1. Types of diabetes
Diabetes is a prevalent endocrine disease associated
with oxidative stress. In 2014, 422 million individuals were
diagnosed with diabetes worldwide, while diabetes was directly
associated with 1,5 million deaths in 2012 and 2,2 million deaths
indirectly through an increased risk of cardiovascular mortality
and other diseases (1).
Diabetes mellitus is categorized into two types
according to insulin dependence. Type 1 diabetes mellitus or
insulin-dependent diabetes mellitus (IDDM) (formerly known as
juvenile diabetes) is characterized by hyperglycemia and
hypoinsulinemia. Type 1 diabetes mellitus is considered an
autoimmune disease, in which T-cells mediate the elimination of
pancreatic β-cells and thereby contribute to the production of low
insulin levels (2). In type 2
diabetes mellitus or non-insulin dependent diabetes mellitus
(NIDDM) (formerly known as adult diabetes), insulin resistance
seems to be the predominant factor and occurs from defects in
insulin secretion and a low tissue sensitivity to insulin (3). Diabetes is also known to cause
complications, such as cardiovascular diseases, neuropathy,
nephropathy, retinopathy, foot ulcers, skin lesions and hearing
impairment (4).
Diabetes mellitus is associated with high blood
sugar levels for a long period of time due to alterations in
carbohydrate, protein and fat metabolism, which results from a
dysfunction in insulin secretion, insulin action, or both (1). In diabetic conditions, excessive
reactive oxygen species (ROS) formation mainly appears to be
responsible for pancreatic β-cell dysfunction and insulin
resistance (5). When ROS are
produced by the mitochondria, they cause an impairment in the
mitochondrial respiration chain activity, which may, in turn, lead
to the excessive formation of superoxide anions
(O2-), and thus contributing to the incidence
and pathogenesis of diabetes (6).
The underlying mechanisms of ROS through the production of
superoxide anions contribute to the pathogenesis of diabetes by
upregulating poly(ADPribose) polymerase (PARP) and suppressing the
action of glyceraldehyde-3 phosphate dehydrogenase (GAPDH), which
constitutes an important glycolytic enzyme (7). Subsequently, hyperglycemia-induced
superoxide anions and hyperglycemia induce the flux of
mitochondrial electron transport chain through four damaging
pathways [generation of advanced glycation end-products (AGEs),
protein kinase C (PKC) activation, polyol formation and hexosamine
pathway stimulation], thus supporting the hypothesis that
mitochondrial-derived ROS is the missing link to the glucose
disturbance observed in diabetes (7). Various mechanisms have also been
proposed to enhance the oxidative stress mediated by diabetes, such
as lipid peroxidation (LPO), decreased antioxidant activity and
reduced glutathione (GSH) levels (8). For example, cerebral cells isolated
from streptozotocin (STZ)-treated rats are characterized by
increased levels of malondialdehyde (MDA), increased LPO and a
concomitant reduction in antioxidant enzyme activity and in the
glutathione-to-glutathione disulfide (GSH/GSSG) ratio (9). In addition to the above, other
mechanisms such as glucose auto-oxidation and protein glycation can
be important factors in determining the incidence of diabetic
complications (10).
Normal metabolism and energy production rely on the
multiple actions of taurine (11).
The antioxidant properties of taurine have been demonstrated in a
wide range of distinct diabetic animal models, where it was shown
to provide protection from the stressful signals of insulin
resistance or obesity, via various mechanisms: i) The upregulation
of antioxidant enzymes, such as superoxide dismutase (SOD),
catalase (CAT) and glutathione peroxidase (GPx); ii) interference
with PKC activity; iii) the downregulation of nicotinamide adenine
dinucleotide phosphate (NADPH) oxidase/cytochrome P450 2E1 (CYP2E1)
expression ratio; iv) the inhibition of protein carbonylated (PC)
content accumulation; v) the inhibition of LPO; vi) the disruption
of the generation of AGEs (12-15).
Based on the above, taurine could be used as an effective
therapeutic agent against diabetic complications, mostly due to its
anti-oxidant activity.
2. Beneficial effects of taurine on type 1
diabetes
Historically, the first line of evidence that
taurine exerted a positive effect on glucose tolerance in diabetic
patients, through the activation of glycolysis and glycogenesis,
was provided in 1976(12). Following
this, an inverse association seemed to exist between taurine and
plasma glucose content in patients with type 1 diabetes (13).
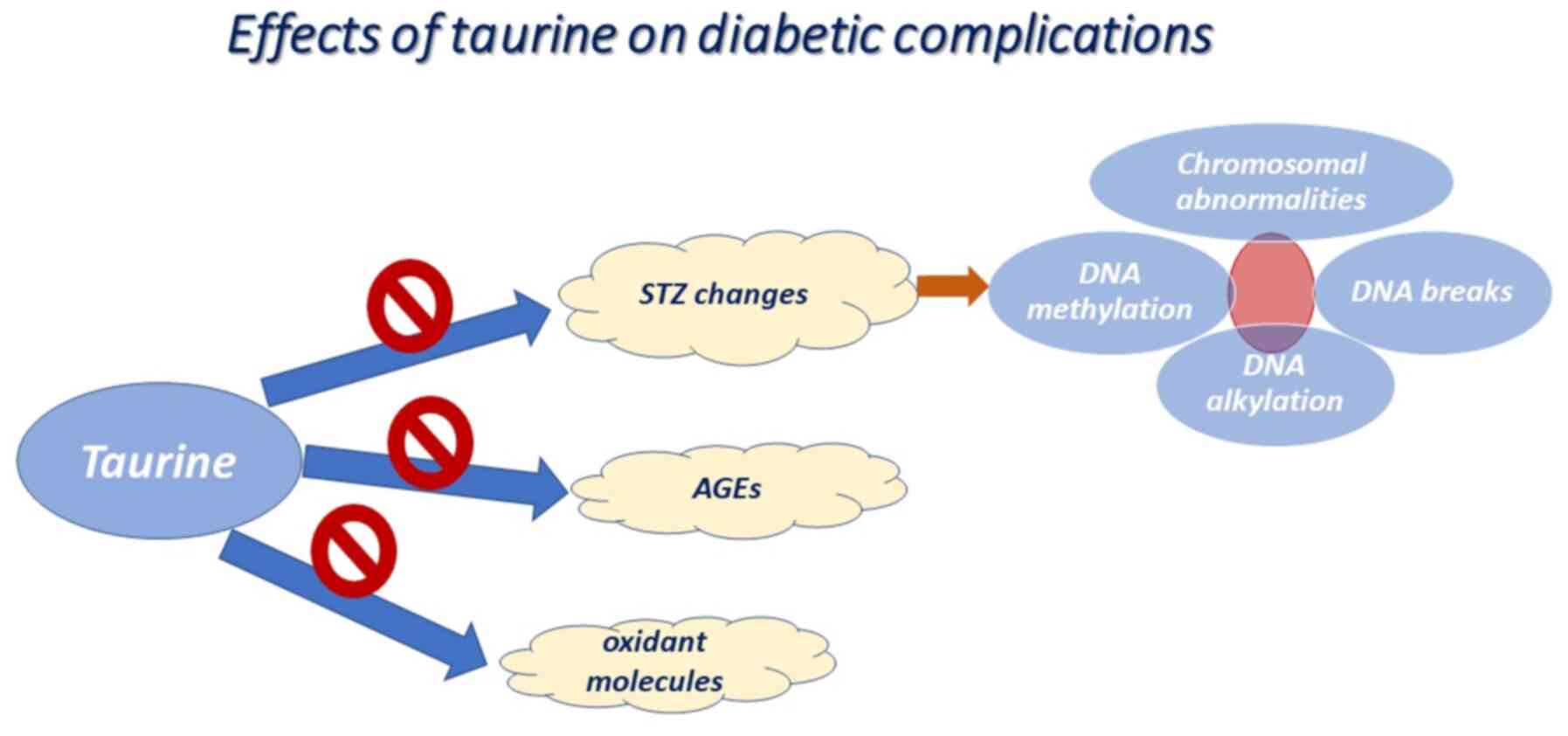
Type 1 diabetes is a condition that can be easily
replicated in animal models using either streptozotocin (STZ) or
alloxan toxins, which impair the function of pancreatic β-cells
(14,15). The underlying mechanisms of toxins is
based on the induction of alkylated DNA damage and cell death
(16), contributing to increased
PARP levels and to a decreased adenosine triphosphate (ATP) content
(15,17). The STZ or alloxan-mediated diabetic
animals present with lower plasma taurine levels than normal
animals (18). In this context, the
livers of diabetic animals are characterized by markedly reduced
concentrations of taurine, which may be due to an impairment in
taurine transporter (TauT) activity under high glucose conditions
and to an intracellular accumulation of sorbitol sufficient to
abrogate intracellular levels of taurine (19). As anticipated, taurine emerged as a
promising therapeutic agent for STZ-treated rats (18), alloxan-treated rats (20,21) and
alloxan-treated rabbits (22), in
terms of eliciting a hypoglycemic effect.
Antioxidant effects of taurine against
type 1 diabetes
The beneficial effects of taurine on hyperglycemia
caused by diabetes, have been validated by a number of studies. For
example, taurine has been shown to provide protection against the
biochemical, functional and morphological changes caused by
diabetes in the plasma, erythrocytes (23) and kidneys of rats previously treated
with STZ (24). In another example,
plasma glycated hemoglobin (HbA1c), cholesterol/triglyceride levels
and plasma LPO products were reduced in STZ-treated diabetic rats
following treatment with taurine prior to the diabetic onset
(25). Similarly, the inhibitory
effect of taurine on glucose levels seems to be achieved by
suppressing hyperglycemia in the type 1 model of diabetes induced
by alloxan, highlighting a potential inhibitory effect of taurine
against hyperglycemia prior to the diabetic onset (22). Notably, the potential of taurine on
altering the hyperglycemic status became evident in various models
of type 1 diabetes, thereby affording protection against diabetic
complications induced by STZ or alloxan, independently of the
diabetic onset (21). In some cases,
the hypoglycemic effect of taurine was evidenced when administered
at the time point of the diabetic onset (26), whereas in other cases, its effect was
manifested after the STZ-induced diabetic onset (27). In both cases, it was demonstrated
that taurine ameliorated the stress signals caused by hyperglycemia
and was thus beneficial in controlling glucose homeostasis
(26,27). In other words, as taurine was able to
reverse the phenotype of aorta rings derived from STZ-treated
diabetic rats, causing a reduction in the response to
norepinephrine and an increase in the response to acetylcholine
(28), it was concluded that it
improves the impaired endothelium-dependent vasodilator response in
hyperglycemia (28).
Ameliorative effects of taurine
against endothelial dysfunction and lipoprotein accumulation in
type 1 diabetes
When taurine is administered for long periods of
time, it appears to confer protection against endothelial
dysfunctional cell-mediated signals, by reducing the levels of AGEs
and of various oxidant molecules, including oxidized low-density
lipoprotein (ox-LDL) and hypochlorous acid (HOCl) (summarized in
Fig. 1) (29,30). It
also appears to ameliorate endothelial dysfunction, leading to the
formation of low quantities of MDA (31). Consistent with the above, taurine
plays an essential role in maintaining the extracellular matrix
(ECM) and the junctions between endothelial cells. When endothelial
cells are cultured under high glucose conditions, the upregulation
of adhesion molecules, including vascular cell adhesion molecule-1
(VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), is
observed (29). In vivo,
taurine supplementation for 5 days appears to be sufficient in
altering leukocyte-endothelial cell interactions and
hyperglycemia-induced endothelial apoptosis (32). In the same frame, the cytoprotective
role of taurine against type 1 diabetes emerged through its effect
on cholesterol levels. It has been demonstrated that taurine
supplementation causes the inhibition of lectin-like ox-LDL
receptor-1 (LOX-1), which is located in the endothelial cells of
aortas, in STZ-treated diabetic rats (33). In addition, the chronic
administration of taurine has been proven to be very helpful in
inhibiting the increase in LDL cholesterol levels in STZ-treated
diabetic mice (34). Accordingly,
taurine has been shown to attenuate the incidence of the
atherosclerosis-associated decrease in high-density lipoprotein
(HDL) cholesterol levels in plasma (34), which occurs when lipid-forming fatty
acids adhere to the walls of arteries in patients with
atherosclerosis.
Ameliorative effects of taurine
against oxidative stress in hepatocytes in type 1 diabetes
In hepatocytes of animal models with type 1
diabetes, the beneficial role of taurine has been highlighted
through its antioxidant effects. Initially, Mohamed and Gawad
(35) observed that taurine reduces
diabetes-mediated oxidative stress, thereby promoting the survival
of hepatocytes from STZ-induced harmful stimuli in rats. There are
numerous mechanisms through which taurine attenuates
diabetes-associated hepatic stress, as summarized in Fig. 2. Firstly, taurine appears to reverse
oxidative stress-related hepatic injury by reducing CYP2E1 activity
and gene expression (18). This
makes sense if one considers that CYP2E1 is a form of cytochrome
P450, which is involved in the catabolism of endogenous compounds
and in free radical reactions (36),
thereby highlighting the potential redox orchestration by taurine.
In the same frame, Fukuda et al (37) supported the antioxidant activity of
taurine, by demonstrating its ability to eliminate LPO products in
an indirect manner; taurine appeared to be involved in increasing
hepatic fatty acid oxidation and therefore hepatic efflux of
hepatic fatty acids to renal cells. Similarly, the beneficial
effect of taurine on the hepatic disturbance was also supported by
the activation of hepatic phosphoinositide 3-kinase (PI3K), protein
kinase B (Akt) and hexokinase, which led to the reduced
translocation of glucose transporter (GLUT)2 to the membrane of
hepatocytes in alloxan-induced diabetic rats, highlighting the
capacity to interfere with multiple signaling pathways (38).
