Introduction
Coronavirus disease 2019 (COVID-19) typically
presents with respiratory signs and symptoms (1). The transmission of COVID-19 is mediated
through air droplets with the recent delta variant suggested to be
airborne (2). Hence, the airway is
typically the first site of infection. The direct pulmonary effects
of COVID-19 are considered to result from the ability of the virus
to invade the cells lining the airway via the
angiotensin-converting enzyme (ACE-2) receptor, assisted by the
endothelial cell surface protein, the transmembrane serine protease
2 (TMPRSS-2) (3). The virus
replicates inside the cells, later causing pyroptosis and
widespread infection.
Extrapulmonary manifestations of COVID-19 were
naturally regarded as a sign of progressive infection following
viremia and aberrant immunological reaction (3). Therefore, clinical evidence of the
cytokine storm [defined as elevated levels of pro-inflammatory
cytokines, including interleukin (IL)-6, IL-10, tumour necrosis
factor-α (TNF-α), monocyte chemoattractant protein (MCP)-1, MCP-3
and others] in patients with COVID-19 has been shown to be
associated with severe infection and is a significant predictor of
mortality (3-5).
Therefore, researchers have suggested the use of immunomodulators
and cytokine antagonists from the early stages of the development
of cytokine storm, in order to improve the survival rate (4).
There is an increasing body of evidence with regards
to the early extrapulmonary manifestations of COVID-19 in the
absence of or with minimal respiratory signs (1). The underlying pathophysiology with
regards to various systemic manifestations of COVID-19 is still
under investigation. The cycle threshold (Ct) values of reverse
transcription-polymerase chain reaction (RT-PCR) are the number of
cycles are required for the fluorescent signal from the device to
cross the threshold, whereby the lower the Ct value is highly
associated with the enhanced ability to recover the virus from the
biological sample (6). Therefore, it
is expected that patients with lower Ct values will present with an
array of COVID-19-associated multisystem involvements.
The present study describes a clinical case of
COVID-19 (with strong positive reaction as per the Ct values),
presenting with marked cardiac and gastrointestinal manifestations,
in the absence of significant respiratory sequelae. The present
study aimed to determine the possible link between COVID-19 and
extrapulmonary manifestations supported by the literature and
