and 17.12±15.50 in the benign, and 8.99±3.75, 7.35±3.26,
5.43±2.40, 12.25±12.18 and 112.41±85.98 in the malignant groups,
respectively (Table III). The
SUVmax, SUVpeak, SUVmean and TLG were higher in the malignant group
than in the benign group, with statistically significant
differences (all P<0.0001). However, there was no statistically
significant difference in MTV (P=0.154) between the groups.
 | Table IIIDiagnostic efficiency of PET/CT
parameters to differentiate between benign and malignant
intravertebral lesions. |
Table III
Diagnostic efficiency of PET/CT
parameters to differentiate between benign and malignant
intravertebral lesions.
| Parameter | Benign group | Malignant group | P-value | AUC (95% CI) | Youden index | Sensitivity (%) | Specificity (%) |
|---|
| SUVmax (g/ml) | 4.27±1.25 | 8.99±3.75 | <0.0001 | 0.919
(0.817-0.974) | 0.7619 | 83.33 | 92.86 |
| SUVpeak (g/ml) | 3.49±1.07 | 7.35±3.26 | <0.0001 | 0.905
(0.799-0.966) | 0.7262 | 83.33 | 89.29 |
| SUVmean (g/ml) | 2.49±0.84 | 5.43±2.40 | <0.0001 | 0.899
(0.792-0.963) | 0.6214 | 80 | 82.14 |
| MTV
(cm3) | 6.58±5.36 | 12.25±12.18 | P=0.154 | 0.609
(0.472-0.734) | 0.2524 | 46.67 | 78.57 |
| TLG (g/ml x
cm3) | 17.12±15.50 | 112.41±85.98 | <0.0001 | 0.786
(0.658-0.883) | 0.4833 | 73.33 | 75 |
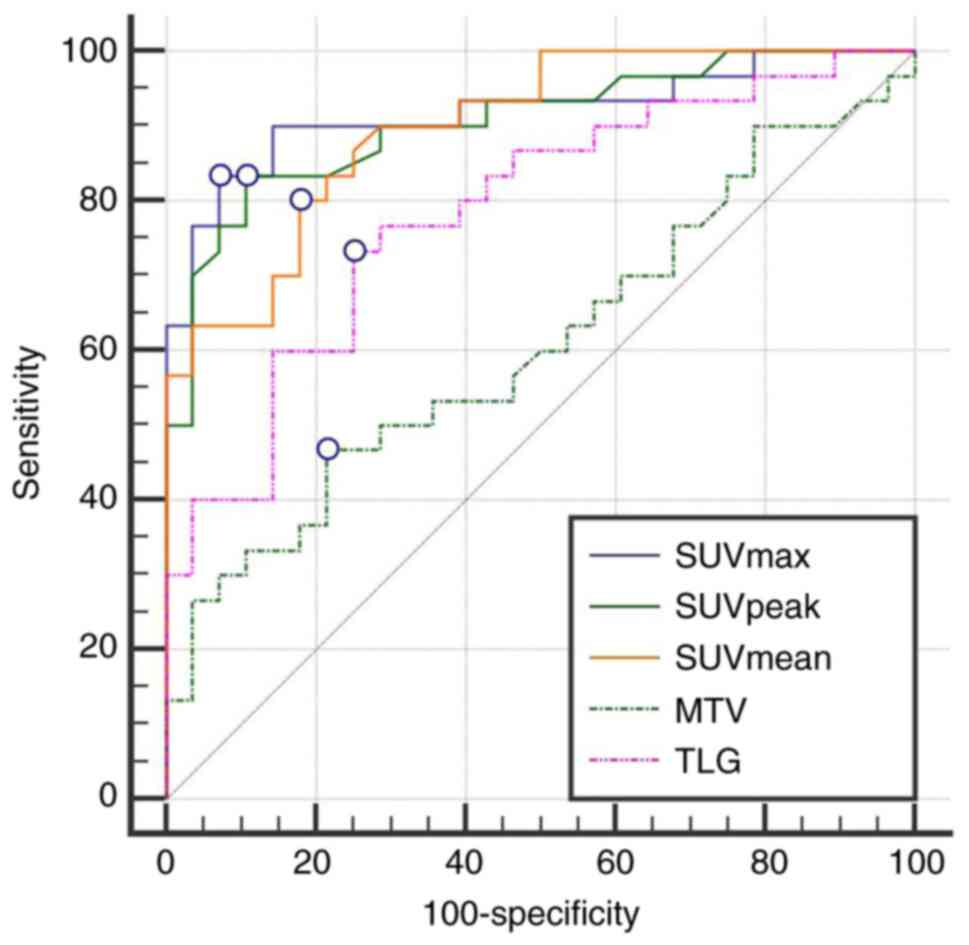
As regards the diagnostic efficacy between the
malignant and benign groups, the area under the ROC curve (AUC) for
SUVmax was 0.919, which was the largest, with a Youden index of
0.762, and 83.3% sensitivity and 92.9% specificity. In addition,
the AUC for SUVpeak was 0.905, with a Youden index of 0.726, and
83.3% sensitivity and 89.3% specificity. The AUC for SUVmean was
0.899, with a Youden index was 0.621, and 80.0% sensitivity and
82.1% specificity. The AUCs of the aforementioned three parameters
were significantly greater than those of MTV and TLG (0.609 and
0.786, respectively; P<0.001), and there was no significant
difference among the three (P>0.05; Table III and Fig. 3).
Discussion
Given that the treatment and prognosis of various