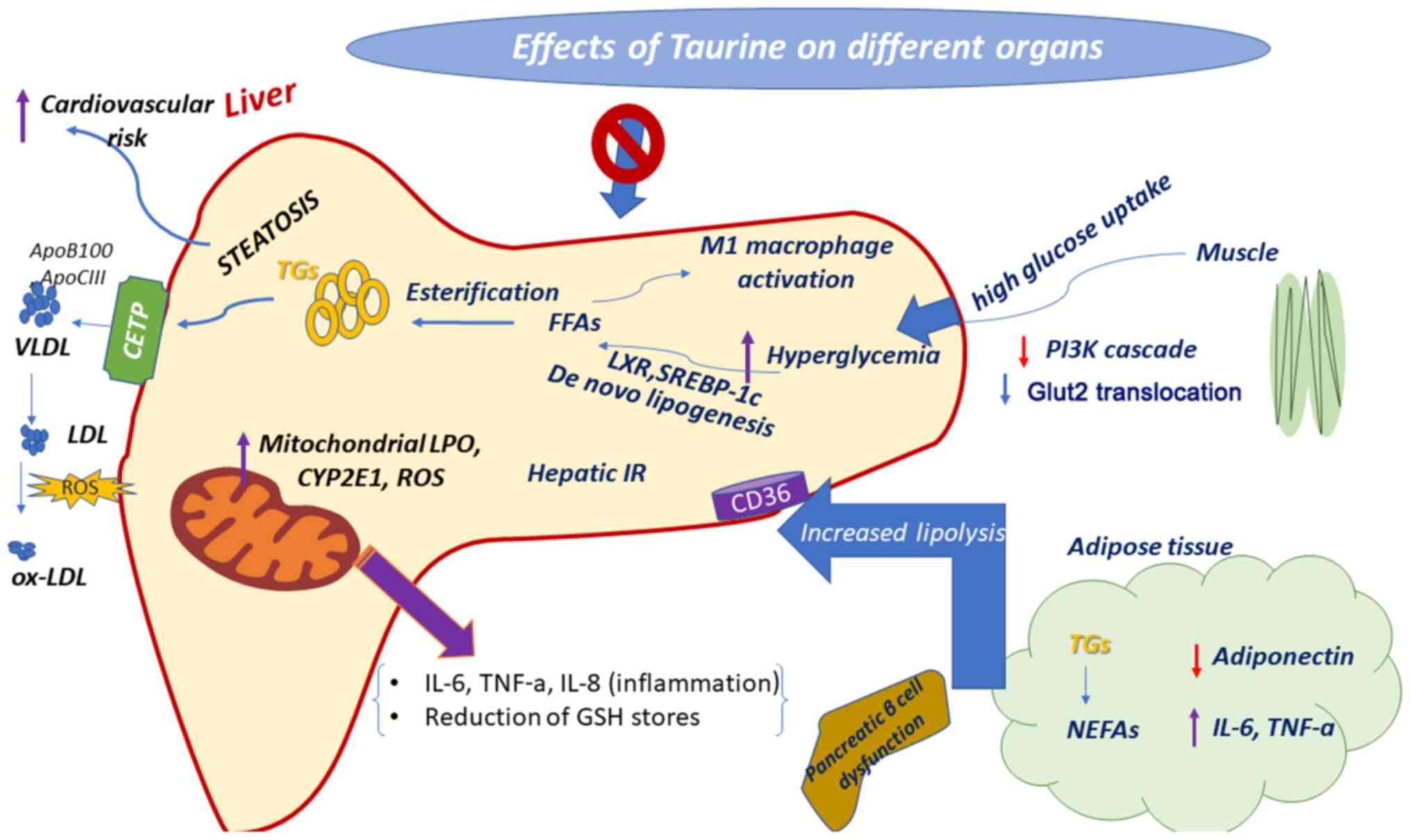 | Figure 2Therapeutic properties of taurine
against hyperglycemia. In the diabetic liver, hepatic glucose
uptake is increased and the blood glucose level, as well liver
disease are induced. Taurine protects against hyperglycemia via
inducing distinct pathways. Purple arrows indicate upregulation and
red arrows indicate downregulation. TNF-α, tumor necrosis factor-α;
IL, interleukin; CYP2E1, cytochrome P450 2E1; LPO, lipid
peroxidation; ROS, reactive oxygen species; FFAs, free fatty acids;
SREBP-1c, sterol regulatory element-binding protein 1c; LXR, liver
X receptor; PI3K, phosphoinositide 3-kinase; GLUT2, glucose
transporter 2; NEFAs, non-esterified fatty acids; TGs,
triglycerides; CETP, cholesteryl-ester transfer protein; LDL,
low-density lipoprotein; VLDL, very-low-density lipoprotein;
ox-LDL, oxidized low-density lipoprotein. |
Ameliorative effects of taurine on GSH
levels in type 1 diabetes
Taurine exerts its beneficial effect on type 1
diabetes through alterations in the cellular GSH content. As
previously demonstrated, following treatment with taurine, the GSH
content and the GSH/GSSG ratio appeared to be increased by 14 and
27%, respectively. In this manner, a protective mode of action by
taurine against STZ-induced oxidative stress in the brain and
spinal cord areas of diabetic rats was suggested (39). In another example, taurine was proven
to restore pancreatic β-cell damage when administered at 1.2 mM per
kg in male Sprague-Dawley rats within 45-75 min prior to the
intraperitoneal injection of STZ in rats (39). In the same frame, it has been
proposed that taurine protects against oxidative stress by serving
as an inducing signaling cue for GSH-related enzymes, such as
glutathione reductase (GR) and glutathione synthetase (GSS),
thereby contributing to the maintenance of the intracellular GSH
stores in the liver (40). Taurine
has been shown to improve hepatic GPx activity and to increase GSH
levels by directing cysteine into the GSH synthesis pathway in the
liver, proving its antioxidant properties in the liver (18). Accordingly, Furfaro et al
(41) proved that the administration
of taurine to STZ-treated rats for 6 months was sufficient to
inhibit the loss of the hepatic GSH content and the decrease in the
GSH/GSSG ratio. In a similar manner, in another study, 1% taurine
supplementation in drinking water was shown to exert an antioxidant
and hypoglycemic effect in alloxan diabetic rabbits, by increasing
the GSH/GSSG ratio and by restoring intracellular GSH levels
through increasing renal GR activity and by inhibiting hydroxyl
radicals, as well as by increasing the activity of antioxidant
enzymes, such as CAT in the serum and renal cortex (22). Taurine has also been shown to exhibit
neuroprotective and antioxidant activity by reducing LPO and
increasing GSH levels, thereby providing protection to rat cerebral
cells subjected to injury from D-galactose-related stress (42).
Positive effects of taurine on insulin
secretion in type 1 diabetes
Τhe inhibitory action of taurine on
insulin-dependent diabetes has been established in the pancreas,
through experiments proving the significant contribution of
taurine, conferring protection to pancreatic β-cells against injury
and the preservation of normal secretory granule functions, thus
delaying the onset of diabetes (43). In an experimental set-up where
taurine was administered to diabetic rats at the onset of diabetes,
in which STZ (60 mg/kg i.p.) was administered for 14 days, there
was a marked decline in plasma/blood glucose levels within 6 weeks
of diet (44). Furthermore,
pancreatic islets isolated from rats fed a high-glucose low-protein
diet, which were characterized by markedly reduced insulin
secretion rates, began to produce insulin again following the
taurine administration (45).
Similarly, in another study, taurine supplementation seemed to play
an important role in promoting insulin secretion in the islets of
malnourished mice fed a high-fat diet (HFD) (46). The long-term administration of
taurine was sufficient to restore the function of impaired
pancreatic islet cells, by inducing insulin secretion. At the
molecular level, Lin et al (47) revealed that the transcriptional
regulation of the insulin response in islet cells from rats with
STZ-induced diabetes occurred in vitro following the taurine
administration. In particular, taurine promoted insulin secretion
by enhancing the transactivation of transcription factors, such as
pancreatic duodenal homeobox- 1 (Pdx-1) and neurogenic
differentiation 1 (NeuroD1) (47).
Consistent with the results obtained in vitro, in another
study, pancreatic islet cells isolated from taurine-treated
diabetic mice were characterized by markedly high Pdx-1 levels of
transcription within 30 days of treatment, resulting in increased
glucose-activated insulin secretion (48). Notably, another study also
demonstrated that the mRNA levels of MafA, neurogenin 3 (Ngn3) and
NeuroD1 transcription factors also appeared to increase in mice
following the administration of taurine (49). Those findings were important
considering that Pdx-1, NeuroD1 and MafA are the crucial
transcription factors that bind to the upstream regions of the
insulin gene promoter, thereby determining the rate of insulin
synthesis (50), with NeuroD1 being
essential for the survival and normal functioning of pancreatic
cells (51). Similar results were
obtained in diabetic mice which had 2% taurine supplemented in
their drinking water for 30 days (48); the animals presented with low blood
glucose levels and supplementation of taurine appeared to increase
both the basal and the insulin-activated tyrosine phosphorylation
of the insulin receptors in the skeletal muscle and liver of
diabetic mice (48). The mice in
both groups (taurine versus no taurine) exhibited glucose-induced
insulin release; however, the mice in the taurine group exhibited
higher levels of insulin secretion compared with the control group.
The expression levels of the genes involved in the activation of
insulin secretion, i.e., GLUT2, glucokinase, sulfonylurea
receptor-1 (SUR-1) and pancreatic duodenal homeobox-1 (Pdx-1)
transcription factor were increased in the taurine group (48). In another example, it was
demonstrated that the protective mode of taurine against
diabetic-associated pancreatic dysfunction relied on its
anti-inflammatory properties. In particular, pregnant non-obese
diabetic (NOD) mice were administered taurine until weaning, in
order to investigate the effect of taurine on pancreatic
alterations in autoimmune type 1 of diabetes (43). The results suggested that taurine
modulates the infiltration of mononuclear leucocytes into the
pancreatic islets, thereby reducing the incidence of diabetes by
20% (43).
Neuroprotective effects of taurine
through the activation of inhibitory neurotransmitters
If one considers that cerebral cells are more
susceptible to oxidative damage than other types of cells mainly
due to their strict oxygen requirements, peroxidative damage to
lipids and proteins, and the lack of antioxidant responses, it is
thus expected that diabetes will have multiple effects on the brain
(52). In the long term, diabetes
exerts detrimental effects on cerebral cells, such as altered redox
potential, tissue damage, accelerated cognitive impairment, brain
atrophy and brain aging (9). Above
all, an imbalance arises between the overproduction of free
radicals/nitrogen species, and the diminished activity of
anti-oxidant enzymes, leading to an altered redox metabolism,
mitochondrial dysfunction and compromised energy metabolism
(53). In this context, taurine
exhibits potent neuroprotective activity and is linked to a lower
risk of diabetic neuropathy. In a previous study, in the
hippocampus of STZ-treated diabetic mice, taurine increased the
transcriptional levels of the GABAA receptor (GABAAR) α2 subunit
and of the brain-derived neurotrophic factor (BDNF), thereby
leading to an increased synthesis of gamma-aminobutyric acid (GABA)
by glutamate decarboxylases (GAD65 and GAD67) (54). In other words, taurine has been shown
to act as a GABAAR agonist in synaptic and extrasynaptic membranes,
by activating the neurotransmitter system and counterbalancing the
lower extracellular levels of GABA in STZ-treated mice (55). Chronic treatment with taurine has
been shown to increase the expression levels of BDNF, which appear
to be very low in the hippocampus of STZ-treated rats, thereby
rescuing neurons from atrophy (56).
Notably, this effect appears to co-exist with alterations in nerve
conductance deficits, hyperalgesia and nerve blood flow (57). For example, taurine has been shown to
attenuate the defects of hind limb sciatic motor and digital
sensory nerve conduction velocity, nerve blood flow and sensory
thresholds in Zucker diabetic fatty rats (58). Moreover, taurine seems to improve
excessive sympathetic nervous system activity and diuretic action
through the activation of sodium secretion and maintenance of
potassium and magnesium (59). The
administration of taurine also targets ROS formation, causing a
remarkable decline in intracellular calcium levels in the
mitochondria of neurons (60).
Taking all of the above-described evidence into account, it can be
suggested that taurine has the potential to bypass diabetic
neuropathy, by mainly activating nerve growth factors as weapons
against excessive oxidative stress.
Neuroprotective effects of taurine
through the attenuation of oxidative stress, inflammation and the
hormonal axis
Taurine exerts potent anti-inflammatory and
antioxidant activity through which it weakens neural responses in
the diabetic context (61). Taurine
reduces the expression levels of nuclear factor κ light chain-
enhancer of activated B cells (NF-κB) and increases the expression
levels of nuclear factor erythroid-derived 2-like 2 (Nrf2), of heme
oxygenase (HO-1) and GLUT1 and 3 in the brains of diabetic rats, as
compared to healthy rats (61). The
administration of taurine seems to partially lower the serum MDA
content and neuroinflammation via the inhibition of NF-κB
expression, and an increase in Nrf2, HO-1 and GLUT1/3 expression
levels in diabetic rats (61).
The regulatory effects of taurine on diabetes have
not only been linked to its antioxidant properties, but also to
potent hormonal alterations caused by the inhibition of the
hypothalamic-pituitary-gonadal axis in male diabetic rats (35). Specifically, it has been proven that
the plasma levels of acetylcholinesterase (AChE), gonadotropic,
gonadotropin-releasing hormone (GnRH), thyroid-stimulating hormone
(TSH), follicle-stimulating hormone (FSH) and luteinizing hormone
(LH) are reduced in diabetic animal models through the exogenous
administration of taurine (62).
These changes are accompanied by a marked elevation in the levels
of thyrotropin-releasing hormone (TRH), T3 and thyroxin (35). In support of this, it has been shown
that taurine decreases hyperglycemia, insulin loss and
mitochondrial oxidative stress, as well as hormone-associated
changes through the inhibition of the
hypothalamic-pituitary-gonadal axis (62).
3. Effects of taurine on type 2
diabetes
Type 2 diabetes is a major contributing factor for
the development of cardiovascular diseases, which are the first
cause of mortality worldwide. To address this challenge,
experimental animals on high-fat or high-sucrose diets have become
models of both liver and skeletal muscle insulin resistance
(63). Nonetheless, there is some
controversy regarding mitochondrial function in type 2 diabetes,
with the majority of studies pointing towards a reduction in the
oxidative potential of mitochondria (64) and other studies pointing towards an
increased oxidative potential (65,66).
Several studies have proposed that disturbed taurine
homeostasis accounts for the high incidence in obesity and diabetic
complications. It has been shown that the taurine concentration is
low in the plasma and platelets of patients with type 2 diabetes
(67), as well as in the plasma of
diabetic animal models (67). In
this context, a lower dietary intake of taurine is associated with
a higher cardiovascular risk (68).
This suggests that diabetes may be a taurine-deficient condition,
as supported by the low intestinal absorption rates and the high
renal excretion rates of taurine in these patients (69).
If one considers that taurine is the key element of
mitochondrial oxidative phosphorylation, it can be suggested that
taurine acts protectively against diabetes mellitus, insulin
resistance and related complications (70). The cytoprotective effect of taurine
has been demonstrated in different cells of type 2 diabetic
animals, through various mechanisms. For example, taurine
supplementation has been shown to improve hyperglycemia and insulin
resistance in Otsuka Long-Evans Tokushima Fatty (OLETF) rats and to
reduce diabetic complications, including retinopathy, nephropathy,
neuropathy and cardiomyopathy (19,71).
However, clinical trials have been conducted in order to
investigate the hypoglycemic effects of taurine on type 2 diabetes,
although no such properties have been reported thus far (72).
The protective mode of taurine against
non-insulin-dependent diabetes mellitus, and insulin resistance,
has been reported via multiple mechanisms (21,73,74).
This is possible as taurine is involved in a number of important
physiological processes; for example, taurine is positively
implicated in glucose homeostasis, exerting a strong hypoglycemic
effect (20), by reducing oxidative
stress (75) and inflammation
(76,77), and by increasing insulin sensitivity
and insulin secretion (78). Another
mode of action through which taurine improves diabetic
complications is the reduction of mitochondrial calcium overload,
usually accompanied by appropriate protein folding (79). For example, taurine has been shown to
reduce hyperalgesia and abnormal calcium signaling in the sensory
neurons of diabetic rats (57).
Advantageous effects of taurine on
diabetes 2 through its antioxidant properties
Taurine has been revealed to exert its anti-diabetic
effects by rescuing pancreatic β-cell dysfunction through its
antioxidant capacity (80). As
regards the antioxidant properties of taurine, it has been reported
that taurine ensures normal electron transport chain (ETC), thereby
protecting the mitochondria from excessive
O2- formation (75). The loss of taurine in the
mitochondria causes a significant reduction in the biosynthesis of
mitochondrial-encoded proteins ND5 and ND6, resulting in the
disruption of complex I and III activities of the respiratory chain
(81). This is probably due to the
inadequate incorporation of taurine into mitochondrial tRNA
(82,83). In the same context, taurine exerts
its anti-oxidant action via its capacity to counteract changes
caused by overproduction of uncoupling protein 2 (UCP2) in
pancreatic β-cells, thereby neutralizing the possibility for
excessive mitochondrial O2- generation
(84). Taurine has also shown to
abrogate LPO products in genetically hyperlipidemic animals,
including apoE-deficient mice (85)
and Watanabe heritable hyperlipidemic (WHHL) rabbits, that mimick
human familial hypercholesterolemia (86). In obese malnourished mice, taurine
maintains whole-body glucose tolerance and promotes liver insulin
signal transduction, as shown by the closely linked regulatory
importance of taurine to redox balance and protein phosphatases
activity (87). Taurine has also
been shown to mediate its antioxidant properties in muscles
isolated from high-glucose fed mice within 6 h of treatment
(88).
Beneficial effects of taurine on
diabetes 2 through its anti-inflammatory properties
Inflammation has been considered a major
contributing factor to the pathogenesis of diabetes; thus, it is
plausible that macrophages infiltrate into the adipose tissue of
patients with type 2 diabetes (89).
It is common knowledge that taurine can be converted into taurine
chloramine, which exerts anti-inflammatory activity by preventing
the nuclear translocation of NF-κB transcription factor and
consequently by inhibiting the expression of pro-inflammatory
cytokines [tumor necrosis factor-α (TNF-α) and monocyte
chemoattractant protein 1 (MCP-1)] (76,77). For
example, taurine has been shown to alleviate hyperglycemia symptoms
in C57BL/6 mice on a HFD, through its anti-inflammatory action. In
particular, taurine has been reported to promote the M1 to M2
conversion of macrophages in the murine adipose tissue and to
inhibit the gene expression of pro-inflammatory cytokines (90). The short-term administration of
taurine appears to be crucial for improving glucose homeostasis
through its anti-inflammatory action, by reducing the expression
levels of pro-inflammatory c-Jun NH-terminal kinase 1 (JNK1) in the
liver of rats on a HFD (91).
Furthermore, You et al (90)
demonstrated that rats with HFD-induced obesity presented a
recession of diabetic signs following 8 weeks of 3% taurine
supplementation in their drinking water, possibly due to the
positive anti-inflammatory action of taurine on adiponectin and
cholesterol levels. In the same context, other researchers have
also supported that taurine improves insulin sensitivity and LPO
through enhancement of adiponectin levels in obese women (92).
Beneficial effects of taurine on
diabetes 2 through its hypoglycemic properties
Taurine exerts its hypoglycemic properties through
its ability to improve peripheral insulin sensitivity, and in this
manner, by enhancing insulin release in response to glucose uptake
(78). Following treatment with
taurine, insulin secretion is induced, by upregulating insulin
receptor substrate 1 (IRS-1/2) tyrosine and Akt serine
phosphorylation in diabetic conditions (91). Experiments with malnourished mice on
a HFD have shown that central insulin signaling is a determinant of
altered food intake (78).