discusses certain learning points with regards to the therapeutic
choices made for the patient described herein.
Case report
A 64-year-old male with a history of polysubstance
abuse, and underlying hypertension and ischaemic heart disease was
transferred to Hospital Tuanku Fauziah (Perlis, Malaysia) for
monitoring due to being positive for COVID-19. This was considered
a high-risk patient, in view of his advanced age and the presence
of co-morbidities. The results of the confirmatory test for
COVID-19 were as follows: RdRP gene: 24.33 (threshold, 38.3) and E
gene: 26.32 (threshold, 34.9) via the QIAstat-Dx®
Respiratory SARS-CoV-2 panel (Qiagen GmbH). The SARS-CoV-2 in this
panel targets two genes from the virus genome, which are detected
with the same fluorescence channel. The two targets are not
differentiated, and the amplification of either or both regions
lead to a fluorescence signal. The panel cartridge includes a full
process internal control, which verifies all steps in the analysis
process, including sample resuspension or homogenization, lysis,
nucleic acid purification, reverse transcription and polymerase
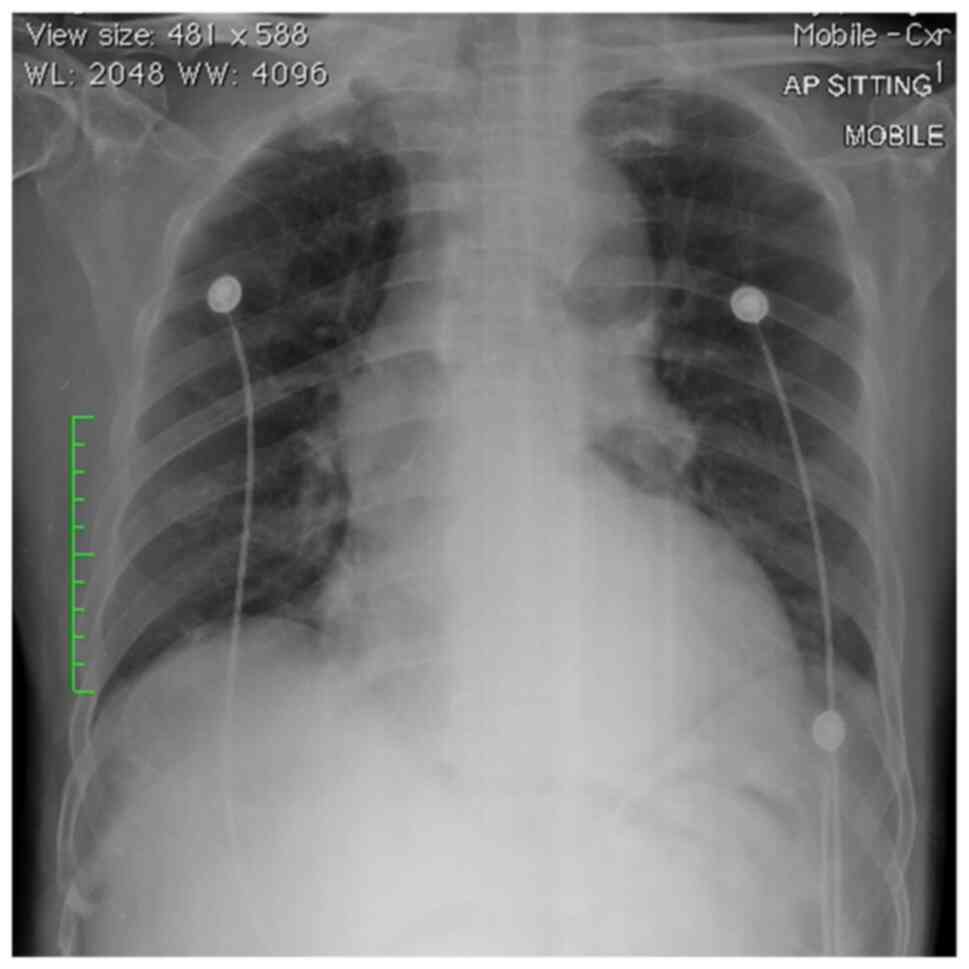
chain reaction. Otherwise, the patient was asymptomatic, and his
chest X-ray results were clear (Fig.
1).
In the ward, he was noted to have asymptomatic sinus
bradycardia [heart rate range, 30-45 beats per minute (BPM)] with
an electrocardiogram (ECG) revealing right heart axis with
incomplete right bundle branch block (QRS, 100-120 msec), with
downsloping depression (1 mm) of the ST-segment and T inversion. He
was otherwise normotensive (blood pressure, 135/54) with a good
pulse volume and was able to maintain good oxygen saturation
[peripheral capillary oxygen saturation (SpO2), 98-99%
in room air]. The jugular venous pressure was not raised, and there
was no radio-radial or radio-femoral delay. There was a pansystolic
murmur, grade III, with the echocardiogram revealing moderate
aortic regurgitation, a thickened aortic valve and a dilated left
ventricular chamber, with an ejection fraction of 70%. There was no
pericardial effusion, and the pulmonary artery systolic pressure
was 10.0+5.0 mmHg. No foot oedema was noted. The baseline
haematological and biochemical parameters were within normal limits
(Table I).
 | Table IHaematological and biochemical
parameters of the patient in the present study upon admission and
the worst derangement. |
Table I
Haematological and biochemical
parameters of the patient in the present study upon admission and
the worst derangement.