benign and malignant lesions in the spinal canal are differ
greatly, the early diagnosis and assessment of their properties are
essential in clinical practice. Early diagnosis facilitates the
selection of individualized treatment schemes and improves the
quality of life of patients with cancer. Traditional techniques,
such as DR and CT are usually not sufficient to correctly identify
spinal canal or spinal cord lesions. With the continuous
development of medical imaging technology, it has become possible
to effectively identify benign or malignant intraspinal lesions
using MRI and PET/CT (2,4,5).
MRI is the preferred examination technique for
evaluating intravertebral lesions due to high soft-tissue
resolution, and multi-sequence and multimodality. Therefore, MRI
plays a prominent role in clinical practice. The present study
found that the accuracy, sensitivity, specificity, positive
predictive value and negative predictive value of MRI were 82.8,
83.3, 82.1, 83.3 and 82.1%, respectively, which was similar to
values previously reported (8).
Conventional MR sequences distinguish neoplastic from
non-neoplastic lesions mainly based on lesion location, signal
intensity and morphological criteria (9). Alternatively, in multiple conditions,
intravertebral lesions caused by various etiologies have a similar
morphology, signal manifestations, or enhancement patterns on MRI,
which are prone to produce similar imaging results, rendering the
diagnosis very challenging or even leading to misdiagnosis
(2). For instance, some benign
lesions are large, have mixed T1 and T2 signals and infiltrative
growth, which cannot be easily distinguished from malignant tumors.
In the present study, in the 30 cases of malignant lesions, MRI
misdiagnosed two metastases as astrocytomas, two cases of lymphoma
as schwannoma, and one case of melanoma as meningioma. On the other
hand, PET/CT correctly diagnosed two of them (one lymphoma and one
metastasis) as malignant lesions, considering the abnormal
concentration of FDG metabolic radioactivity.