Specifically, as previously demonstrated, mice which had already
been on a balanced diet or on a protein-restricted diet for 6
weeks, were fed a HFD for another 8 weeks (78). Taurine supplementation (5%) appeared
to reverse the features of obesity only in mice fed a normal diet
before being fed a HFD (78). A
decrease in hyperglycemia was only observed in taurine-treated mice
previously fed a balanced diet, possibly due to taurine reducing
the activated form of insulin receptor [phosphorylated IRS-1
(p-IRS-1)] by half, without altering its total protein levels in
the murine hypothalamus (78). In
other words, taurine seemed to promote insulin secretion and to
restore metabolic disturbances in experimental animals fed a
balanced diet before being fed a HFD, possibly due to the
accumulation of protein constituents which are sufficient to
compensate for the metabolic alterations caused by a HFD (78). In another case, taurine
supplementation appeared to improve insulin sensitivity by
restoring the phosphorylation status of IRS and Akt in rats
subjected to lipid infusion-induced insulin resistance; the
underlying mechanism was considered to have occurred via inhibition
of the inflammatory JNK1 in the liver of rats (91). The insulin-like properties of taurine
also seem to be mediated by a reduction of ATP-sensitive
K+ channels, which are crucial for insulin secretion
(93,94). Other possible mechanisms for the
taurine-mediated increase in insulin sensitivity include increased
glycogen synthesis in the liver and glucose uptake in the
peripheral tissues, both of which imply an indirect association
between taurine and insulin sensitivity (88).
Furthermore, the high potency of taurine in
preventing diabetic complications is considered to stem from its
ability to significantly decrease glucagon, thereby contributing to
the maintenance of glucose homeostasis (49). Numerous studies have described the
beneficial hypoglycemic effect of taurine on increasing insulin
availability, through the activation of hepatic glucose
accumulation as glycogen (21) and
the inhibition of gluconeogenesis in animal models of diabetes
(95). The positive effect of
taurine on glucose regulation has also been confirmed via a more
prominent formation of islet-like cell aggregates (ICAs) from
autologous adipose-derived mesenchymal stem cells (ADMSCs),
following the administration of taurine (96).
Beneficial effect of taurine on
diabetes 2 through its interference with energy expenditure
The anti-obesity effects of taurine have been
well-documented in genetically modified diabetic KK mice (97), OLETF rats (71) and mice on a HFD (98). The observation that taurine inhibits
postprandial glucose oxidation suggests that it may be responsible
for altering the proportion of energy expenditure in glycogen
synthesis or lipogenesis (29). The
main underlying mechanism is the increase in oxygen consumption
rates (98). Specifically, the
dietary supplementation of taurine has been shown to increase the
expression levels of energy expenditure-related genes, such as
peroxisome proliferator-activated receptor α (PPARα), peroxisome
proliferator-activated receptor gamma co-activator 1 (PGC-1α) and
Nrf2, and their target genes [lipoprotein lipase (LPL), acyl-CoA
oxidase (ACO), acyl-CoA synthetase (ACS) and medium-chain acyl-CoA
dehydrogenase (MCAD)] in adipose tissue (98). If one considers the already
established cholesterol and lipid-lowering properties of taurine,
it is only reasonable to suggest that taurine is also actively
involved in increasing the rate of lipolysis in adipocytes, thereby
acting against obesity. This property may be due to either direct
activation of cyclic adenosine monophosphate (cAMP)-dependent
protein kinase A (PKA) catalytic activity or to an indirect PKA
stimulation through the formation of cAMP and interference with
hydrogen peroxide (H2O2) accumulation
(99). For example, 2.5% taurine
supplementation in the drinking water of rats with mono-sodium
glutamate (MSG)-induced obesity for 10 weeks is sufficient to
attenuate lipid accumulation, inhibiting total fat and triglyceride
formation, without altering glucose homeostasis (100). In rats with MSG-induced obesity,
taurine appears to exert a significant effect on energy
expenditure, by decreasing the expression of PGC-1α in the white
adipose tissue (WAT) and by increasing its expression in brown
adipose tissue (BAT), this way favoring thermogenesis (101). Focusing on the taurine-mediated
regulation of transcription factors, it has been proven that
taurine supplementation causes an increase in the transcriptional
activation of PPARα and UCP2, thereby preventing WAT formation and
promoting BAT formation in high-fat/cholesterol-fed mice (102). Therefore, taurine prevents fat
hyperplasia through its regulatory impact on PGC-1α, an important
source of energy expenditure in adipose tissues (102). In addition, it has been shown that
taurine supplementation prevents adipocyte differentiation
(103) and modulates the expression
of adipokines by inhibiting the signal transducer and activator of
transcription STAT3 signaling pathway (104). Notably, taurine modulates the
expression levels of adipokines through its conversion to taurine
chloramine (104). In addition, it
should be noted that the duration of taurine treatment appears to
be a major determinant for the control of obesity, in the sense
that prolonged administration of taurine has produced more stable
and prominent results than taurine's temporary treatment for 6
weeks (105).
Treating hyperlipidemia is one of the main targets
in the management of type 2 diabetes. In stroke-prone spontaneously
hypertensive rats (SHRSP) on a high-cholesterol diet (106), mice on a high-cholesterol diet
(107), rats on a high-cholesterol
diet (108) and ovariectomized rats
(109), the administration of
taurine was shown to exert both a hypolipidemic and a
hypocholesterolemic effect, via three distinct mechanisms. The
first mechanism of taurine seems to be based on a reduction in bile
acid absorption from the intestine (110), by increasing the cholesterol 7
alpha-hydroxylase (CYP7A1) bile acid synthesis enzyme and by
stimulating the fecal release of bile, in rats fed a
high-cholesterol diet (106). The
second mechanism includes an increase in the binding of LDL to the
LDL receptor from blood (111), and
the third mechanism has been shown to involve the inhibition of
very-low-density lipoprotein (VLDL) secretion from the liver
(112), accompanied by the
inhibition of hepatic acyl-CoA:cholesterol acyltransferase (ACAT)
activity (111). In addition,
taurine appears to exert its hypolipidemic effect through the
upregulation of hepatic LDL receptors and by ensuring accelerated
LDL turnover (113) and a reduction
in serum leptin levels (70).
Another hypolipidemic mechanism has been associated with a
reduction in triglycerides; as the administration of taurine has
been shown to enhance the activity of LPL in both plasma and liver,
thereby promoting the peripheral clearance of triglycerides
(114). Indeed, taurine
supplementation has been shown to enhance peroxisomal fatty acid
β-oxidation and to reduce fatty acid synthase activity in the
livers of type 2 diabetic/obese mice, contributing to normal redox
homeostasis with low MDA formation (115). As a result, the hypolipidemic
effect of taurine has been attributed to its ability to activate
mitochondrial fatty acid oxidation.
Hence, the hypolipidemic effect of taurine is
accompanied by a marked decline in leptin levels and by bypassing
insulin resistance in type 2 diabetic rats (70). In OLETF rats with type 2 diabetes,
taurine has been noted to ameliorate hyperglycemia and
dyslipidemia, by enhancing insulin sensitivity and by inhibiting
leptin secretion (70). In the same
frame, chronic taurine administration (9 weeks) to 2-month-old
OLETF rats has been shown to lead to the elimination of serum
triglycerides and cholesterol without altering LPO (116). This makes sense if one considers
that taurine has been shown to positively modulate hypothalamic
neuropeptide expression (117) and
to normalize the 24-h pattern of leptin production via the
regulation of insulin levels (118). Therefore, it has been revealed that
taurine functions in the hypothalamus of OLETF rats by suppressing
food intake and locomotor activity, and by stimulating signal
transduction through the protein kinase B/Forkhead box (Akt/FOX)
(117). Another study by Carneiro
et al (48) demonstrated that
taurine regulated glucose homeostasis through the control of leptin
gene expression levels, which are required for glucose-stimulated
insulin secretion.
Protective effect of taurine in animal
models subjected to glucose or fatty acid infusion
The protective effects of taurine against diabetic
stress-mediated pancreatic islet dysfunction have been evaluated in
animal models subjected to glucose or fatty acid infusion. Both
in vivo and in vitro experiments have demonstrated
that taurine inhibits oxidative stress, improves pancreatic β-cell
dysfunction, thereby conferring enhanced sensitivity to insulin in
rats following lipid infusion (48 h of oleate infusion) (80). Other studies have supported that
taurine stimulates Akt in hepatic cells, causing alterations that
lead to restoring the function of pancreatic β-cells and to
ameliorating insulin resistance in mice fed a high-fat diet (HFD)
(102,119). Particular emphasis has been given
on the ability of taurine to enhance insulin sensitivity by
negatively affecting hyperglycemia through the activation of the
PI3K/Akt signal transduction pathway (102,119,120).
In a previous study, a 48-h combination treatment scheme, including
both oleate and taurine was shown to restore insulin release from
pancreatic islets, mainly due to the ROS-scavenging capacity of
taurine (80). Additional
experiments have proven that taurine contributes to the maintenance
of insulin signaling by interfering with fatty acid-induced
oxidative stress, JNK1 activation in pancreatic islets and livers
subjected to intravenous infusion of fatty acids (91).
Furthermore, several studies have supported the
significance of taurine as a promising agent in controlling
oxidative stress in diabetic conditions; however, the vast majority
of studies have only evaluated its efficacy in animal models and
clinical trials in the very narrow window of up to 6 months
(73,80,121).
The long-term administration of taurine has a distinct differential
effect on glucose and lipid levels in diabetic conditions. For
example, Branco et al (122)
demonstrated that taurine supplementation over 12 months caused
adverse effects in lipid and glucose tolerance in mice fed a HFD.
Similarly, long-term 5% taurine supplementation caused ectopic
lipid accumulation, hyperglycemia, pancreatic islet hyperfunction,
insulin resistance and signs of renal damage. Renal injury in
HFD-fed mice that received taurine was identified by increased
rates of urinary proteins and albumin (123). However, the persistently high
concentration of taurine interfered with glomerular and tubular
processes, negatively affecting glomerular filtration barrier
integrity, causing the development of vacuoles in renal tubules,
ultimately resulting in disturbed renal reabsorption and renal
dysfunction (124). The development
of vacuolar structures were shown to be involved in the storage of
phospholipids and cholesteryl esters in proximal tubular cells
(124). By contrast, short-term
taurine supplementation appeared to decrease renal damage and to
prevent the increase of blood urea nitrogen (BUN) and serum
creatinine levels in diabetic rats (125). Consequently, taurine-based
therapies for obese and diabetic subjects should be carefully
designed to confer the desired therapeutic effect, which aims at
the impaired glucose control due to prolonged taurine
supplementation.
The beneficial effect of taurine against diabetic
stress has also emerged in various combination schemes. For
example, dietary fish oil containing n-3 PUFAs (eicosapentaenoic
acid and docosahexaenoic acids) in combination with 4% taurine
dietary supplementation proved to be very successful against white
adipose tissue formation and high blood glucose levels in KK-Ay
mice (115). Characteristically,
the effect caused by the combination scheme was significantly more
prominent than that mediated by soybean oil treatment alone
(115). In another study where 2%
taurine was administered in combination with fish oil to obese
mice, it was shown that fat metabolism was mediated by fatty acid
oxidation and glucose uptake (115,126).
Accordingly, Kishida et al (109) proved that taurine was capable of
decreasing plasma total cholesterol concentration in rats that were
fed with corn oil, but not in rats fed with coconut oil. Taurine
appeared to be efficient in maintaining normal liver lipid levels
by enhancing the activity of LDL receptor (LDL-R) in the liver
(109).
4. Effect of taurine on the fructose-fed rat
model
Dyslipidemia, insulin resistance and type 2
diabetes can be simulated with the administration of a
high-fructose diet to rodents (127), as also indicated by studies on
humans (128). A high-fructose diet
in rats has been shown to cause a marked decline in plasma and
liver taurine levels (114). In
this context, previous studies have highlighted the important
contribution of taurine to bypass fructose-mediated insulin
resistance (129,130).
Taurine supplementation has been proven to be
highly efficient in controlling glucose production in fructose-fed
rats, independently of treatment duration frames (30 or 182 days).
On the one hand, improved glucose intolerance and insulin
resistance were observed in Wistar rats following long-term (26
weeks) fructose and 2% taurine supplementation (131). Despite being able to restore
triglycerides to normal cells in fructose-fed rats, taurine
supplementation did not seem to alter hepatic phosphoenolpyruvate
carboxykinase (PCK1) mRNA levels (131). No effect was also observed on
phosphorylated Akt levels in the skeletal muscle of fructose-fed
rats, which was in accordance with the observation that taurine
acts as an agonist of insulin signaling (74). In the same frame, there was no
recorded activation of the rate-determining gluconeogenic enzyme
PCK1(132), thereby excluding the
possibility that increased glycogenolysis is the main factor for
the increase in glucose. Those findings were not in agreement with
previous results that supported the inhibitory action of taurine on
glycogenolysis (133), even though
such discrepancies could be due to differences in animal strains,
and doses and duration of taurine treatment and so on. Therefore,
the improvement in glucose tolerance in taurine/fructose-fed rats
was attributed to the increased release rates of insulin (48). Taurine has also been shown to confer
insulin sensitivity, by reducing lipid peroxidation in fructose-fed
rats (129,134). In accordance with this, taurine
supplementation to high fructose-fed rats has been shown to prevent
the formation of lipid peroxidation products, to enhance insulin
sensitivity and to suppress the accumulation of glycated proteins,
such as fructosamine and HbA1c, which are localized in the plasma
of these animals (115,129,134).
5. Therapeutic effect of taurine on
diabetes
Clinical effectiveness of taurine on
type 1 diabetes
From a historical point of view, Franconi et
al (67) were the first to
provide insight into the significance of taurine against type 1
diabetes, based on the observation that taurine expression levels
were very low in the plasma of diabetic patients. Specifically,
patients with type 1 diabetes (n=39) who were administered taurine
at doses of 1.5 grammars per day for 90 days (long-term treatment)
did not exhibit any alterations in their glucose metabolism
(67). The ability of taurine to
reduce platelet aggregation in diabetic patients was also
highlighted, as shown by the dose-dependent restoration of taurine
concentration in the plasma and platelets of type 1 diabetes
subjects (67). Following this,
Elizarova and Nedosugova evaluated the effects of taurine on a
small subset of patients with type 1 diabetes (n=10), who had
already been treated with insulin (13); in this case, taurine was administered
in doses of 0.5 g per day for 30 days and was shown to improve the
symptoms of type 1 diabetes, as demonstrated by an increase in
carbohydrate metabolism and a decline in triglyceride content
(13). Another clinical study
demonstrated the therapeutic effectiveness of taurine against
insulin resistance due to its ability to reduce oxidative stress
(73). Specifically, a 2-week
taurine supplementation of 3 g per day for 48 h before
lipid-infusion was shown to prevent the formation of LPO products
and to improve pancreatic β-cell function (73).
It is well-established that the intact vascular
endothelium produces nitric oxide (NO), which is regarded as a
vasculoprotective molecule, by inhibiting platelet, as well as
leukocyte adhesion to the vascular endothelium (135). In diabetic conditions,
hyperglycemia is responsible for suppressing the endothelial NO
synthase (NOS), contributing to excessive ROS formation; however,
it also enhances the overproduction of vasoconstrictor substances,
such as endothelin 1 and the activation of the renin-angiotensin
system (136). In this context, a
cross-over study employing male patients with type 1 diabetes who
were administered taurine at doses of 1.5 g per day, revealed that
the particular treatment scheme was able to reverse early,
detectable conduit vessel abnormalities in the endothelium of these
patients (137); in support of
this, a study using diabetic animal models suggested a possible
role for taurine on suppressing monocyte-endothelium interaction
(138). In another study, the
co-culture of human umbilical vein endothelial cells (HUVECs) with
monocytes isolated from smokers revealed signs of impaired
monocyte-endothelium interaction (139). Taurine administration appears to
restore this interaction by decreasing the efflux of NO from the
monocytes of smokers, leading to elevated endothelin-1 in HUVECs
(139). In a similar context, the
hypoglycemic effect observed in patients with type 1 diabetes who
received taurine, has been attributed to improved endothelial
function (137). Specifically,
taurine supplementation at doses of 1.5 g per day for 2 weeks was
able to reverse all the alterations related to arterial stiffness
in diabetic patients (137).
Notably, the same group had previously reported that this
particular mode of taurine supplementation (1.5 g per day for 2
weeks) has been shown to improve the disturbed flow-mediated
dilatation flow of the brachial artery in young cigarette smokers
(139).