| Laboratory
result | Upon admission | The worst
derangement | Reference range |
|---|
| Haematological
panel | | | |
|
Total white
blood cell (x103/µl) | 7.54 | 11.2 | 4.0-10.0 |
|
Haemoglobin
(g/dl) | 15.4 | 17.37 | 13.0-17.0 |
|
Mean
corpuscular volume (fl) | 90.5 | 88.7 | 83-101 |
|
Mean
corpuscular haemoglobin (pg) | 32.0 | 31.5 | 27.0-32.0 |
|
Platelet
(x103/µl) | 215 | 70 | 150-450 |
|
ANC
(x103/µl) | 4.52 | 8.78 | 2.0-7.0 |
|
ALC
(x103/µl) | 2.34 | 1.21 | 1.0-3.0 |
| Biochemical
panel | | | |
|
Urea
(mmol/l) | 5.1 | 16.5 | 2.8-7.2 |
|
Creatinine
(µmol/l) | 86 | 343 | 59-104 |
|
Albumin
(g/l) | 42 | 38 | 35-52 |
|
LDH
(U/l) | 21 | 11,655 | <248 |
|
ALT
(U/l) | 21 | 4,944 | <50 |
|
AST
(U/l) | 35 | 16,722 | <50 |
| Creatine kinase
(U/l) | 158 | 5,211 | <171 |
| Creatine kinase-MB
(U/l) | 16 | 291 U/l | <24 |
| Inflammatory
markers | | | |
|
CRP
(mg/l) | <5 | 42 | <5 |
|
D-dimer
(µg/ml) | Negative | Positive | <0.5 |
The trial of bolus intravenous atropine at 0.5 mg
was unsuccessful, followed by a subsequent attempt with an
intravenous infusion of dopamine at 5 mg, slowly uptitrated to 15
mg, which also led to no improvement in the baseline heart rate. In
view of the persistent wide pulse pressure, it was decided that the
patient be subjected to transcutaneous cardiac pacing. The
intravenous infusion of dopamine was slowly weaned off with the
overlapping titration of noradrenaline infusion. His heart rate
gradually improved to 45-60 BPM, with the systolic blood pressure
ranging from 110-150 mmHg and diastolic blood pressure ranging from
60-90 mmHg. However, during the ensuing 48 h post-pacing, his blood
pressure gradually decreased, the lowest reaching 88/54 mmHg with A
heart rate of 54 BPM. A transvenous pacing through a subclavian
access (heart rate setting, 80; output, 10) was implanted. However,
the patient developed shortness of breath, chest pain and sweating
within the following hours; hence, the pacer was removed after
attempts to readjust the pacing wire failed. The re-implantation of
the transvenous pacing was subsequently performed via jugular
access.
On day 4 of admission, the patient complained of
lower abdominal pain with no bowel movements documented since
admission. He had vomited once, and streaks of blood were noted on
diapers. Blood gas analysis revealed severe metabolic acidosis [pH