With the rapid development of PET/CT in recent
years, its clinical application has been widespread for the
detection of malignant tumors, and to differentiate between
neoplastic and non-neoplastic lesions. Particular advantages are
the ability to assess the grading of brain tumors, identify tumor
recurrence and radiation necrosis following radiotherapy with
‘one-stop’ evaluating anatomical structure and systemic functional
metabolism (10). Previous studies
on intraspinal tumors (mesenchymal astrocytomas, ventricular
meningiomas, etc.) have confirmed the feasibility of PET/CT in
intradural lesions and that it can further improve the diagnostic
efficiency (10,11). In addition, relevant studies
(12,13) have also demonstrated that PET/CT
combines the advantages of the high sensitivity of PET for the
detection and accurate anatomical localization of CT, and can
simultaneously evaluate soft tissue involvement. This is of
particular value in the diagnosis of intradural and vertebral
metastases with a higher sensitivity than MRI, providing richer
imaging information for clinical diagnosis and treatment.
The present study demonstrated that PET/CT had a
high diagnostic efficacy with a sensitivity of 90.0%, an accuracy
of 77.6%, and a negative predictive value of 85.7% for the
diagnosis of intravertebral lesions. Among the 30 malignant
lesions, 27 cases were correctly diagnosed, apart from two cases of
lymphoma and one case of a metastatic tumor with an insignificant
increase in FDG metabolism, which led to a false-negative
diagnosis. False-negatives may be due to highly differentiated or
low-grade malignant tumors with poor FDG metabolism or sparse
radioactive distribution. Primary intramedullary lymphoma is rare,
accounting for only 1% of central nervous system lymphomas. PET/CT
shows intravertebral occupations that may involve adjacent
vertebral and paravertebral areas. Metastases usually have a
history of malignancy, presenting in multiple nodular lesions,
which can be accompanied by vertebral and accessory bone
destruction and perineural edema. Both pathologies exhibit an
increased FDG uptake; however, intradural astrocytomas and
schwannomas usually have a lower FDG uptake than lymphomas or
metastases (14,15). False-positives of PET/CT imaging in
intraspinal lesions are primarily due to tuberculosis, abscess,
inflammatory demyelinating lesions (16,17),
where FDG metabolism levels are generally increased. Intramedullary
tuberculoma is rare, with a low or slightly high density, single or
multiple nodules, ~1 cm in diameter, a circumferential or
homogeneous enhancement, and a high FDG uptake. PET/CT is helpful
for determining cases involving tuberculosis of other systems and
the extent of involvement; in spite of this however, it has limited
specificity in distinguishing tuberculomas from tumors (16).
The findings of the present study demonstrated the
sensitivity of PET/CT diagnosis was 90.0%, and the negative
predictive value was 85.7%, which was higher than the MRI
sensitivity (83.3%) and negative predictive value (82.1%). The
PET/CT specificity (64.3%) and positive predictive value (72.9%)
were lower than the MRI specificity (82.1%) and positive predictive
value (83.8%). Compared with MRI, PET/CT has a higher sensitivity,
particularly in screening metastases, where sensitivity is more
valuable than specificity, as false-negative results often have
severe consequences for patients, and delay timely and effective
treatment. Mostardi et al (18), by linking PET and MR imaging
features, also confirmed that PET could detect the majority of
intramedullary metastases. Moreover, for the monitoring of
post-operative and post-treatment changes in some cases, even with
MRI with contrast enhancement, it can be challenging to assess
recurrence. By contrast, FDG-PET can provide additional information
in this regard. In patients diagnosed with benign astrocytoma or
schwannoma by MRI, PET is also useful for follow-up. When the
uptake of FDG increases, it may indicate a malignant transformation
of the tumor (5,15).