Clinical effectiveness of taurine on
type 2 diabetes
Taurine has been shown to exert a therapeutic
effect on obesity and lipid profile in clinical trials. For
example, the administration of taurine at doses of 3 g per day for
7 weeks has been shown to improve plasma triglyceride and total
cholesterol content in overweight non-diabetic subjects (140). In another case, the administration
of taurine at doses of 6 g per day in subjects on high-fat and
high-cholesterol diets appeared to improve the symptoms of diabetic
complications (141). Furthermore,
other trials have reported that taurine supplementation at doses of
3 g per day for 4 months does not have any effect on glucose and
lipid peroxide levels on patients with type 2 diabetes (72). However, the HbA1C appears unaltered
in diabetic patients following the taurine supplementation of 1.5 g
per day for 8 weeks, therefore preventing pancreatic β-cells from
being more sensitive to insulin (72); these findings are not in agreement
with the results of certain animal studies. In terms of diabetic
nephropathy, Nakamura et al (142) reported that the long-term
supplementation of taurine, (3 g per day) did not have any effect
on the phenotype of patients with microalbuminuria related to type
2 diabetes within 12 months, as demonstrated by the relative
expression levels of fibrotic markers [serum collagen IV and plasma
matrix metalloproteinase-9 (MMP)-9]. Notably, the accuracy and
validity of the results obtained in clinical studies are questioned
by the presence of certain limitations, such as the concomitant
administration of other medications, the severity of the disease,
the correct patient characterization and stratification, the
taurine dosage scheme, and the duration of trials among others
(59).
6. Functional significance of taurine in
renal disorders
Initial studies on taurine using ion-exchange
chromatography in 1960 revealed that a significant amount was
localized in the urine of patients with renal tubular disorders or
with hereditary aminoaciduria (143). Following this, the increased
excretion of taurine was also detected in patients with X-linked
disorders and genetic syndromes such as Fanconi anemia and
cystinosis (143).
Taurine is a key modulator of several physiologic
functions in renal cells. It has been shown to positively affect
ion reabsorption and secretion, urine composition, renal blood
flow, osmoregulation and glomerular filtration (144). In renal cells, taurine has been
shown to exert major non-ionic osmolarity capacity. Several lines
of evidence have pointed out that taurine, along with betaine,
myoinositol and a-glycerolphosphate, enable the normal flux of the
renal medullary tonicity gradient (145). In this context, it has been
hypothesized that taurine may represent an important therapeutic
agent in cases of renal dysfunction.
7. Effects of taurine on hypertension
Hypertension is a prevalent symptom in renal
disorders, and it is considered to occur through defects in the
renin-angiotensin-aldosterone system (RAAS), monogenic
abnormalities of ion transporters and acute kidney inflammation
(146).
As taurine is well-known for its antioxidant
properties, its potent osmoregulatory potential and its regulatory
importance for cation transport in renal cells (147), it has also been extensively
investigated in hypertension. Taurine supplementation seems to
reverse the adverse effects of hypertension in animal models
(71,121,148-151)
and importantly, the onset of hypertension in rats has been
accelerated in conditions of taurine loss (152). In this direction, it has been
demonstrated that taurine exerts an inhibitory effect on
adriamycin-mediated proteinuria and hyperlipidemia, by preventing
urinary taurine excretion (153).
Other researchers have reported that taurine negatively affects
lipid levels, reducing the efficacy of enzymes [lecithin
cholesterol acyltransferase (LCAT), LPL] or serum factors
[platelet-activating factor (PAF)] or GSH values, in an attempt to
counterbalance renal dysfunction (154). In a previous study, in a
fawn-hooded hypertensive rat model of spontaneous hypertension,
taurine supplementation appeared to suppress proteinuria, through
its capacity to increase urinary NO excretion, as well as sodium
(Na+) and potassium (K+) excretion (155).
An important mechanism through which taurine exerts
its hypotensive effect is through the RAAS, which is classified as
the major reason for the development of hypertension (156). The hypotensive ability of taurine
mainly relies on antagonizing angiotensin II, thereby prohibiting
the activation of the RAAS, as demonstrated in both cell cultures
(157,158) and animal models, such as i)
spontaneously hypertensive rats (SHR) (159,160);
ii) rats fed a high-fructose diet (129); and iii) deoxycorticosterone acetate
(DOCA)-salt hypertensive rats (161). On a similar note, taurine has been
shown to be effective against lead-induced hypertension (162) and cyclosporine A-induced
hypertension (148).
The anti-oxidant and anti-inflammatory properties
of taurine also appear to protect renal cells in models of
hypertension. In the case of N-nitro-L-arginine methyl ester
in Sprague-Dawley rats, taurine appears to be an attractive agent
against the form of hypertension, by reducing pro-inflammatory
cytokine expression levels, whilst promoting NOS activity, thereby
positively regulating serum NO levels (151).
Taurine supplementation has been shown to attenuate
the advancement of chronic kidney disease (CKD), most probably due
to positively affecting renal blood flow dynamics, as well as
reducing hypertension and proteinuria. While focusing on the
molecular mechanisms underlying the beneficial effect of taurine
against hypertension, it has been proposed that taurine reduces
vascular resistance and positively controlling arterial blood
pressure through regulation of the autonomic nervous system
(151,163). Τaurine has been reported to be
involved in the regulation of the sympathetic nervous system, where
it has been shown to lead to a marked decline in hypertension in
both rats and humans (164,165). Considering that the RAAS and renal
sympathetic nerve activities participate in the renal excretion of
fluids and sodium (166), it can be
hypothesized that the diuretic and natriuretic properties of
taurine are the direct results of the suppression of either the
RAAS or the renal sympathetic nerve activity.
Taurine appears to negatively affect hypertension
through its action on the hydrogen sulfide (H2S)
content. Of note, increased taurine levels have been closely
associated with increased H2S levels, thereby leading to
hypotension manifested by reduced transient receptor potential
channel 3 (TRPC3)-induced signaling in the vasculature (167).
Taurine has been shown to improve vessel function
and endothelial function. The hypotensive properties of taurine
have been observed in both animal models and in hypertensive human
subjects, where taurine reduces blood pressure through its effects
on endothelial cells (167,168). Following taurine supplementation,
improved endothelial function and reduced oxidative stress have
been observed (167,168). For example, the taurine-mediated
attenuating effect on endothelial-dependent vasodilation was
documented in young smokers following taurine supplementation (1.5
g) for 5 days (139) and in rats
following treatment with 1% taurine for 8 weeks (169). The underlying mechanism of
taurine's action was based on reducing the calcium overload,
attenuating oxidative stress and reducing sympathetic/inflammatory
action, resulting in improved kidney function (71,147,148,151,152,161,168,170,171).
Consistent with the above, it has been shown that taurine can
possess diuretic and natriuretic properties in saline-loaded rats
(152).
In a clinical setting, Ogawa et al (172) supported the view that patients with
hypertension are characterized by low plasma levels of taurine.
Accordingly, an epidemiological study indicated that there was an
inverse association between taurine intake and blood pressure
(173). The administration of
taurine (6 g) in young patients with borderline hypertension led to
a reduction in both systolic and diastolic blood pressure within 7
days (121). The same favorable
effect was reported when taurine (3 g) was administered for a
longer period (2 months) (174).
Recently, Sun et al (167)
demonstrated a marked decline in diastolic/systolic pressure in 120
pre-hypertensive subjects following taurine supplementation.; in
particular, high diastolic pressure (80-89 mmHg) was reduced to 4.7
mmHg following taurine administration (1.6 g/day) to a
pre-hypertensive subject for 12 weeks (167). In the same patient, systolic
pressure ranged from 120 to 139 mmHg prior to taurine
administration, whereas it appeared to decrease to 7.2 mmHg
following its administration (167). Moreover, taurine supplemented (6
g/3 weeks) to healthy volunteers has been shown to be inversely
associated with levels of urinary norepinephrine excretion
(141)
8. Effects of taurine on acute kidney
injury
Acute kidney injury (AKI) is a very severe health
issue in humans, constituting a major cause of mortality (175). A significant number of studies have
highlighted the potential of taurine to be used as a novel
therapeutic agent, as well as a significant diagnostic factor in
renal injury.
Several nephrotoxins can lead to the development of
AKI and include heavy metals, such as lead, cadmium, mercury,
uranium and gold. In this respect, taurine has been shown to
eradicate renal toxin-induced symptoms, thereby acting as a potent
reno-protective agent (40). For
example, in a subchronic lead intoxication rat model, exposure to
lead caused the animals to be more susceptible to brain oxidative
stress, as shown by reduced delta-aminolevulinic acid dehydratase
(ALAD) activity, diminished GSH values and upregulated zinc
protoporphyrin (176); taurine
appeared to salvage rats from the harmful injuries of lead exposure
via normalization of the GSH status (176). In another study investigating the
penetration rates of trace elements (Se, Cu, Fe2+, and
Mn) using ICP-MS in arsenic-exposed rats, the administration of
taurine appeared to reverse the toxic changes in the kidneys and
livers of these animals (177).
This makes sense if one considers that taurine is composed of an
amino group and a sulfonate group instead of a carboxyl group,
thereby promoting the excretion of heavy metals and their
conjugation to other compounds (178), as well as arsenic excretion.
Among toxins, arsenic is an established factor in
causing acute kidney injury due to mitogen-activated protein kinase
(MAPK)/NF-kB activation and mitochondrial-dependent pathway
alterations (179). Even in low
concentration doses, arsenic is able to cause an oxidative burst
and to enhance LPO and protein carboxylation in renal cells
(179), as well as to cause
oxidative DNA damage to vascular smooth muscle cells (180). Taurine has been shown to confer
significant kidney protection from oxidative DNA damage in
arsenic-intoxicated mice (181).
Following the administration of taurine to mice, renal tissues were
characterized by low 8-hydroxy-2-deoxyguanosine (8-OHdG) in their
glomeruli and renal tubule areas (181). The weak distribution of 8-OHdG
immunoreactivity was in agreement with the histopathological
changes observed in arsenic-exposed mice that received taurine, as
demonstrated by the lack of DNA strand breaks in the renal tissues
in immunohistochemistry data (181). Consistent with the above, in
another study, taurine appeared to reverse intestinal DNA damage in
rats following potassium bromate exposure (182) and to prevent testicular oxidative
injury in diabetic rats, by mitigating LPO and DNA damage levels
(62).
ΑKI can also be caused experimentally by the
administration of acetaminophen (183). Acetaminophen-administered swiss
albino mouse strains are usually characterized by renal necrosis
and significant alterations in normal oxidative status (183). Treatment of these animals with
taurine has been shown to improve the signs of nephrotoxicity, as
evidenced by a reduction in CYP2E1 expression (183).
In cases of antibiotic-mediated nephrotoxicity,
evidence of kidney injury includes elevated BUN and urinary
N-acetyl-glucosamine (NAG) and reduced Na+
K+ ATPase activity (184). For example, the co-administration
of taurine and quercetin has been shown to normalize creatinine
clearance and to decrease protein urinary excretion, uronic acids,
and urinary NAG in animal models induced by the combination of
gentamicin and diclofenac (184).
Cortical LPO products appear to increase after administration of
gentamicin and to decline after the administration of taurine and
quercetin (184).
In radiation-induced kidney injury, taurine and its
transporter can be restored to normal levels in renal cells
following taurine supplementation (185). In glomerular disease, taurine is
converted to taurine chloramine, via the increased intracellular
activity of myeloperoxidase in invaded polymorphonuclear leukocytes
(186). Taurine chloramine has been
shown to function as an oxidant reservoir, exhibiting antioxidant
effects in both proximal or distant sites (186). This response is more prominent in
phagocytes, which contain a high number of taurine-related
anti-oxidants (187), particularly
during the early stages of inflammation in the glomeruli and
tubules of the renal tissues (188).
9. Effects of taurine on diabetic
nephropathy
Human diabetic nephropathy is a severe health
concern that often requires renal dialysis (189). End-stage renal disease requiring
dialysis is most often caused by diabetic nephropathy. A number of
different mechanisms have been identified as essential components
to the development and progression of diabetic nephropathy.
Hyperglycemia is considered to be the common underlying driving
force for diabetic nephropathy through the following mechanisms:
The overexpression of glucose transporters, glucose accumulation in
mesangial cells and ECM production (189). In this manner, signals are
transmitted in specific transduction pathways that lead to an
overproduction of ROS, pro-inflammatory cytokines and hormones,
thereby conferring pro-inflammatory characteristics to patients
with diabetes (190). The aberrant
glomerular and tubular structural changes are observed in diabetic
nephropathy. In particular, glomerular basement membrane
thickening, glomerulosclerosis and tubulointerstitial fibrosis seem
to occur due to the accumulation of AGEs in the kidneys (191). Furthermore, persistent
hyperglycemia accounts for the activation of PKC, which in turn
leads to both transforming growth factor β1 (TGF-β1)-mediated-ECM
production in mesangial cells and increased eicosanoid release
linked to glomerular hyperfiltration (192). Notably, moderate hyperglycemia
without glycosuria can enhance plasma renin activity and mean
glomerular pressure, resulting in hyperfiltration and
glomerulosclerosis (189).
Taurine has been shown to exhibit great potential
in inhibiting the progression of diabetic nephropathy (19), given that taurine deficiency is
considered a key characteristic in diabetic patients (69). Of all renal disorders, taurine has
been studied most extensively in diabetic nephropathy as a possible
new therapeutic strategy (summarized in Fig. 3). This has been accomplished in
several diabetic animal models induced by STZ or alloxan, where the
administration of taurine has caused a regression of diabetic
symptoms by inducing hypoglycemic, anti-oxidant and renoprotective
activity, including improved hyperglycemia, dyslipidemia, as well
as reduced blood HbA1c 1c levels and oxidative stress (24). It should be highlighted that the
beneficial effect of taurine in STZ-animal models is crucial
(33), given that the particular
animal model can mimic all the complications of human diabetic
nephropathy (193).
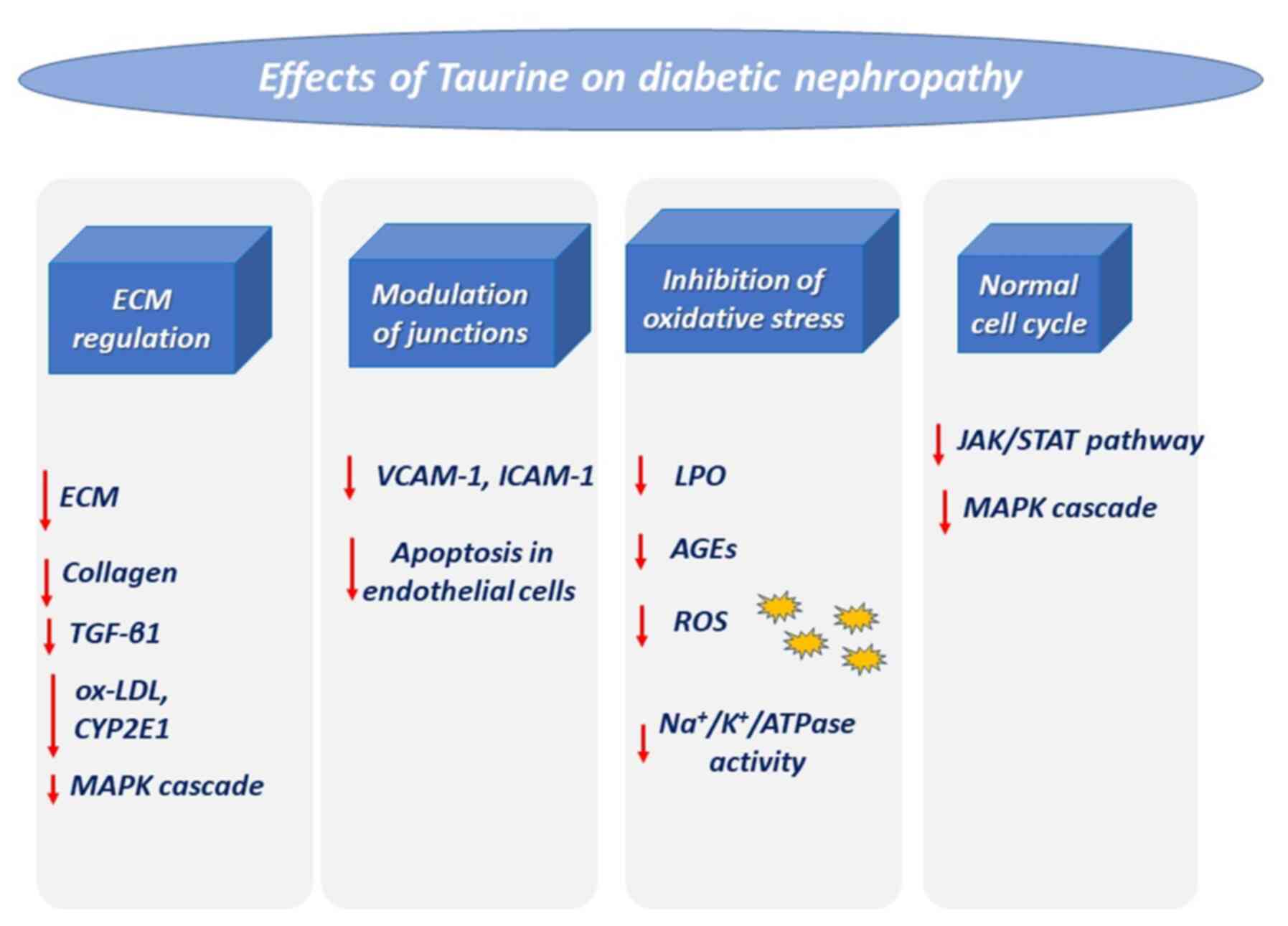 | Figure 3Effects of taurine on diabetic
nephropathy. Diabetes triggers impaired kidney function via the
mentioned pathways, which are downregulated via treatment with
taurine. ECM, extracellular matrix; TGF-β1, transforming growth
factor β1; ox-LDL, oxidized low-density lipoprotein; CYP2E1,
cytochrome P450 2E1; MAPK, mitogen-activated protein kinase;
VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intercellular
adhesion molecule-1; LPO, lipid peroxidation; AGEs, advanced
glycation end-products; ROS, reactive oxygen species. |
The major mechanism underlying the cytoprotective
role of taurine against diabetic nephropathy is the activation of
antioxidant enzymes. Of note, the protein expression levels of HO-1
appear to be elevated in the renal glomeruli of diabetic rats
(194). The administration of
taurine alone or in combination with other anti-oxidants is able to
relieve common symptoms of diabetes, mainly by normalizing HO-1
expression (194). In this manner,
taurine can salvage renal glomerular cells from pathological
changes and inhibit renal disturbances such as proteinuria and
hypertension (194). Furthermore,
the nephroprotective property of taurine can be attributed to its
ability to decrease renal nicotinamide adenine dinucleotide
phosphate (NADPH) oxidase activity (22) and to counteract the upregulation of
plasminogen activator inhibitor-1(195).