7.013; partial pressure of oxygen (pO2), 71.9; partial
pressure of carbon dioxide (pCO2), 43.5; bicarbonate
(HCO3)-, 11.0) with a high anion gap (33.1
mEq/l). Free-flow gastric decompression yielded 665 millilitres of
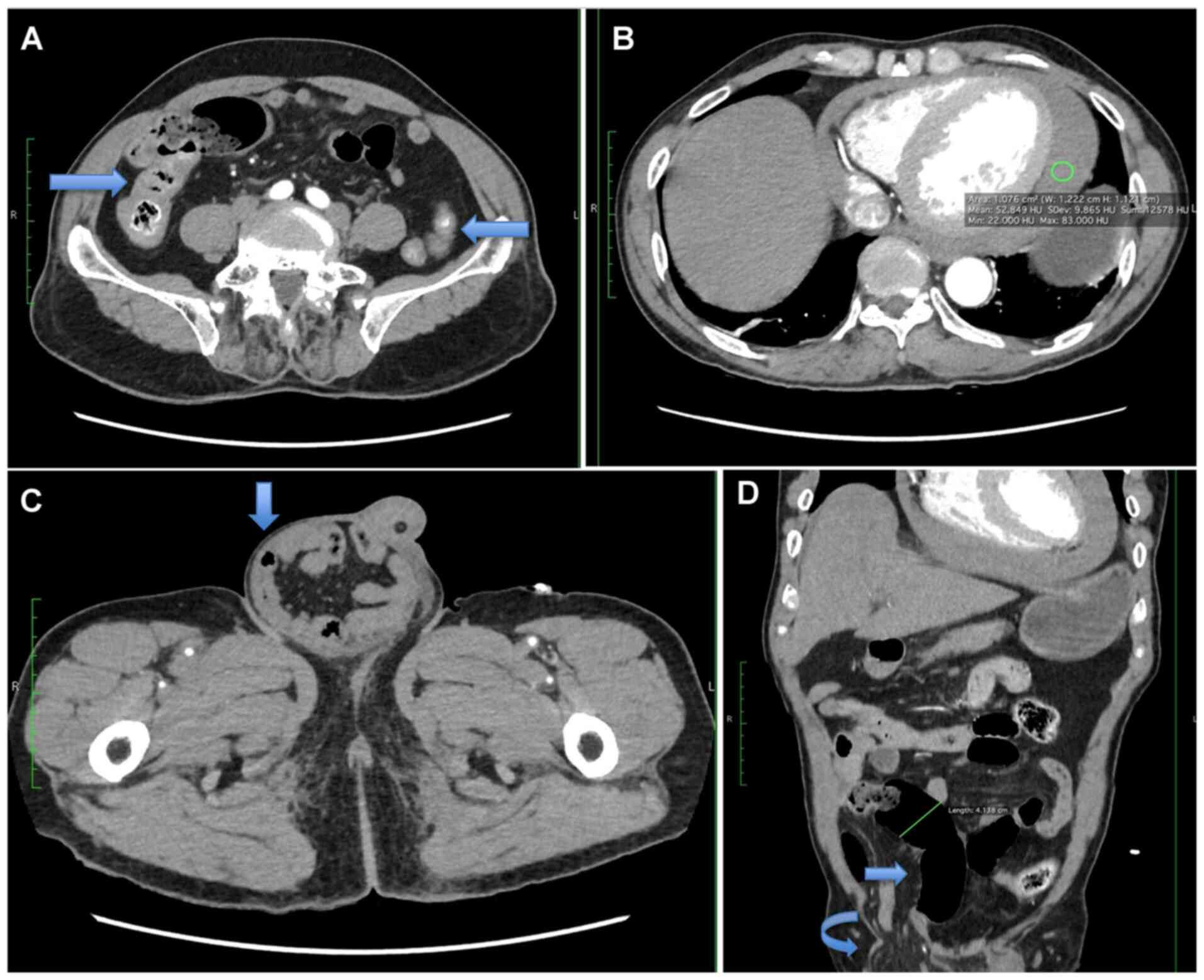
yellowish faecal material. The computed tomography examination of
the abdomen revealed a short segment circumferential wall
thickening at the proximal ascending colon, suggestive of
inflammatory or infective changes. There was also a right inguinal
hernia with small bowel and mesenteric content associated with
proximal bowel dilatation, suggestive of obstruction (Fig. 2). Clinically, the right inguinal
hernia was soft, reducible and non-tender (not painful), measuring
7x5 cm with no overlying skin changes. It was decided not to
subject the patient to surgical intervention.
From this point onwards, his clinical condition
deteriorated with the evidence of multiorgan failure; he had
persistent hyperglycaemia (Dextrostix, 14.0 mmol/l; Normal, <7.8
mmol/l), cardiorenal syndrome with anuria and worsening metabolic
acidosis (highest urea, 16.5 mmol/l; normal range, 2.8-7.2 mmol/l;
highest creatinine levels, 343 µmol/l; normal range, 59-104
µmol/l). He also had features of fulminant hepatic failure with
worsening transaminitis [alanine transaminase (ALT), 4,944 U/l;
normal, <50 U/l; aspartate aminotransferase (AST), 16,722 U/l;
normal, <50 U/l), hyperbilirubinemia (total bilirubin, 63
µmol/l; range, 5-21), thrombocytopaenia (platelet count,
73x103/µl; normal range, 150-450x103/µl) and
coagulopathy and was administered the N-acetylcysteine (NAC)
regime (150 mg/kg in D5 over 15 min followed by 50 mg/kg in D5 over
4 h, then 6.25 mg/kg/h in D5 for 67 h). There was evidence of third
space loss with global pericardial effusion, measuring 2.0-2.5 cm,
with evidence of right ventricular wall thickness. He required
double strengths of triple inotropic support, and haemodialysis
with sustained slow-efficiency dialysis (SLEDD), and continuous
veno-venous hemofiltration (CVVH) to correct the acidosis.
Repeated cycles of fresh-frozen plasma, packed cells
and platelet concentrate were administered, and empirical
antibiotics were escalated to meropenem. The patient eventually
succumbed to the disease after 6 days of hospitalization.