The application of PET/CT in intravertebral lesions
is limited, possibly due to the slightly lower spatial resolution
of PET, whereas, with the continuous improvement of resolution,
PET/CT can now clearly confirm the location. The mechanism of FDG
uptake is complex, reflecting the process of glucose transport and
consumption. Glucose depletion is related to tumor grade, cell
attenuation and bioinvasiveness (19). The significant increase in FDG uptake
highly suggests malignant tumors, although it may also occur in
tissues with high cell proliferation, such as in aggressive benign
tumors, inflammatory infections, etc. Unlike in brain tissue, an
increased FDG uptake is not common in the normal spinal cord. The
uptake of FDG by intraspinal lesions can be quantitatively
evaluated. The most commonly used semi-quantitative index parameter
of glucose metabolism is SUVmax, which is the largest SUV value of
a single voxel in the target volume. It has a simple operation and
good repeatability. The results of the present study indicated that
the SUVmax, SUVpeak and SUVmean values were higher in the malignant
group (8.99±3.75, 7.35±3.26 and 5.43±2.40, respectively) than in
the benign group (4.27±1.25, 3.49±1.07 and 2.49±0.84, respectively)
and the differences were statistically significant (all
P<0.0001). In particular, in distinguishing the diagnostic
efficiency, the AUC for SUVmax was 0.919, which was the largest,
with a Youden index of 0.762, and a 83.3% sensitivity and 92.9%
specificity. It was significantly higher than the AUC values for
MTV or TLG (0.609 and 0.786, respectively; P<0.001). Similarly,
the study by Tomura et al (11) revealed that benign intraspinal tumors
exhibited a slight to moderate increase in FDG uptake, while
malignant tumors, such as mesenchymal astrocytoma had an abnormally
high SUVmax.
The present study has some limitations which should
be mentioned. Retrospective analysis may result in selection bias.
In addition, the current sample size was small; hence, further
prospective studies with larger sample sizes are required. It is
considered that the coordination effect of MRI and PET-CT will be
higher than that of a single-mode, and the authors aim to closely
plan any future studies.
In conclusion, as demonstrated in the present study,
MRI remains a reliable examination for the diagnosis of benign or
malignant intravertebral lesions. 18F-FDG PET/CT, as a
useful supplement, also has a high sensitivity and accuracy for the
qualitative diagnosis and differentiation. The synergistic effect
of both techniques reflects different aspects of the lesions and
contributes to a more accurate diagnosis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ was involved in the conception and design of the
study, in the writing of the original draft, and was a major
contributor to the preparation of the manuscript. XW was involved
in the literature research and in the statistical analysis. WS, WT
and ZL were involved in image and data analysis, writing, reviewing
and editing of the manuscript. FZ and XW confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures performed in the present study
involving human participants were in accordance with the ethical
standards and declarations of Helsinki. The present retrospective
study was approved by Review Committee of the 4th People's Hospital
of Shenyang (no. 2021-011) and all patients enrolled gave informed
consent.
Patient consent for publication
For the images presented in the study, informed
consent was obtained from the patients and descriptions with
obvious indications of identity were removed.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xia LL, Tang J and Huang SL: Primary
intraspinal benign tumors treated surgically: an analysis from
China. Br J Neurosurg. 1–4. 2021.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
2
|
Watts J, Box GA, Galvin A, Van Tonder F,
Trost N and Sutherland T: Magnetic resonance imaging of
intramedullary spinal cord lesions: A pictorial review. J Med
Imaging Radiat Oncol. 58:569–581. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dammacco F, Rubini G, Ferrari C, Vacca A
and Racanelli V: 18F-FDG PET/CT: A review of diagnostic
and prognostic features in multiple myeloma and related disorders.
Clin Exp Med. 15:1–18. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shen G, Ma H, Pan L, Su M and Kuang A:
Primary spinal poorly differentiated neuroendocrine tumor displayed
on FDG PET/CT. Clin Nucl Med. 44:e586–e587. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Naito K, Yamagata T, Arima H, Abe J,
Tsuyuguchi N, Ohata K and Takami T: Qualitative analysis of spinal
intramedullary lesions using PET/CT. J Neurosurg Spine. 23:613–619.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sher A, Lacoeuille F, Fosse P, Vervueren
L, Cahouet-Vannier A, Dabli D, Bouchet F and Couturier O: For avid
glucose tumors, the SUV peak is the most reliable parameter for
[(18)F]FDG-PET/CT quantification, regardless of acquisition time.