The beneficial effect of taurine on diabetic renal
symptoms may also occur via the modulation of various signal
transduction pathways. For example, in high glucose conditions,
hypertrophic renal tubular epithelial cells seem to revert to their
normal size following administration of taurine, which inhibits
JAK2, signal transducers and STAT1/STAT3), as well as
extracellular-regulated kinase (ERK)1/2 kinases (196). Taurine has been shown to inhibit
fibronectin and type IV collagen synthesis and expression of cyclin
D/cdk4 and to reduce p21 Waf1/Cip1 and p27 (Kip1) levels (196). In this manner, taurine has been
shown to slow down the hypertrophy of high-glucose-treated renal
cells, and to induce their proliferation through alterations in
signal transduction pathways and the ECM (196). Hence, taurine improves the protein
values for reactive AGEs in renal cells grown in the high-glucose
culture medium, due to its anti-fibrotic action, which is mediated
by the inactivation of Raf-1/ERK (197).
Furthermore, taurine has been shown to be a
significant renoprotective agent in rats with alloxan-induced
diabetes due to its potent antioxidant and anti-inflammatory
properties. In particular, it protects renal cells from apoptosis
and inflammation, by significantly reducing the expression levels
of pro-inflammatory cytokines [TNF-α, interleukin (IL)-6 and IL-1β]
and by decreasing NADPH levels (22).
Importantly, taurine mitigates diabetic
complications by interfering with leukocyte adhesion molecules
(33). In STZ-treated diabetic rats
characterized by renal injury through increased levels of BUN,
serum creatinine and renal MDA, taurine has been shown to reverse
all the histological changes through the downregulation of LOX-1
and ICAM-1(33). In rats with
STZ-induced diabetes, following 4 months of established diabetic
nephropathy, the administration of taurine (1%) appeared to improve
diabetic complications, as evidenced by diminished proteinuria.
This was accompanied by a marked decline in TGF-β1 expression in
the renal glomeruli of diabetic rats, improved mesangial ECM
expansion, and a further increase in protein urinary excretion
(198). Accordingly, LPO and TGF-β1
values also seem to decrease following the administration of
taurine in renal proximal tubule cells grown under high glucose
conditions (199). Additional
mechanisms accounting for the beneficial effects of taurine on
high-glucose-cultured renal cells include changes in the MAPK
signal transduction cascade and STAT3 transcription factor
(196). Such data provide
sufficient evidence to support the immense potential of taurine to
be used as an effective therapeutic agent in patients with
established diabetic nephropathy. Indeed, it has already been
demonstrated to protect renal tubular epithelial cells of
STZ-treated diabetic rats or renal tubular cells from hypertrophy
and fibrosis (200). It has also
been proposed that renal tubular cell hypertrophy may be caused by
the accumulation of AGEs via reduced NO/cyclic guanosine
monophosphate/cGMP-dependent protein kinase (NO/cGMP/PKG)
signaling. Even in this case, taurine also seems to reverse
hypotrophy, mediating the activation of NO/cGMP/PKG signaling
(200). Nephropathy is improved via
CYP2E1 expression and activity in diabetic rat kidneys, as CYP2E1
activity is capable of creating an oxidative environment and
inducing the metabolism of various endogenous and exogenous
compounds, ultimately resulting in formation of AGE products and
ROS accumulation (201).
Apart from the protective action of taurine alone,
it has been shown that a metformin and taurine combination scheme
enhances insulin sensitivity, causing a marked decline in the high
glucose levels observed in diabetic patients (80). The underlying mechanisms of action
probably involve the activation of ECM degradation and the
attenuation of vascular oxidative stress. The combination scheme
has proven to be efficient in inhibiting the renin-angiotensin
system that is mediated by hyperglycemia and in releasing smaller
amounts of TGF-β1, which is ultimately involved the development of
interstitial fibrosis and mesangial and tubular hypertrophy
(202). These findings are in
agreement with the findings of experiments where taurine has been
used alone and not in combination; i.e., effective in reducing the
TGF-β1 mRNA expression in rats with experimental non-alcoholic
steatohepatitis (203) and in rats
with carbon tetrachloride (CCL4)-induced hepatic
fibrosis (204). This protection
was also attained with the metformin/taurine combination treatment
scheme, through a reduction in MDA formation and an improvement of
the GSH/GSSG ratio in the plasma and liver of diabetic rats
(202). Indeed, it has been
documented that taurine administration reduces gluconeogenesis and
contributes to normal homeostasis, through the normalization of GSH
levels and a reduction in hydroxyl free radicals (202).
Despite reported advancements being made in the
field of therapeutics for diabetes, novel approaches are required
for a more accurate diagnosis and the prevention of renal injury.
Understanding more precisely the underlying molecular mechanisms of
the pathogenesis of diabetic nephropathy is a prerequisite for
devising preventive strategies and in enhancing the therapeutic
efficacy of existing drugs. Some progress has been made in animal
modeling, strain analyses, genotype associations and pathologic
processes (205); however, the
ideal model of diabetes has yet not been created.
10. Effects of taurine on renal
transplantation
Renal allografts currently constitute the main
option for the restoration of normal renal function. A previous
study including 11 children who had received kidney transplants,
demonstrated that plasma taurine and leucine concentrations were
lower in those children at 97±14 days post-transplantation surgery,
as compared to 10 age-matched controls. Several muscle amino acids,
including taurine, were increased post-transplantation. The authors
postulated that classical used glucocorticoid (prednisone) may have
affected the plasma taurine status (206). In this manner, donor
preconditioning with taurine has been shown to rescue kidney grafts
from the harmful consequences associated with transplantation
procedures (206).
A significant challenge in transplantation is the
composition of the perfusion solution that is injected in cadaveric
kidneys prior to the allograft procedure. Initially, the addition
of taurine to the perfusion solution seems to significantly improve
hypoxic changes, to restore reoxygenation and to allow the recovery
of energy metabolism in LLC-PK1 cells (207). Those properties were also
reinforced by the regulatory effects of taurine on calcium levels,
thereby suggesting that taurine can enhance the cellular growth of
transplants (207). Nonetheless,
ischemia/reperfusion constitutes a significant issue during renal
transplantation, as blood vessel clamping seems to activate
antioxidant injury to the renal vasculature, including the
endothelium. For example, in a previous study, in rats subjected to
ischemia for 60 min, followed by 90 min of reperfusion, energy
biosynthesis seems to slow down, as evidenced by increased
creatinine and decreased ATP values; in this case, the taurine
administration of 40 mg/kg was reported to decrease only
creatinine, and not ATP levels (208). In support of the beneficial effects
of taurine on ischemia/reperfusion, another study demonstrated that
taurine improved endothelial damage, as indicated by the increased
formation of ROS, the disrupted calcium regulation and reduced
endothelial NOS activity. Accordingly, taurine has been
demonstrated to improve existing histopathological renal injury
abnormalities, by increasing the BUN and creatinine content in
Wistar rats (209). In these
animals, treatment with taurine was shown to protect kidney
transplants from apoptotic cell death and triggers anti-oxidant
responses. Following transplantation, the taurine-treated tissue
was characterized by decreased caspase-3 levels as compared to the
controls, while SOD and heat shock protein levels were increased.
The serum levels of creatinine, aspartate aminotransferase (AST)
and lactate dehydrogenase (LDH) appeared to be markedly reduced in
a dose-dependent manner following the treatment of kidney
transplants with taurine (210).
Moreover, the use of the calcineurin inhibitors cyclosporine (CsA)
and tacrolimus (FK506) seems to be essential in eliminating the
danger of organ rejection. Despite their reported necessity, the
use of calcineurin inhibitors is hindered by ROS accumulation,
which may ultimately lead to the induction of hypertension and
nephrotoxicity (148). This
damaging action may be prevented with taurine, as in vivo
experiments in rats have demonstrated the restoration of
antioxidant responses (GSH, GPx and SOD), as well as normal serum
creatinine and proteinuria levels following taurine
supplementation, thereby contributing to normal renal transplant
functioning. In this case, the underlying mechanism in
counteracting CsA-mediated cytotoxicity was postulated to be the
ROS-scavenging activity of taurine.
11. Conclusions
In diabetes, the beneficial effects of taurine have
been reported in several animal models and multiple mechanisms seem
to be involved. In type 1 diabetes, taurine exerts its beneficial
effects through its antioxidant, anti-inflammatory effects and its
effect on increasing glutathione levels, augmenting insulin
secretion, activating inhibitory neurotrasmitters and interfering
hypothalamic-pituitary-gonadal axis.
In type 2 diabetes, taurine can act either as a
hypoglycemic or an anti-obesity or a hypo-lipidemic agent. The
hypoglycemic effect of taurine is manifested through its
antioxidant, anti-inflammatory and insulin-sensitizing properties.
The anti-obesity effect is manifested via increasing the oxygen
consumption rate, whereas the hypo-lipidemic effect relies on
promoting cholesterol degradation or increasing LDL uptake from
blood and on lowering cholesterol release from the liver or
reducing bile acid absorption from the intestine. Even though the
beneficial effects of taurine on glucose and lipid metabolism have
been reported, there is an inconsistency in the results of various
studies, mainly due to the concentration of taurine used in each
experimental set-up. Some other differences are focused on the
different animal strains, variations in the supplementation, the
duration of treatment, different handling and living conditions of
animal models, the different types of dietary fatty acids and other
random events during experimental procedures. Epidemiological
studies have indicated the potential therapeutic usefulness of
taurine in ameliorating diabetic 1 and 2 complications in humans.
Nevertheless, the clinical trials conducted to date are limited,
and there is an insufficient amount of data on the correct dosage
of administration, the duration of trials, sample size, the
severity of the disease, the presence of metabolic syndrome-related
diseases, the basal level of taurine intake from food, lifestyle
factors and genetic background. Additional studies need to be
designed with optimized clinical trial conditions, in order to
overcome the aforementioned hurdles. This will provide important
information as to whether taurine can be recommended unequivocally
as a nutraceutical for the prevention of diabetes.
Taurine exerts a cytoprotective and multifaceted
effect on the homeostasis of renal cells due to its antioxidant and
its osmoregulatory properties. Indeed, the protective nature of
taurine is proved to be invaluable in animal models of renal
disorders, mainly through its antioxidant and osmoregulatory
properties. For example, the protective effect of taurine against
hypertension, which is commonly observed in renal disorders, is
substantiated through its inhibitory action on the RAAS and in the
sympathetic nerve system, as well as by its antioxidant, its
anti-inflammatory and its ameliorative effect on endothelial
dysfunction. Furthermore, the beneficial role of taurine against
diabetic nephropathy is advocated through its interference with the
MAPK pathways, and its antioxidant and its anti-inflammatory
nature. The present review article highlights the importance of
taurine in circumventing side effects in patients with kidney
transplantation. Even though taurine has been employed in multiple
nephrotoxic animal models, its therapeutic use is limited, enabling
for further research regarding the molecular mechanisms underlying
the ameliorative nature against signs of renal injury.
Acknowledgements
Not applicable.
Availability of data and materials
Not applicable.
Authors' contributions
All authors (SB, MA, PI, AP, MIP, IC, DAS, AMK and
VZ) were involved in the conception and design of the study. SB
performed the literature search, wrote the manuscript. SB and PI
critically analyzed the existing knowledge and edited the
manuscript. SB, IC and AMK designed the figures. SB, MA, PI, AP,
MIP, DAS, AMK and VZ contributed to editing the manuscript. SB and
PI confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor in Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
American Diabetes Association. Diagnosis
and classification of diabetes mellitus. Diabetes Care. 37 (Suppl
1):S81–S90. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Atkinson MA, Eisenbarth GS and Michels AW:
Type 1 diabetes. Lancet. 383:69–82. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Henning RJ: Type-2 diabetes mellitus and
cardiovascular disease. Future Cardiol. 14:491–509. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chaudhury A, Duvoor C, Reddy Dendi VS,
Kraleti S, Chada A, Ravilla R, Marco A, Shekhawat NS, Montales MT,
Kuriakose K, et al: Clinical review of antidiabetic drugs:
implications for type 2 diabetes mellitus management. Front
Endocrinol (Lausanne). 8(6)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lamb RE and Goldstein BJ: Modulating an
oxidative-inflammatory cascade: Potential new treatment strategy
for improving glucose metabolism, insulin resistance, and vascular
function. Int J Clin Pract. 62:1087–1095. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Victor VM, Rocha M, Herance R and
Hernandez-Mijares A: Oxidative stress and mitochondrial dysfunction
in type 2 diabetes. Curr Pharm Des. 17:3947–3958. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625.
2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Asmat U, Abad K and Ismail K: Diabetes
mellitus and oxidative stress-A concise review. Saudi Pharm J.
24:547–553. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mastrocola R, Restivo F, Vercellinatto I,
Danni O, Brignardello E, Aragno M and Boccuzzi G: Oxidative and
nitrosative stress in brain mitochondria of diabetic rats. J
Endocrinol. 187:37–44. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Robertson RP: Chronic oxidative stress as
a central mechanism for glucose toxicity in pancreatic islet beta
cells in diabetes. J Biol Chem. 279:42351–42354. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ito T, Yoshikawa N, Ito H and Schaffer SW:
Impact of taurine depletion on glucose control and insulin
secretion in mice. J Pharmacol Sci. 129:59–64. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dokshina GA, Silaeva TIu and Lartsev EI:
Insulin-like effects of taurine. Vopr Med Khim. 22:503–507.
1976.PubMed/NCBI(In Russian).
|
|
13
|
Elizarova EP and Nedosugova LV: First
Experiments in Taurine Administration for Diabetes Mellitus. In:
Taurine 2. Huxtable RJ, Azuma J, Kuriyama K, Nakagawa M and Baba A
(eds). Springer, Boston, MA, pp583-588, 1996.
|
|
14
|
Damasceno DC, Netto AO, Iessi IL, Gallego
FQ, Corvino SB, Dallaqua B, Sinzato YK, Bueno A, Calderon IM and
Rudge MV: Streptozotocin-induced diabetes models:
Pathophysiological mechanisms and fetal outcomes. BioMed Res Int.
2014(819065)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lenzen S: The mechanisms of alloxan- and
streptozotocin-induced diabetes. Diabetologia. 51:216–226.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Szkudelski T: The mechanism of alloxan and
streptozotocin action in B cells of the rat pancreas. Physiol Res.
50:537–546. 2001.PubMed/NCBI
|
|
17
|
Sandler S and Swenne I: Streptozotocin,
but not alloxan, induces DNA repair synthesis in mouse pancreatic
islets in vitro. Diabetologia. 25:444–447. 1983.PubMed/NCBI View Article : Google Scholar
|
|
18
|
El-Batch M, Hassan AM and Mahmoud HA:
Taurine is more effective than melatonin on cytochrome P450 2E1 and
some oxidative stress markers in streptozotocin-induced diabetic
rats. J Agric Food Chem. 59:4995–5000. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hansen SH: The role of taurine in diabetes
and the development of diabetic complications. Diabetes Metab Res
Rev. 17:330–346. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Das J, Vasan V and Sil PC: Taurine exerts
hypoglycemic effect in alloxan-induced diabetic rats, improves
insulin-mediated glucose transport signaling pathway in heart and
ameliorates cardiac oxidative stress and apoptosis. Toxicol Appl
Pharmacol. 258:296–308. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gavrovskaya LK, Ryzhova OV, Safonova AF,
Matveev AK and Sapronov NS: Protective effect of taurine on rats
with experimental insulin-dependent diabetes mellitus. Bull Exp
Biol Med. 146:226–228. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Winiarska K, Szymanski K, Gorniak P,
Dudziak M and Bryla J: Hypoglycaemic, antioxidative and
nephroprotective effects of taurine in alloxan diabetic rabbits.