Discussion
The case presented herein describes the challenging
clinical management of a patient with COVID-19 who presented with
predominant extrapulmonary manifestation. His Ct values of RT-PCR
signified a strong positive reaction, denoting a high amount of
target nucleic acid of the COVID-19 virus in the body (6). Upon arrival to the hospital, he was
noted to have asymptomatic bradycardia requiring pacing. After
several days of admission, he then complained of abdominal pain
accompanied by days of no bowel movements. Despite aggressive
resuscitative effort, the patient eventually succumbed to
mortality. Therein, the present study discussed the prevalence and
pathophysiology of extrapulmonary manifestation of COVID-19 cases,
and the learning points from the therapeutic choices made for the
patient described herein.
The patient did not present with overt respiratory
symptoms and his chest X-ray did not reveal typical ground-glass
appearance pathognomonic of COVID-19 infection upon arrival.
However, reports of extrapulmonary manifestation of COVID-19 cases
were not uncommon with the haematological (67-90%),
gastrointestinal system (22-30%), cardiovascular (20-30%), and
renal (up to 29%) been commonly reported (7). The underlying pathophysiology of
multiorgan involvement was hypothesized to result from the systemic
viral entry as the ACE-2 receptors are virtually expressed in most
organ systems. Apart from the direct viral toxicity and cellular
injury, other hypothetical mechanisms include thromboinflammation
and endothelial injury, aberrant immunological response, and
dysregulation of the renin-angiotensin-aldosterone system (7), apart from the anticipated progression
of sepsis-related sequelae.
Cardiac complications from COVID-19 include viral
myocarditis, ST-changes on electrocardiogram, arrhythmias and
persistently raised cardiac enzyme levels (1). Such changes were also evident in the
patient described herein who had an elevated creatine kinase level
of 5,211 U/l (reference range, <171 U/l) and creatine kinase-MB
isozyme level of 291 U/l (reference range, <24 U/l). Cardiac
complications with COVID-19 are found to increase in the presence
of cardiovascular comorbidities, including hypertension,
cardiomyopathy and coronary artery disease (8), as evidenced in the patient described
herein. In such cases, the myocardial localization of SARS-CoV-2
leading to cardiogenic shock was hypothesized as the core
underlying pathophysiology (8).
On the other hand, gastrointestinal manifestations
of COVID-19 are usually prevalent with diarrhoea and abdominal
distension being the commonly reported symptoms (1,9).
Furthermore, COVID-19 has been found to have a specific tropism
towards the gastrointestinal system, with the recovery of COVID-19
nucleic acid in the stool of infected patients in the early course
of the illness (1). The patient in
the present study complained of abdominal pain after 4 days of
hospitalization with no bowel movements. The computed tomography
examination revealed features of obstruction with focal
inflammatory changes, reflecting the commonly reported
gastrointestinal complications of COVID-19, which include
hypomotility and bowel ischaemia (9).
Apart from that, there were indeed several learning
points with regards to the clinical management of the patient. It
was noted that within hours of the first implantation of the
transvenous cardiac pacing, the patient exhibited signs suggestive
of possible cardiac perforation, pericarditis, or a pneumothorax,
with the sudden onset of chest pain, shortness of breath, sweating
and cold peripheries. The early complication of transvenous pacing
is not uncommon, particularly with subclavian access performed by
an inexperienced operator (10).
Furthermore, all patients with temporary pacing should have
continuous electrocardiographic monitoring and be examined by
trained personnel who would be able to discern detection failure or
recognise an incipient or a dysfunctional pacemaker.
There was also a consideration for the role of
surgical management, as there were features suggestive of
intestinal obstruction, possibly due to the inguinal hernia.
However, the clinical association suggests otherwise; hence, the
surgical team deemed that the anticipated risks and complications
from the surgery far outweighed the potential benefits. Apart from
that, there was also a focal area suggestive of inflammatory
changes that could potentially be attributed to COVID-19. Whether
early surgical intervention could have changed the course of his
illness remains elusive.
The patient also developed features of fulminant
hepatic failure, requiring the use of the NAC regime. Acute hepatic
failure is rare, although a very severe medical emergency. There is
no established treatment to date for non-acetaminophen-induced
hepatic failure apart from liver transplant; however a recent
placebo-controlled study assessing the clinical course of patients
with acute liver failure treated with NAC determined that the
mortality rate among those treated was reduced to 28% as compared
to 53% in the placebo group (P=0.023) (11). In spite of this, the patient
described herein did not exhibit signs of clinical improvement
following treatment with the NAC regime.