EJNMMI Res. 6(21)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Duarte PS, Zhuang H, Castellucci P and
Alavi A: The receiver operating characteristic curve for the
standard uptake value in a group of patients with bone marrow
metastasis. Mol Imaging Biol. 4:157–160. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fawzy MF, Almassry HN and Ismail AM: What
can be achieved by using MR-DWI and ADC value in cases of
intramedullary spinal cord lesions of non-traumatic causes? Egyp J
Radio Nucl Med. 49:711–718. 2018.
|
|
9
|
Kessler J, Pawha P, Shpilberg K and
Tanenbaum L: Diffusion-weighted Imaging Facilitates Detection of
Spinal Multiple Myeloma and Assists in Diagnosing Equivocal
Lesions. Radiological Society of North America 2011 Scientific
Assembly and Annual Meeting, November 26 - December 2, 2011,
Chicago, IL. http://archive.rsna.org/2011/11001777.html. Accessed
April 15, 2021.
|
|
10
|
Piroth MD, Pinkawa M, Holy R, Klotz J,
Nussen S, Stoffels G, Coenen HH, Kaiser HJ, Langen KJ and Eble MJ:
Prognostic value of early [18F]fluoroethyltyrosine positron
emission tomography after radiochemotherapy in glioblastoma
multiforme. Int J Radiat Oncol Biol Phys. 80:176–184.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tomura N, Ito Y, Matsuoka H, Saginoya T,
Numazawa SI, Mizuno Y and Watanabe K: PET findings of
intramedullary tumors of the spinal cord using [18F] FDG and [11C]
methionine. AJNR Am J Neuroradiol. 34:1278–1283. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Laufer I, Lis E, Pisinski L, Akhurst T and
Bilsky MH: The accuracy of [(18)F]fluorodeoxyglucose positron
emission tomography as confirmed by biopsy in the diagnosis of
spine metastases in a cancer population. Neurosurgery. 64:107–113;
discussion 113-4. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schmidt GP, Schoenberg SO, Schmid R, Stahl
R, Tiling R, Becker CR, Reiser MF and Baur-Melnyk A: Screening for
bone metastases: Whole-body MRI using a 32-channel system versus
dual-modality PET-CT. Eur Radiol. 17:939–949. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Martins ES, Duque C, Rebelo O and Batista
S: Primary intramedullary spinal-cord lymphoma (PISCL): A rare
entity with a challenging diagnosis. BMJ Case Rep.
14(e242548)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sahel OA, Bazine A, Nabih SO, Benameur Y,
Biyi A and Doudouh A: Unsuspected intramedullary spinal cord
metastasis detected by FDG PET/CT. Indian J Nucl Med. 35:353–354.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu M, Lu L, Liu Q, Bai Y and Dong A: FDG
PET/CT in disseminated intracranial and intramedullary spinal cord
tuberculomas. Clin Nucl Med. 46:266–269. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu Q, Liu M, Bai Y and Dong A: Solitary
acute inflammatory demyelinating lesion of the cervical spinal cord
mimicking malignancy on FDG PET/CT. Clin Nucl Med. 45:1023–1025.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mostardi PM, Diehn FE, Rykken JB, Eckel
LJ, Schwartz KM, Kaufmann TJ, Wood CP, Wald JT and Hunt CH:
Intramedullary spinal cord metastases: Visibility on PET and
correlation with MRI features. AJNR Am J Neuroradiol. 35:196–201.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Viel T, Talasila KM, Monfared P, Wang J,
Jikeli JF, Waerzeggers Y, Neumaier B, Backes H, Brekka N, Thorsen
F, et al: Analysis of the growth dynamics of angiogenesis-dependent
and -independent experimental glioblastomas by multimodal
small-animal PET and MRI. J Nucl Med. 53:1135–1145. 2012.PubMed/NCBI View Article : Google Scholar
|