Biochimie. 91:261–270. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Budhram R, Pandya KG and Lau-Cam CA:
Protection by Taurine and Thiotaurine Against Biochemical and
Cellular Alterations Induced by Diabetes in a Rat Model. In:
Taurine 8. El Idrissi A and L'Amoreaux WJ (eds). Springer, New
York, NY, pp321-343, 2013.
|
|
24
|
Pandya KG, Budhram R, Clark G and Lau-Cam
CA: Comparative Evaluation of Taurine and Thiotaurine as
Protectants Against Diabetes-Induced Nephropathy in a Rat Model.
In: Taurine 8. El Idrissi A and L'Amoreaux WJ (eds). Springer, New
York, NY, pp371-394, 2013.
|
|
25
|
Alvarado-Vásquez N, Zamudio P, Cerón E,
Vanda B, Zenteno E and Carvajal-Sandoval G: Effect of glycine in
streptozotocin-induced diabetic rats. Comp Biochem Physiol C
Toxicol Pharmacol. 134:521–527. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Goodman HO and Shihabi ZK: Supplemental
taurine in diabetic rats: Effects on plasma glucose and
triglycerides. Biochem Med Metab Biol. 43:1–9. 1990.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Di Leo MAS, Santini SA, Silveri NG,
Giardina B, Franconi F and Ghirlanda G: Long-term taurine
supplementation reduces mortality rate in streptozotocin-induced
diabetic rats. Amino Acids. 27:187–191. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Abebe W: Effects of taurine on the
reactivity of aortas from diabetic rats. Life Sci. 82:279–289.
2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ulrich-Merzenich G, Zeitler H, Vetter H
and Bhonde RR: Protective effects of taurine on endothelial cells
impaired by high glucose and oxidized low density lipoproteins. Eur
J Nutr. 46:431–438. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang CF, Yuan JR, Qin D, Gu JF, Zhao BJ,
Zhang L, Zhao D, Chen J, Hou XF, Yang N, et al: Protection of
tauroursodeoxycholic acid on high glucose-induced human retinal
microvascular endothelial cells dysfunction and
streptozotocin-induced diabetic retinopathy rats. J Ethnopharmacol.
185:162–170. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ogasawara M, Nakamura T, Koyama I, Nemoto
M and Yoshida T: Reactivity of taurine with aldehydes and its
physiological role. Chem Pharm Bull (Tokyo). 41:2172–2175.
1993.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Casey RG, Gang C, Joyce M and
Bouchier-Hayes DJ: Taurine attenuates acute hyperglycaemia-induced
endothelial cell apoptosis, leucocyte-endothelial cell interactions
and cardiac dysfunction. J Vasc Res. 44:31–39. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang LJ, Yu YH, Zhang LG, Wang Y, Niu N,
Li Q and Guo LM: Taurine rescues vascular endothelial dysfunction
in streptozocin-induced diabetic rats: Correlated with
downregulation of LOX-1 and ICAM-1 expression on aortas. Eur J
Pharmacol. 597:75–80. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mochizuki H, Takido J and Yokogoshi H:
Effect of dietary taurine on endogenous hypercholesterolemia in
rats fed on phenobarbital-containing diets. Biosci Biotechnol
Biochem. 63:1298–1300. 1999.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mohamed NA and Gawad HSA: Taurine dietary
supplementation attenuates brain, thyroid, testicular disturbances
and oxidative stress in streptozotocin-induced diabetes mellitus in
male rats. Beni Suef Univ J Basic Appl Sci. 6:247–252. 2017.
|
|
36
|
Lieber CS: Cytochrome P-4502E1: Its
physiological and pathological role. Physiol Rev. 77:517–544.
1997.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fukuda N, Yoshitama A, Sugita S, Fujita M
and Murakami S: Dietary taurine reduces hepatic secretion of
cholesteryl ester and enhances fatty acid oxidation in rats fed a
high-cholesterol diet. J Nutr Sci Vitaminol (Tokyo). 57:144–149.
2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rashid K, Das J and Sil PC: Taurine
ameliorate alloxan induced oxidative stress and intrinsic apoptotic
pathway in the hepatic tissue of diabetic rats. Food Chem Toxicol.
51:317–329. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Patel SN and Lau-Cam CA: The Effect of
Taurine and Its Immediate Homologs on Diabetes-Induced Oxidative
Stress in the Brain and Spinal Cord of Rats. In: Taurine 10. Lee
DH, Schaffer SW, Park E and Kim HW (eds). Springer, Dordrecht,
pp337-351, 2017.
|
|
40
|
Acharya M and Lau-Cam CA: Comparison of
the protective actions of N-acetylcysteine, hypotaurine and taurine
against acetaminophen-induced hepatotoxicity in the rat. J Biomed
Sci. 17 (Suppl 1)(S35)2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Furfaro AL, Nitti M, Marengo B,
Domenicotti C, Cottalasso D, Marinari UM, Pronzato MA and Traverso
N: Impaired synthesis contributes to diabetes-induced decrease in
liver glutathione. Int J Mol Med. 29:899–905. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Aydın AF, Çoban J, Doğan-Ekici I,
Betül-Kalaz E, Doğru-Abbasoğlu S and Uysal M: Carnosine and taurine
treatments diminished brain oxidative stress and apoptosis in
D-galactose aging model. Metab Brain Dis. 31:337–345.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Arany E, Strutt B, Romanus P, Remacle C,
Reusens B and Hill DJ: Taurine supplement in early life altered
islet morphology, decreased insulitis and delayed the onset of
diabetes in non-obese diabetic mice. Diabetologia. 47:1831–1837.
2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Pandya KG, Budhram R, Clark G and Lau-Cam
CA: Comparative evaluation of taurine and thiotaurine as
protectants against diabetes-induced nephropathy in a rat model.
Adv Exp Med Biol. 775:371–394. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Batista TM, Ribeiro RA, Amaral AG, de
Oliveira CAM, Boschero AC and Carneiro EM: Taurine supplementation
restores glucose and carbachol-induced insulin secretion in islets
from low-protein diet rats: Involvement of Ach-M3R, Synt 1 and
SNAP-25 proteins. J Nutr Biochem. 23:306–312. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Vettorazzi JF, Ribeiro RA, Santos-Silva
JC, Borck PC, Batista TM, Nardelli TR, Boschero AC and Carneiro EM:
Taurine supplementation increases K(ATP) channel protein content,
improving Ca2+ handling and insulin secretion in islets from
malnourished mice fed on a high-fat diet. Amino Acids.
46:2123–2136. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lin S, Wu G, Zhao D, Han J, Yang Q, Feng
Y, Liu M, Yang J and Hu J: Taurine increases insulin expression in
STZ-treated rat islet cells in vitro. Adv Exp Med Biol.
975:319–328. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Carneiro EM, Latorraca MQ, Araujo E,
Beltrá M, Oliveras MJ, Navarro M, Berná G, Bedoya FJ, Velloso LA
and Soria B: Taurine supplementation modulates glucose homeostasis
and islet function. J Nutr Biochem. 20:503–511. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Santos-Silva JC, Ribeiro RA, Vettorazzi
JF, Irles E, Rickli S, Borck PC, Porciuncula PM, Quesada I, Nadal
A, Boschero AC, et al: Taurine supplementation ameliorates glucose
homeostasis, prevents insulin and glucagon hypersecretion, and
controls β, α, and δ-cell masses in genetic obese mice. Amino
Acids. 47:1533–1548. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kataoka K: Multiple mechanisms and
functions of maf transcription factors in the regulation of
tissue-specific genes. J Biochem. 141:775–781. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kim JY, Chu K, Kim HJ, Seong HA, Park KC,
Sanyal S, Takeda J, Ha H, Shong M, Tsai MJ, et al: Orphan nuclear
receptor small heterodimer partner, a novel corepressor for a basic
helix-loop-helix transcription factor BETA2/neuroD. Mol Endocrinol.
18:776–790. 2004.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yildirim Z and Kilic N: Effects of Taurine
and Age on Cerebellum Antioxidant Status and Oxidative Stress. Int
J Gerontol. 5:166–170. 2011.
|
|
53
|
Raza H, John A and Howarth FC: Increased
oxidative stress and mitochondrial dysfunction in zucker diabetic
rat liver and brain. Cell Physiol Biochem. 35:1241–1251.
2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
El Idrissi A and L'Amoreaux WJ: Selective
resistance of taurine-fed mice to isoniazide-potentiated seizures:
In vivo functional test for the activity of glutamic acid
decarboxylase. Neuroscience. 156:693–699. 2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
L'Amoreaux WJ, Marsillo A and El Idrissi
A: Pharmacological characterization of GABAA receptors in
taurine-fed mice. J Biomed Sci. 17 (Suppl 1)(S14)2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Caletti G, Almeida FB, Agnes G, Nin MS,
Barros HMT and Gomez R: Antidepressant dose of taurine increases
mRNA expression of GABAA receptor α2 subunit and BDNF in the
hippocampus of diabetic rats. Behav Brain Res. 283:11–15.
2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Li F, Obrosova IG, Abatan O, Tian D,
Larkin D, Stuenkel EL and Stevens MJ: Taurine replacement
attenuates hyperalgesia and abnormal calcium signaling in sensory
neurons of STZ-D rats. Am J Physiol Endocrinol Metab. 288:E29–E36.
2005.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Li F, Abatan OI, Kim H, Burnett D, Larkin
D, Obrosova IG and Stevens MJ: Taurine reverses neurological and
neurovascular deficits in Zucker diabetic fatty rats. Neurobiol
Dis. 22:669–676. 2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Das J, Roy A and Sil PC: Mechanism of the
protective action of taurine in toxin and drug induced organ
pathophysiology and diabetic complications: A review. Food Funct.
3:1251–1264. 2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Chen WQ, Jin H, Nguyen M, Carr J, Lee YJ,
Hsu CC, Faiman MD, Schloss JV and Wu JY: Role of taurine in
regulation of intracellular calcium level and neuroprotective
function in cultured neurons. J Neurosci Res. 66:612–619.
2001.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Agca CA, Tuzcu M, Hayirli A and Sahin K:
Taurine ameliorates neuropathy via regulating NF-κB and Nrf2/HO-1
signaling cascades in diabetic rats. Food Chem Toxicol. 71:116–121.
2014.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Abd El-Twab SM, Mohamed HM and Mahmoud AM:
Taurine and pioglitazone attenuate diabetes-induced testicular
damage by abrogation of oxidative stress and up-regulation of the
pituitary-gonadal axis. Can J Physiol Pharmacol. 94:651–661.
2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Buettner R, Schölmerich J and Bollheimer
LC: High-fat diets: Modeling the metabolic disorders of human
obesity in rodents. Obesity (Silver Spring). 15:798–808.
2007.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Schrauwen P and Hesselink MKC: Oxidative
capacity, lipotoxicity, and mitochondrial damage in type 2
diabetes. Diabetes. 53:1412–1417. 2004.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Iossa S, Mollica MP, Lionetti L, Crescenzo
R, Botta M and Liverini G: Skeletal muscle oxidative capacity in
rats fed high-fat diet. Int J Obes. 26:65–72. 2002.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Rani AJ and Mythili SV: Study on total
antioxidant status in relation to oxidative stress in type 2
diabetes mellitus. J Clin Diagn Res. 8:108–110. 2014.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Franconi F, Bennardini F, Mattana A,
Miceli M, Ciuti M, Mian M, Gironi A, Anichini R and Seghieri G:
Plasma and platelet taurine are reduced in subjects with
insulin-dependent diabetes mellitus: Effects of taurine
supplementation. Am J Clin Nutr. 61:1115–1119. 1995.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Yamori Y, Liu L, Mori M, Sagara M,
Murakami S, Nara Y and Mizushima S: Taurine as the Nutritional
Factor for the Longevity of the Japanese Revealed by a World-Wide
Epidemiological Survey. Adv Exp Med Biol. 643:13–25.
2009.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Merheb M, Daher RT, Nasrallah M, Sabra R,
Ziyadeh FN and Barada K: Taurine intestinal absorption and renal
excretion test in diabetic patients: A pilot study. Diabetes Care.
30:2652–2654. 2007.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Kim KS, Oh DH, Kim JY, Lee BG, You JS,
Chang KJ, Chung HJ, Yoo MC, Yang HI, Kang JH, et al: Taurine
ameliorates hyperglycemia and dyslipidemia by reducing insulin
resistance and leptin level in Otsuka Long-Evans Tokushima fatty
(OLETF) rats with long-term diabetes. Exp Mol Med. 44:665–673.
2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Harada H, Tsujino T, Watari Y, Nonaka H,
Emoto N and Yokoyama M: Oral taurine supplementation prevents
fructose-induced hypertension in rats. Heart Vessels. 19:132–136.
2004.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Chauncey KB, Tenner TE Jr, Lombardini JB,
Jones BG, Brooks ML, Warner RD, Davis RL and Ragain RM: The effect
of taurine supplementation on patients with type 2 diabetes
mellitus. Adv Exp Med Biol. 526:91–96. 2003.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Xiao C, Giacca A and Lewis GF: Oral
taurine but not N-acetylcysteine ameliorates NEFA-induced
impairment in insulin sensitivity and beta cell function in obese
and overweight, non-diabetic men. Diabetologia. 51:139–146.
2008.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Nakaya Y, Minami A, Harada N, Sakamoto S,
Niwa Y and Ohnaka M: Taurine improves insulin sensitivity in the
Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous type
2 diabetes. Am J Clin Nutr. 71:54–58. 2000.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Parvez S, Tabassum H, Banerjee BD and
Raisuddin S: Taurine prevents tamoxifen-induced mitochondrial
oxidative damage in mice. Basic Clin Pharmacol Toxicol.
102:382–387. 2008.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Liu Y and Quinn MR: Chemokine production
by rat alveolar macrophages is inhibited by taurine chloramine.
Immunol Lett. 80:27–32. 2002.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Barua M, Liu Y and Quinn MR: Taurine
chloramine inhibits inducible nitric oxide synthase and TNF-alpha
gene expression in activated alveolar macrophages: decreased
NF-kappaB activation and IkappaB kinase activity. J Immunol.
167:2275–2281. 2001.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Camargo RL, Batista TM, Ribeiro RA,
Velloso LA, Boschero AC and Carneiro EM: Effects of Taurine
Supplementation Upon Food Intake and Central Insulin Signaling in
Malnourished Mice Fed on a High-Fat Diet. In: Taurine 8. El Idrissi
A and L'Amoreaux WJ (eds). Springer, New York, NY, pp93-103,
2013.
|
|
79
|
El Idrissi A and Trenkner E: Growth
factors and taurine protect against excitotoxicity by stabilizing
calcium homeostasis and energy metabolism. J Neurosci.
19:9459–9468. 1999.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Oprescu AI, Bikopoulos G, Naassan A,
Allister EM, Tang C, Park E, Uchino H, Lewis GF, Fantus IG,
Rozakis-Adcock M, et al: Free fatty acid-induced reduction in
glucose-stimulated insulin secretion: Evidence for a role of
oxidative stress in vitro and in vivo. Diabetes. 56:2927–2937.
2007.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Jong CJ, Azuma J and Schaffer S: Mechanism
underlying the antioxidant activity of taurine: Prevention of
mitochondrial oxidant production. Amino Acids. 42:2223–2232.
2012.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Suzuki T, Suzuki T, Wada T, Saigo K and
Watanabe K: Taurine as a constituent of mitochondrial tRNAs: New
insights into the functions of taurine and human mitochondrial
diseases. EMBO J. 21:6581–6589. 2002.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Kirino Y, Yasukawa T, Ohta S, Akira S,
Ishihara K, Watanabe K and Suzuki T: Codon-specific translational
defect caused by a wobble modification deficiency in mutant tRNA
from a human mitochondrial disease. Proc Natl Acad Sci USA.
101:15070–15075. 2004.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Krauss S, Zhang CY, Scorrano L, Dalgaard
LT, St-Pierre J, Grey ST and Lowell BB: Superoxide-mediated
activation of uncoupling protein 2 causes pancreatic β cell
dysfunction. J Clin Invest. 112:1831–1842. 2003.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Kondo Y, Toda Y, Kitajima H, Oda H, Nagate
T, Kameo K and Murakami S: Taurine inhibits development of
atherosclerotic lesions in apolipoprotein E-deficient mice. Clin
Exp Pharmacol Physiol. 28:809–815. 2001.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Murakami S, Kondo Y, Sakurai T, Kitajima H
and Nagate T: Taurine suppresses development of atherosclerosis in
Watanabe heritable hyperlipidemic (WHHL) rabbits. Atherosclerosis.