Clinical deterioration in the present case appeared
to have been inevitable, as supportive therapeutic modalities
failed to alter the course of his illness. Multiorgan failure is
indeed the hallmark of the impending shut down of organ systems
with a high mortality rate. Therefore, it is suggested that
patients with complex clinical presentations be transferred at an
early stage to specialised healthcare facilities with adequate
expertise and resources to accommodate intensive care.
In conclusion, the case described in the present
study underscores the challenges in the clinical management of
patients with COVID-19 with multisystem involvement. Extrapulmonary
manifestations of COVID-19 are not uncommon, and a high index of
suspicion is required for monitoring the spectrum of
manifestations. The shared learning points may serve as a future
guide for other healthcare providers to plan for therapeutic
strategies when managing complex clinical syndromes.
Acknowledgements
The authors would like to thank the Director General
of Health Malaysia for his permission to publish the article.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors have made substantial contributions to
the study conception and design, and agreed to be accountable for
all aspects of the work. KK and KKA participated in the acquisition
and interpretation of the data, and in the drafting the first
manuscript. SAW and KKA revised the manuscript critically for
important intellectual content. KK, SAW and KKA confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was registered with the National
Medical Research Register of the Ministry of Health Malaysia
(NMRR-21-904-59980). Informed consent was not obtained since the
patient was deceased and the family members were out of reach. All
efforts were made to ensure anonymity. Additionally, approval was
obtained from the National Institute of Health (NIH) Ministry of
Health Malaysia for the use of patient clinical data for
publication purposes [NIH.800-4/4/1 Jld. 100(56)].
Patient consent for publication
Informed consent was not obtained since the patient
was deceased and the family members were out of reach. All efforts
were made to ensure anonymity.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Y, Geng X, Tan Y, Li Q, Xu C, Xu J,
Hao L, Zeng Z, Luo X, Liu F and Wang H: New understanding of the
damage of SARS-CoV-2 infection outside the respiratory system.
Biomed Pharmacother. 127(110195)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chan D and Harun HN: Delta variant can
infect a person within 15 seconds, says Health DG. New Straits
Times [Internet]. https://www.nst.com.my/news/nation/2021/07/708683/delta-variant-can-infect-person-within-15-seconds-says-health-dg.
Accessed July 22, 2021.
|
|
3
|
Brosnahan SB, Jonkman AH, Kugler MC,
Munger JS and Kaufman DA: COVID-19 and respiratory system
disorders: Current knowledge, future clinical and translational
research questions. Arterioscler Thromb Vasc Biol. 40:2586–2597.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hu B, Huang S and Yin L: The cytokine
storm and COVID-19. J Med Virol. 93:250–256. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen L, Wang G, Tan J, Cao Y, Long X, Luo
H, Tang Q, Jiang T, Wang W and Zhou J: Scoring cytokine storm by
the levels of MCP-3 and IL-8 accurately distinguished COVID-19
patients with high mortality. Signal Transduct Target Ther.
5(292)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Singanayagam A, Patel M, Charlett A,
Bernal JL, Saliba V, Ellis J, Ladhani S, Zambon M and Gopal R:
Duration of infectiousness and correlation with RT-PCR cycle
threshold values in cases of COVID-19, England, January to May
2020. Euro Surveill. 25(2001483)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gupta A, Madhavan MV, Sehgal K, Nair N,
Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan
EY, et al: Extrapulmonary manifestations of COVID-19. Nat Med.
26:1017–1032. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shafi AMA, Shaikh SA, Shirke MM, Iddawela
S and Harky A: Cardiac manifestations in COVID-19 patients-A
systematic review. J Card Surg. 35:1988–2008. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kaafarani HMA, El Moheb M, Hwabejire JO,
Naar L, Christensen MA, Breen K, Gaitanidis A, Alser O, Mashbari H,
Bankhead-Kendall B, et al: Gastrointestinal complications in
Critically Ill patients with COVID-19. Ann Surg. 272:e61–e62.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Betts TR: Regional survey of temporary
transvenous pacing procedures and complications. Postgrad Med J.
79:463–465. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nabi T, Nabi S, Rafiq N and Shah A: Role
of N-acetylcysteine treatment in non-acetaminophen-induced acute
liver failure: A prospective study. Saudi J Gastroenterol.
23:169–175. 2017.PubMed/NCBI View Article : Google Scholar
|