163:79–87. 2002.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Cappelli AP, Zoppi CC, Barbosa-Sampaio HC,
Costa JM Jr, Protzek AO, Morato PN, Boschero AC and Carneiro EM:
Taurine-induced insulin signalling improvement of obese
malnourished mice is associated with redox balance and protein
phosphatases activity modulation. Liver Int. 34:771–783.
2014.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Haber CA, Lam TKT, Yu Z, Gupta N, Goh T,
Bogdanovic E, Giacca A and Fantus IG: N-acetylcysteine and taurine
prevent hyperglycemia-induced insulin resistance in vivo: Possible
role of oxidative stress. Am J Physiol Endocrinol Metab.
285:E744–E753. 2003.PubMed/NCBI View Article : Google Scholar
|
|
89
|
González-Chávez A, Elizondo-Argueta S,
Gutiérrez-Reyes G and León-Pedroza JI: Pathophysiological
implications between chronic inflammation and the development of
diabetes and obesity. Cir Cir. 79:209–216. 2011.PubMed/NCBI
|
|
90
|
You JS, Zhao X, Kim SH and Chang KJ:
Positive Correlation Between Serum Taurine and Adiponectin Levels
in High-Fat Diet-Induced Obesity Rats. In: Taurine 8. El Idrissi A
and L'Amoreaux WJ (eds). Springer, New York, NY, pp105-111,
2013.
|
|
91
|
Wu N, Lu Y, He B, Zhang Y, Lin J, Zhao S,
Zhang W, Li Y and Han P: Taurine prevents free fatty acid-induced
hepatic insulin resistance in association with inhibiting JNK1
activation and improving insulin signaling in vivo. Diabetes Res
Clin Pract. 90:288–296. 2010.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Rosa FT, Freitas EC, Deminice R, Jordão AA
and Marchini JS: Oxidative stress and inflammation in obesity after
taurine supplementation: A double-blind, placebo-controlled study.
Eur J Nutr. 53:823–830. 2014.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Lim JG, Lee HY, Yun JE, Kim SP, Park JW,
Suh SI, Jang BC, Cho CH, Bae JH, Kim SS, et al: Taurine block of
cloned ATP-sensitive K+ channels with different sulfonylurea
receptor subunits expressed in Xenopus laevis oocytes. Biochem
Pharmacol. 68:901–910. 2004.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Park EJ, Bae JH, Kim SY, Lim JG, Baek WK,
Kwon TK, Suh SI, Park JW, Lee IK, Ashcroft FM, et al: Inhibition of
ATP-sensitive K+ channels by taurine through a benzamido-binding
site on sulfonylurea receptor 1. Biochem Pharmacol. 67:1089–1096.
2004.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Koh JH, Lee ES, Hyun M, Kim HM, Choi YJ,
Lee EY, Yadav D and Chung CH: Taurine alleviates the progression of
diabetic nephropathy in type 2 diabetic rat model. Int J
Endocrinol. 2014(397307)2014.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Thadani JM, Marathe A, Vakodikar S,
Kshatriya P, Modi D and Vyas R: Treatment of Type I Diabetes using
Autologous Adipose Derived Mesenchymal Stem Cells Translated to
Insulin Secreting Islet like Cell Aggregates. J Case Rep.
7:235–238. 2017.
|
|
97
|
Fujihira E, Takahashi H and Nakazawa M:
Effect of long-term feeding of taurine in hereditary hyperglycemic
obese mice. Chem Pharm Bull (Tokyo). 18:1636–1642. 1970.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Tsuboyama-Kasaoka N, Shozawa C, Sano K,
Kamei Y, Kasaoka S, Hosokawa Y and Ezaki O: Taurine
(2-aminoethanesulfonic acid) deficiency creates a vicious circle
promoting obesity. Endocrinology. 147:3276–3284. 2006.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Piña-Zentella G, de la Rosa-Cuevas G,
Vázquez-Meza H, Piña E and de Piña MZ: Taurine in adipocytes
prevents insulin-mediated H2O2 generation and
activates Pka and lipolysis. Amino Acids. 42:1927–1935.
2012.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Nardelli TR, Ribeiro RA, Balbo SL, Vanzela
EC, Carneiro EM, Boschero AC and Bonfleur ML: Taurine prevents fat
deposition and ameliorates plasma lipid profile in monosodium
glutamate-obese rats. Amino Acids. 41:901–908. 2011.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Cao PJ, Jin YJ, Li ME, Zhou R and Yang MZ:
PGC-1α may associated with the anti-obesity effect of taurine on
rats induced by arcuate nucleus lesion. Nutr Neurosci. 19:86–93.
2016.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Batista TM, Ribeiro RA, da Silva PMR,
Camargo RL, Lollo PCB, Boschero AC and Carneiro EM: Taurine
supplementation improves liver glucose control in normal protein
and malnourished mice fed a high-fat diet. Mol Nutr Food Res.
57:423–434. 2013.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Kim KS, Choi HM, Ji HI, Kim C, Kim JY,
Song R, Kim SM, Lee YA, Lee SH, Yang HI, et al: Effect of taurine
chloramine on differentiation of human preadipocytes into
adipocytes. Adv Exp Med Biol. 775:247–257. 2013.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Kim KS, Ji HI, Chung H, Kim C, Lee SH, Lee
YA, Yang HI, Yoo MC and Hong SJ: Taurine chloramine modulates the
expression of adipokines through inhibition of the STAT-3 signaling
pathway in differentiated human adipocytes. Amino Acids.
45:1415–1422. 2013.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Kim KS, You JS, Kim JY, Chang KJ, Yoo MC,
Song R, Lee YA, Lee SH, Hong SJ and Yang HI: Taurine ameliorates
hypercholesterolemia but not obesity in rats fed a lard-based,
high-fat diet. Adv Exp Med Biol. 803:271–278. 2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Murakami S, Nara Y and Yamori Y: Taurine
accelerates the regression of hypercholesterolemia in stroke-prone
spontaneously hypertensive rats. Life Sci. 58:1643–1651.
1996.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Chen W, Matuda K, Nishimura N and
Yokogoshi H: The effect of taurine on cholesterol degradation in
mice fed a high-cholesterol diet. Life Sci. 74:1889–1898.
2004.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Choi MJ, Kim JH and Chang KJ: The effect
of dietary taurine supplementation on plasma and liver lipid
concentrations and free amino acid concentrations in rats fed a
high-cholesterol diet. Adv Exp Med Biol. 583:235–242.
2006.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Kishida T, Miyazato S, Ogawa H and Ebihara
K: Taurine prevents hypercholesterolemia in ovariectomized rats fed
corn oil but not in those fed coconut oil. J Nutr. 133:2616–2621.
2003.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Nishimura N, Yamamoto T and Ota T: Taurine
feeding inhibits bile acid absorption from the ileum in rats fed a
high cholesterol and high fat diet. Adv Exp Med Biol. 643:285–291.
2009.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Murakami S, Kondo Y, Toda Y, Kitajima H,
Kameo K, Sakono M and Fukuda N: Effect of taurine on cholesterol
metabolism in hamsters: Up-regulation of low density lipoprotein
(LDL) receptor by taurine. Life Sci. 70:2355–2366. 2002.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Yanagita T, Han SY, Hu Y, Nagao K,
Kitajima H and Murakami S: Taurine reduces the secretion of
apolipoprotein B100 and lipids in HepG2 cells. Lipids Health Dis.
7(38)2008.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Ahmadian M, Roshan VD, Aslani E and
Stannard SR: Taurine supplementation has anti-atherogenic and
anti-inflammatory effects before and after incremental exercise in
heart failure. Ther Adv Cardiovasc Dis. 11:185–194. 2017.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Nandhini AT and Anuradha CV: Taurine
modulates kallikrein activity and glucose metabolism in insulin
resistant rats. Amino Acids. 22:27–38. 2002.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Mikami N, Hosokawa M and Miyashita K:
Dietary combination of fish oil and taurine decreases fat
accumulation and ameliorates blood glucose levels in type 2
diabetic/obese KK-A(y) mice. J Food Sci. 77:H114–H120.
2012.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Harada N, Ninomiya C, Osako Y, Morishima
M, Mawatari K, Takahashi A and Nakaya Y: Taurine alters respiratory
gas exchange and nutrient metabolism in type 2 diabetic rats. Obes
Res. 12:1077–1084. 2004.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Solon CS, Franci D, Ignacio-Souza LM,
Romanatto T, Roman EA, Arruda AP, Morari J, Torsoni AS, Carneiro EM
and Velloso LA: Taurine enhances the anorexigenic effects of
insulin in the hypothalamus of rats. Amino Acids. 42:2403–2410.
2012.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Figueroa ALC, Figueiredo H, Rebuffat SA,
Vieira E and Gomis R: Taurine Treatment Modulates Circadian Rhythms
in Mice Fed A High Fat Diet. Sci Rep. 6(36801)2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Ribeiro RA, Santos-Silva JC, Vettorazzi
JF, Cotrim BB, Mobiolli DDM, Boschero AC and Carneiro EM: Taurine
supplementation prevents morpho-physiological alterations in
high-fat diet mice pancreatic β-cells. Amino Acids. 43:1791–1801.
2012.PubMed/NCBI View Article : Google Scholar
|
|
120
|
McCarthy FP, Delany AC, Kenny LC and Walsh
SK: PPAR-γ - a possible drug target for complicated pregnancies. Br
J Pharmacol. 168:1074–1085. 2013.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Fujita T and Sato Y: Changes in blood
pressure and extracellular fluid with taurine in DOCA-salt rats. Am
J Physiol. 250:R1014–R1020. 1986.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Branco RC, Batista TM, Camargo RL, Borck
PC, Ribeiro RA, Zoppi CC, Lollo PC, Morato PN, Boschero AC and
Carneiro EM: Long-term taurine supplementation leads to enhanced
hepatic steatosis, renal dysfunction and hyperglycemia in mice fed
on a high-fat diet. Adv Exp Med Biol. 803:339–351. 2015.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Ware LB, Johnson ACM and Zager RA: Renal
cortical albumin gene induction and urinary albumin excretion in
response to acute kidney injury. Am J Physiol Renal Physiol.
300:F628–F638. 2011.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Declèves AE, Zolkipli Z, Satriano J, Wang
L, Nakayama T, Rogac M, Le TP, Nortier JL, Farquhar MG, Naviaux RK,
et al: Regulation of lipid accumulation by AMP-activated kinase
[corrected] in high fat diet-induced kidney injury. Kidney Int.
85:611–623. 2014.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Wang L, Zhang L, Yu Y, Wang Y and Niu N:
The protective effects of taurine against early renal injury in
STZ-induced diabetic rats, correlated with inhibition of renal
LOX-1-mediated ICAM-1 expression. Ren Fail. 30:763–771.
2008.PubMed/NCBI View Article : Google Scholar
|
|
126
|
He X, Duan Y, Yao K, Li F, Hou Y, Wu G and
Yin Y: β-Hydroxy-β-methylbutyrate, mitochondrial biogenesis, and
skeletal muscle health. Amino Acids. 48:653–664. 2016.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Tran LT, Yuen VG and McNeill JH: The
fructose-fed rat: A review on the mechanisms of fructose-induced
insulin resistance and hypertension. Mol Cell Biochem. 332:145–159.
2009.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Bantle JP, Raatz SK, Thomas W and
Georgopoulos A: Effects of dietary fructose on plasma lipids in
healthy subjects. Am J Clin Nutr. 72:1128–1134. 2000.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Nandhini ATA, Thirunavukkarasu V and
Anuradha CV: Potential role of kinins in the effects of taurine in
high-fructose-fed rats. Can J Physiol Pharmacol. 82:1–8.
2004.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Rahman MM, Park HM, Kim SJ, Go HK, Kim GB,
Hong CU, Lee YU, Kim SZ, Kim JS and Kang HS: Taurine prevents
hypertension and increases exercise capacity in rats with
fructose-induced hypertension. Am J Hypertens. 24:574–581.
2011.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Larsen LH, Orstrup LKH, Hansen SH, Grunnet
N, Quistorff B and Mortensen OH: The effect of long-term taurine
supplementation and fructose feeding on glucose and lipid
homeostasis in Wistar rats. Adv Exp Med Biol. 776:39–50.
2013.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Pilkis SJ and Granner DK: Molecular
physiology of the regulation of hepatic gluconeogenesis and
glycolysis. Annu Rev Physiol. 54:885–909. 1992.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Lau-Cam CA and Patel JP: Comparison of the
effects of taurine with those of related sulfur-containing
compounds on pyridoxal-induced adrenomedullary catecholamine
release and glycogenolysis in the rat. Adv Exp Med Biol.
583:203–212. 2006.PubMed/NCBI View Article : Google Scholar
|
|
134
|
El Mesallamy HO, El-Demerdash E, Hammad LN
and El Magdoub HM: Effect of taurine supplementation on
hyperhomocysteinemia and markers of oxidative stress in high
fructose diet induced insulin resistance. Diabetol Metab Syndr.
2(46)2010.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Devasagayam TPA, Tilak JC, Boloor KK, Sane
KS, Ghaskadbi SS and Lele RD: Free radicals and antioxidants in
human health: Current status and future prospects. J Assoc
Physicians India. 52:794–804. 2004.PubMed/NCBI
|
|
136
|
Rask-Madsen C and King GL: Mechanisms of
Disease: Endothelial dysfunction in insulin resistance and
diabetes. Nat Clin Pract Endocrinol Metab. 3:46–56. 2007.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Moloney MA, Casey RG, O'Donnell DH,
Fitzgerald P, Thompson C and Bouchier-Hayes DJ: Two weeks taurine
supplementation reverses endothelial dysfunction in young male type
1 diabetics. Diab Vasc Dis Res. 7:300–310. 2010.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Ito T, Schaffer SW and Azuma J: The
potential usefulness of taurine on diabetes mellitus and its
complications. Amino Acids. 42:1529–1539. 2012.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Fennessy FM, Moneley DS, Wang JH, Kelly CJ
and Bouchier-Hayes DJ: Taurine and vitamin C modify monocyte and
endothelial dysfunction in young smokers. Circulation. 107:410–415.
2003.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Zhang M, Bi LF, Fang JH, Su XL, Da GL,
Kuwamori T and Kagamimori S: Beneficial effects of taurine on serum
lipids in overweight or obese non-diabetic subjects. Amino Acids.
26:267–271. 2004.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Mizushima S, Nara Y, Sawamura M and Yamori
Y: Effects of Oral Taurine Supplementation on Lipids and
Sympathetic Nerve Tone. In: Taurine 2. Huxtable RJ, Azuma J,
Kuriyama K, Nakagawa M and Baba A (eds). Springer, Boston, MA,
pp615-622, 1996.
|
|
142
|
Nakamura T, Ushiyama C, Suzuki S, Shimada
N, Ohmuro H, Ebihara I and Koide H: Effects of taurine and vitamin
E on microalbuminuria, plasma metalloproteinase-9, and serum type
IV collagen concentrations in patients with diabetic nephropathy.
Nephron J. 83:361–362. 1999.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Cusworth DC and Dent CE: Renal clearances
of amino acids in normal adults and in patients with aminoaciduria.
Biochem J. 74:550–561. 1960.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Mozaffari MS and Schaffer D: Taurine
modulates arginine vasopressin-mediated regulation of renal
function. J Cardiovasc Pharmacol. 37:742–750. 2001.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Burg MB: Molecular basis of osmotic
regulation. Am J Physiol. 268:F983–F996. 1995.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Gomez RA, Tufro-McReddie A, Everett AD and
Pentz ES: Ontogeny of renin and AT1 receptor in the rat. Pediatr
Nephrol. 7:635–638. 1993.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Han X and Chesney RW: The role of taurine
in renal disorders. Amino Acids. 43:2249–2263. 2012.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Hagar HH, El Etter E and Arafa M: Taurine
attenuates hypertension and renal dysfunction induced by
cyclosporine A in rats. Clin Exp Pharmacol Physiol. 33:189–196.
2006.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Ideishi M, Miura S, Sakai T, Sasaguri M,
Misumi Y and Arakawa K: Taurine amplifies renal kallikrein and
prevents salt-induced hypertension in Dahl rats. J Hypertens.
12:653–661. 1994.PubMed/NCBI
|
|
150
|
Dawson R Jr, Biasetti M, Messina S and
Dominy J: The cytoprotective role of taurine in exercise-induced
muscle injury. Amino Acids. 22:309–324. 2002.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Hu J, Xu X, Yang J, Wu G, Sun C and Lv Q:
Antihypertensive Effect of Taurine in Rat. In: Taurine 7. Azuma J,
Schaffer SW and Ito T (eds). Springer, New York, NY, pp75-84,
2009.
|
|
152
|
Mozaffari MS, Abdelsayed R, Patel C and
Schaffer SW: Effects of Dietary Salt and Fat on Taurine Excretion
in Healthy and Diseased Rats. In: Taurine 6. Oja SS and Saransaari
P (eds). Springer, Boston, MA, pp173-180, 2006.
|
|
153
|
Venkatesan N, Venkatesan P, Karthikeyan J
and Arumugam V: Protection by taurine against adriamycin-induced
proteinuria and hyperlipidemia in rats. Proc Soc Exp Biol Med.
215:158–164. 1997.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Chesney RW, Han X and Patters AB: Taurine
and the renal system. J Biomed Sci. 17 (Suppl 1)(S4)2010.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Koeners MP, Braam B, van der Giezen DM,
Goldschmeding R and Joles JA: Perinatal micronutrient supplements
ameliorate hypertension and proteinuria in adult fawn-hooded
hypertensive rats. Am J Hypertens. 23:802–808. 2010.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Hsueh WA and Wyne K:
Renin-Angiotensin-aldosterone system in diabetes and hypertension.
J Clin Hypertens (Greenwich). 13:224–237. 2011.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Takahashi K, Azuma M, Taira K, Baba A,
Yamamoto I, Schaffer SW and Azuma J: Effect of taurine on
angiotensin II-induced hypertrophy of neonatal rat cardiac cells. J
Cardiovasc Pharmacol. 30:725–730. 1997.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Azuma M, Takahashi K, Fukuda T, Ohyabu Y,
Yamamoto I, Kim S, Iwao H, Schaffer SW and Azuma J: Taurine
attenuates hypertrophy induced by angiotensin II in cultured
neonatal rat cardiac myocytes. Eur J Pharmacol. 403:181–188.
2000.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Nara Y, Yamori Y and Lovenberg W: Effect
of dietary taurine on blood pressure in spontaneously hypertensive
rats. Biochem Pharmacol. 27:2689–2692. 1978.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Yamamoto J, Akabane S, Yoshimi H, Nakai M
and Ikeda M: Effects of taurine on stress-evoked hemodynamic and
plasma catecholamine changes in spontaneously hypertensive rats.
Hypertension. 7:913–922. 1985.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Sato Y, Ogata E and Fujita T: Hypotensive
action of taurine in DOCA-salt rats--involvement of sympathoadrenal
inhibition and endogenous opiate. Jpn Circ J. 55:500–508.
1991.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Patrick L: Lead toxicity part II: The role
of free radical damage and the use of antioxidants in the pathology
and treatment of lead toxicity. Altern Med Rev. 11:114–127.
2006.PubMed/NCBI
|
|
163
|
Roysommuti S, Suwanich A, Lerdweeraphon W,
Thaeomor A, Jirakulsomchok D and Wyss JM: Sex dependent effects of
perinatal taurine exposure on the arterial pressure control in
adult offspring. Adv Exp Med Biol. 643:135–144. 2009.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Malpas SC, Ramchandra R, Guild SJ, McBryde
F and Barrett CJ: Renal sympathetic nerve activity in the
development of hypertension. Curr Hypertens Rep. 8:242–248.
2006.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Hano T, Kasano M, Tomari H and Iwane N:
Taurine Suppresses Pressor Response Through the Inhibition of
Sympathetic Nerve Activity and the Improvement in Baro-Reflex
Sensitivity of Spontaneously Hypertensive Rats. In: Taurine 7.
Azuma J, SchafferSW and Ito T (eds). Springer, New York, NY,
pp57-63, 2009.
|
|
166
|
Iimura O and Shimamoto K: Salt and
Hypertension: Water-Sodium Handling in Essential Hypertension. Ann
N Y Acad Sci. 676:105–121. 1993.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Sun Q, Wang B, Li Y, Sun F, Li P, Xia W,
Zhou X, Li Q, Wang X, Chen J, et al: Taurine Supplementation Lowers
Blood Pressure and Improves Vascular Function in Prehypertension:
Randomized, Double-Blind, Placebo-Controlled Study. Hypertension.
67:541–549. 2016.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Katakawa M, Fukuda N, Tsunemi A, Mori M,
Maruyama T, Matsumoto T, Abe M and Yamori Y: Taurine and magnesium
supplementation enhances the function of endothelial progenitor
cells through antioxidation in healthy men and spontaneously
hypertensive rats. Hypertens Res. 39:848–856. 2016.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Abebe W and Mozaffari MS: Effects of
chronic taurine treatment on reactivity of the rat aorta. Amino
Acids. 19:615–623. 2000.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Dawson R Jr, Liu S, Jung B, Messina S and
Eppler B: Effects of high salt diets and taurine on the development
of hypertension in the stroke-prone spontaneously hypertensive rat.
Amino Acids. 19:643–665. 2000.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Maia AR, Batista TM, Victorio JA, Clerici
SP, Delbin MA, Carneiro EM and Davel AP: Taurine supplementation
reduces blood pressure and prevents endothelial dysfunction and
oxidative stress in post-weaning protein-restricted rats. PLoS One.
9(e105851)2014.PubMed/NCBI View Article : Google Scholar
|
|
172
|
Ogawa M, Takahara A, Ishijima M and Tazaki
S: Decrease of plasma sulfur amino acids in essential hypertension.
Jpn Circ J. 49:1217–1224. 1985.PubMed/NCBI View Article : Google Scholar
|
|
173
|
Yamori Y, Taguchi T, Hamada A, Kunimasa K,
Mori H and Mori M: Taurine in health and diseases: Consistent
evidence from experimental and epidemiological studies. J Biomed
Sci. 17 (Suppl 1)(S6)2010.PubMed/NCBI View Article : Google Scholar
|
|
174
|
Yamori Y, Nara Y, Ikeda K and Mizushima S:
Is Taurine a Preventive Nutritional Factor of Cardiovascular
Diseases or Just a Biological Marker of Nutrition. In: Taurine 2.
Huxtable RJ, Azuma J, Kuriyama K, Nakagawa M and Baba A (eds).
Springer, Boston, MA, pp623-629, 1996.
|
|
175
|
Parikh CR, Coca SG, Thiessen-Philbrook H,
Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD,
Zappitelli M, et al: TRIBE-AKI Consortium: Postoperative biomarkers
predict acute kidney injury and poor outcomes after adult cardiac
surgery. J Am Soc Nephrol. 22:1748–1757. 2011.PubMed/NCBI View Article : Google Scholar
|
|
176
|
Flora SJS, Pande M, Bhadauria S and Kannan
GM: Combined administration of taurine and meso
2,3-dimercaptosuccinic acid in the treatment of chronic lead
intoxication in rats. Hum Exp Toxicol. 23:157–166. 2004.PubMed/NCBI View Article : Google Scholar
|
|
177
|
Bai J, Yao X, Jiang L, Zhang Q, Guan H,
Liu S, Wu W, Qiu T, Gao N, Yang L, et al: Taurine protects against
As2O3-induced autophagy in livers of rat offsprings through PPARγ
pathway. Sci Rep. 6(27733)2016.PubMed/NCBI View Article : Google Scholar
|
|
178
|
Yeh YH, Lee YT, Hsieh YL and Hwang DF:
Dietary taurine reduces zinc-induced toxicity in male Wistar rats.
J Food Sci. 76:T90–T98. 2011.PubMed/NCBI View Article : Google Scholar
|
|
179
|
Roy A, Manna P and Sil PC: Prophylactic
role of taurine on arsenic mediated oxidative renal dysfunction via
MAPKs/ NF-kappaB and mitochondria dependent pathways. Free Radic
Res. 43:995–1007. 2009.PubMed/NCBI View Article : Google Scholar
|
|
180
|
Bera AK, Rana T, Das S, Bhattacharya D,
Pan D, Bandyopadhyay S and Das SK: Mitigation of arsenic-mediated
renal oxidative stress in rat by Pleurotus florida lectin.
Hum Exp Toxicol. 30:940–951. 2011.PubMed/NCBI View Article : Google Scholar
|
|
181
|
Zheng Y, Qu H, Wang D, Li S, Zhang C and
Piao F: Protection of Taurine Against Arsenic-Induced DNA Damage of
Mice Kidneys. Adv Exp Med Biol. 975:917–927. 2017.PubMed/NCBI View Article : Google Scholar
|
|
182
|
Ahmad MK, Khan AA, Ali SN and Mahmood R:
Chemoprotective effect of taurine on potassium bromate-induced DNA
damage, DNA-protein cross-linking and oxidative stress in rat
intestine. PLoS One. 10(e0119137)2015.PubMed/NCBI View Article : Google Scholar
|
|
183
|
Das J, Ghosh J, Manna P and Sil PC:
Taurine protects acetaminophen-induced oxidative damage in mice
kidney through APAP urinary excretion and CYP2E1 inactivation.
Toxicology. 269:24–34. 2010.PubMed/NCBI View Article : Google Scholar
|
|
184
|
Eldin AAK, Shaheen AA, Abd Elgawad HM and
Shehata NI: Protective effect of taurine and quercetin against
renal dysfunction associated with the combined use of gentamycin
and diclofenac. Indian J Biochem Biophys. 45:332–340.
2008.PubMed/NCBI
|
|
185
|
Ma N, Kato T, Isogai T, Gu Y and Yamashita
T: The Potential Effects of Taurine in Mitigation of Radiation
Nephropathy. In: Taurine 11. Hu J, Piao F, Schaffer SW, El Idrissi
A and Wu JY (eds). Springer Singapore, Singapore, pp497-505,
2019.
|
|
186
|
Ogino T, Than TA, Hosako M, Ozaki M, Omori
M and Okada S: Taurine chloramine: A possible oxidant reservoir.
Adv Exp Med Biol. 643:451–461. 2009.PubMed/NCBI View Article : Google Scholar
|
|
187
|
Kim C and Cha YN: Production of reactive
oxygen and nitrogen species in phagocytes is regulated by taurine
chloramine. Adv Exp Med Biol. 643:463–472. 2009.PubMed/NCBI View Article : Google Scholar
|
|
188
|
Trachtman H, Futterweit S, Prenner J and
Hanon S: Antioxidants reverse the antiproliferative effect of high
glucose and advanced glycosylation end products in cultured rat
mesangial cells. Biochem Biophys Res Commun. 199:346–352.
1994.PubMed/NCBI View Article : Google Scholar
|
|
189
|
Stanton RC: Oxidative stress and diabetic
kidney disease. Curr Diab Rep. 11:330–336. 2011.PubMed/NCBI View Article : Google Scholar
|
|
190
|
Dronavalli S, Duka I and Bakris GL: The
pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol
Metab. 4:444–452. 2008.PubMed/NCBI View Article : Google Scholar
|
|
191
|
Goh SY and Cooper ME: Clinical review: The
role of advanced glycation end products in progression and
complications of diabetes. J Clin Endocrinol Metab. 93:1143–1152.
2008.PubMed/NCBI View Article : Google Scholar
|
|
192
|
Riser BL, Ladson-Wofford S, Sharba A,
Cortes P, Drake K, Guerin CJ, Yee J, Choi ME, Segarini PR and
Narins RG: TGF-β receptor expression and binding in rat mesangial
cells: Modulation by glucose and cyclic mechanical strain. Kidney
Int. 56:428–439. 1999.PubMed/NCBI View Article : Google Scholar
|
|
193
|
Kitada M, Ogura Y and Koya D: Rodent
models of diabetic nephropathy: Their utility and limitations. Int
J Nephrol Renovasc Dis. 9:279–290. 2016.PubMed/NCBI View Article : Google Scholar
|
|
194
|
Koya D, Hayashi K, Kitada M, Kashiwagi A,
Kikkawa R and Haneda M: Effects of antioxidants in diabetes-induced
oxidative stress in the glomeruli of diabetic rats. J Am Soc
Nephrol. 14 (Suppl 3):S250–S253. 2003.PubMed/NCBI View Article : Google Scholar
|
|
195
|
Lee EA, Seo JY, Jiang Z, Yu MR, Kwon MK,
Ha H and Lee HB: Reactive oxygen species mediate high
glucose-induced plasminogen activator inhibitor-1 up-regulation in
mesangial cells and in diabetic kidney. Kidney Int. 67:1762–1771.
2005.PubMed/NCBI View Article : Google Scholar
|
|
196
|
Huang JS, Chuang LY, Guh JY, Huang YJ and
Hsu MS: Antioxidants attenuate high glucose-induced hypertrophic
growth in renal tubular epithelial cells. Am J Physiol Renal
Physiol. 293:F1072–F1082. 2007.PubMed/NCBI View Article : Google Scholar
|
|
197
|
Huang JS, Chuang LY, Guh JY, Yang YL and
Hsu MS: Effect of taurine on advanced glycation end
products-induced hypertrophy in renal tubular epithelial cells.
Toxicol Appl Pharmacol. 233:220–226. 2008.PubMed/NCBI View Article : Google Scholar
|
|
198
|
Higo S, Miyata S, Jiang QY, Kitazawa R,
Kitazawa S and Kasuga M: Taurine administration after appearance of
proteinuria retards progression of diabetic nephropathy in rats.
Kobe J Med Sci. 54:E35–E45. 2008.PubMed/NCBI
|
|
199
|
Park SH, Choi HJ, Lee JH, Woo CH, Kim JH
and Han HJ: High glucose inhibits renal proximal tubule cell
proliferation and involves PKC, oxidative stress, and TGF-beta 1.
Kidney Int. 59:1695–1705. 2001.PubMed/NCBI View Article : Google Scholar
|
|
200
|
Huang JS, Chuang LY, Guh JY and Huang YJ:
Effects of nitric oxide and antioxidants on advanced glycation end
products-induced hypertrophic growth in human renal tubular cells.
Toxicol Sci. 111:109–119. 2009.PubMed/NCBI View Article : Google Scholar
|
|
201
|
Yao HT, Lin P, Chang YW, Chen CT, Chiang
MT, Chang L, Kuo YC, Tsai HT and Yeh TK: Effect of taurine
supplementation on cytochrome P450 2E1 and oxidative stress in the
liver and kidneys of rats with streptozotocin-induced diabetes.
Food Chem Toxicol. 47:1703–1709. 2009.PubMed/NCBI View Article : Google Scholar
|
|
202
|
Pandya KG, Budhram R, Clark GJ and Lau-Cam
CA: Taurine can enhance the protective actions of metformin against
diabetes-induced alterations adversely affecting renal function.
Adv Exp Med Biol. 803:227–250. 2015.PubMed/NCBI View Article : Google Scholar
|
|
203
|
Chen SW, Chen YX, Shi J, Lin Y and Xie WF:
The restorative effect of taurine on experimental nonalcoholic
steatohepatitis. Dig Dis Sci. 51:2225–2234. 2006.PubMed/NCBI View Article : Google Scholar
|
|
204
|
Miyazaki T, Karube M, Matsuzaki Y, Ikegami
T, Doy M, Tanaka N and Bouscarel B: Taurine inhibits oxidative
damage and prevents fibrosis in carbon tetrachloride-induced
hepatic fibrosis. J Hepatol. 43:117–125. 2005.PubMed/NCBI View Article : Google Scholar
|
|
205
|
Schlöndorff D: Choosing the right mouse
model for diabetic nephropathy. Kidney Int. 77:749–750.
2010.PubMed/NCBI View Article : Google Scholar
|
|
206
|
Perfumo F, Canepa A, Divino Filho JC,
Nilsson E, Carrea A, Verrina E, Gusmano R and Bergström J: Muscle
and plasma amino acids and nutritional status in
kidney-transplanted children. Nephrol Dial Transplant. 9:1778–1785.
1994.PubMed/NCBI
|
|
207
|
Wingenfeld P, Minor T, Gehrmann U,
Strübind S, Isselhard W and Michalk D: Hypoxic cellular
deterioration and its prevention by the amino acid taurine in a
transplantation model with renal tubular cells (LLC-PK1). In Vitro
Cell Dev Biol Anim. 31:483–486. 1995.PubMed/NCBI View Article : Google Scholar
|
|
208
|
Michalk DV, Hoffmann B and Minor Th:
Taurine Reduces Renal Ischemia/Reperfusion Injury in the Rat. In:
Taurine 5. Lombardini JB, Schaffer SW and Azuma J (eds). Springer,
Boston, MA, pp49-56, 2003.
|
|
209
|
Guz G, Oz E, Lortlar N, Ulusu NN, Nurlu N,
Demirogullari B, Omeroglu S, Sert S and Karasu C: The effect of
taurine on renal ischemia/reperfusion injury. Amino Acids.
32:405–411. 2007.PubMed/NCBI View Article : Google Scholar
|
|
210
|
Guan X, Dei-Anane G, Liang R, Gross ML,
Nickkholgh A, Kern M, Ludwig J, Zeier M, Büchler MW, Schmidt J, et
al: Donor preconditioning with taurine protects kidney grafts from
injury after experimental transplantation. J Surg Res. 146:127–134.
2008.PubMed/NCBI View Article : Google Scholar
|