Introduction
The 5-year relative survival rate for all stages
combined in pancreatic cancer is lower (9%) than that for other
types of cancer reported in the United States (1). Early-stage pancreatic cancer is
difficult to detect owing to vague symptoms and its anatomical
location, and it often develops into inoperable lesions, such as
locally advanced and metastatic cancer (2). Although targeted therapies based on
genetic profiles have been developed for inoperable pancreatic
cancer, targeted genes including germline BRCA1 or BRCA2 mutations,
and oncogenic neurotrophic receptor tyrosine kinase (NTRK)1, NTRK2
and NTRK3 fusions are highly rare (3,4). Thus,
cytotoxic chemotherapy remains the mainstream treatment choice for
patients with inoperable pancreatic cancer.
Although gemcitabine monotherapy had long been
established as a standard, the combination of leucovorin,
5-fluorouracil, irinotecan and oxaliplatin (FOLFIRINOX) and
gemcitabine plus Nab-paclitaxel (GnP) has been shown to
significantly improve the overall survival (OS), progression-free
survival (PFS) and response rate of patients in phase 3 trials
(5,6). Compared with gemcitabine, the hazard
ratio for mortality in the FOLFIRINOX group was 0.57 [95%
confidence interval (CI), 0.45-0.73; P<0.001], while that in the
GnP group was 0.72 (95% CI, 0.62-0.83; P<0.001) (5,6).
However, each group of patients who participated in these studies
had distinct background characteristics; therefore, whether
FOLFIRINOX or GnP should be used as first-line chemotherapy remains
an open research conundrum. Additionally, there are few reports on
the effects of patient background characteristics on treatment
options and prognosis in practice.
Liposomal irinotecan (nal-IRI) with 5-fluorouracil
and leucovorin following gemcitabine-based therapy has been
approved in several countries due to its high antitumor activity
and feasibility for use in patients with inoperable pancreatic
cancer (7). It can be presumed that
combination therapy will be more commonly used following GnP
treatment failure, whereas the clinical validity of FOLFIRINOX,
which includes irinotecan, 5-fluorouracil and leucovorin, as a
second-line therapy following GnP in practice, remains
controversial (8,9).
In the present study, the influence of patient
characteristics on the selection of either FOLFIRINOX or GnP as a
first-line therapy and survival benefits were determined using the
data of patients with inoperable pancreatic cancer at Kanazawa
University Hospital.
Patients and methods
Patients
The present study used the clinical data of patients
with inoperable pancreatic cancer treated with modified FOLFIRINOX
or Nab-paclitaxel plus gemcitabine as first-line therapy between
April, 2014 and January, 2019 at Kanazawa University Hospital. All
patients were followed-up once a week or every 2 weeks until they
succumbed to the disease. The tumor response was determined in
accordance with the Response Evaluation Criteria in Solid Tumors
(version 1.1) (10). The present
study was approved by the Ethics Board of Kanazawa University
(trial no. 2019-178).
UDP glucuronosyltransferase family 1
member A1 (UGT1A1) gene polymorphism
SN-38 (7-ethyl-10-hydroxycamptothecin), which is an
active form of irinotecan, is metabolized by UGT1A1. The gene
mutations (UGT1A1*28 and UGT1A1*6) impair its
activity, and thus induce severe hematotoxicities in patients
treated with irinotecan-based chemotherapy. To analyze UGT1A1
status, genomic DNA was extracted from the peripheral blood
leukocytes of the patients.
Treatment
Modified FOLFIRINOX, consisting of 85 mg
oxaliplatin, 400 mg leucovorin, 180 mg irinotecan and
5-fluorouracil administered via continuous intravenous infusion for
46 h at 2,400 mg/m2 of body surface area, was repeated
every 2 weeks. 5-Fluorouracil through bolus intravenous infusion
was excluded in all patients treated with FOLFIRINOX.
Nab-paclitaxel plus gemcitabine, consisting of 1,000 mg gemcitabine
and 125 mg/m2 Nab-paclitaxel of body surface area, was
administered on days 1, 8 and 15, and suspended on day 22 every 4
weeks (cycle 1). Chemotherapy dose reduction and delay were
performed depending on any observed toxicities such as pneumonitis
and neutropenia.
Statistical analysis
Patient clinical data were analyzed using GraphPad
Prism ver. 6.05 (GraphPad Software Inc.). Qualitative variables
were compared using Fisher's exact test. OS and PFS were analyzed
using the Kaplan-Meier method with a stratified log-rank test. All
tests were two-sided, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
Between April, 2014 and January, 2019, a total of 45
patients received either FOLFIRINOX (n=21) or GnP (n=24) as
first-line chemotherapy at Kanazawa University Hospital. The male
to female ratio, age, number of metastatic sites at diagnosis and
the type of UGT1A1 gene polymorphism were well-balanced between the
2 groups (Table I). The number of
patients with either primary lesions in the head of the pancreas or
with an Eastern Cooperative Oncology Group performance status (PS)
of 2 was greater in the GnP group than in the FOLFIRINOX group.
Additionally, biliary stents were more commonly placed in half of
the patients in the GnP group than in the FOLFIRINOX group
(Table I).
 | Table IClinical characteristics of the
patients in the present study. |
Table I
Clinical characteristics of the
patients in the present study.
| | First-line
chemotherapy |
|---|
| Characteristic | FOLFIRINOX, n=21
(%) | GnP, n=24 (%) |
|---|
| Sex | | |
|
Male | 15(71) | 17(71) |
|
Female | 6(29) | 7(29) |
| Age, years (median
range) | 65 (55-75) | 67 (53-79) |
| ECOG PS | | |
|
0 | 7(33) | 5(21) |
|
1 | 13(62) | 14(58) |
|
2 | 1(5) | 5(21) |
| Pancreatic tumor
location | | |
|
Head | 4(19) | 13(54) |
|
Uncinate
process | 1(5) | 1(4) |
|
Body | 7(33) | 5(21) |
|
Tail | 7(33) | 3(13) |
|
Body and
tail | 2(10) | 2(8) |
| Biliary stent
placement | 4(19) | 12(50) |
| Number of metastatic
sites | | |
|
0 | 6(29) | 5(21) |
|
1 | 9(42) | 11(46) |
|
2 | 6(29) | 6(25) |
|
≥3 | 0 (0) | 2(8) |
| UGT1A1 gene
polymorphism | | |
|
Wild | 11(53) | 8(33) |
|
Heterozygous | 9(42) | 10(42) |
|
Homozygous | 1(5) | 5(21) |
|
Unknown | 0 (0) | 1(4) |
Treatment efficacy and adverse events
(AEs)
The response rate (partial response) and disease
control rate of the FOLFIRINOX group (19 and 85%, respectively)
were similar to those of the GnP group (21 and 79%, respectively)
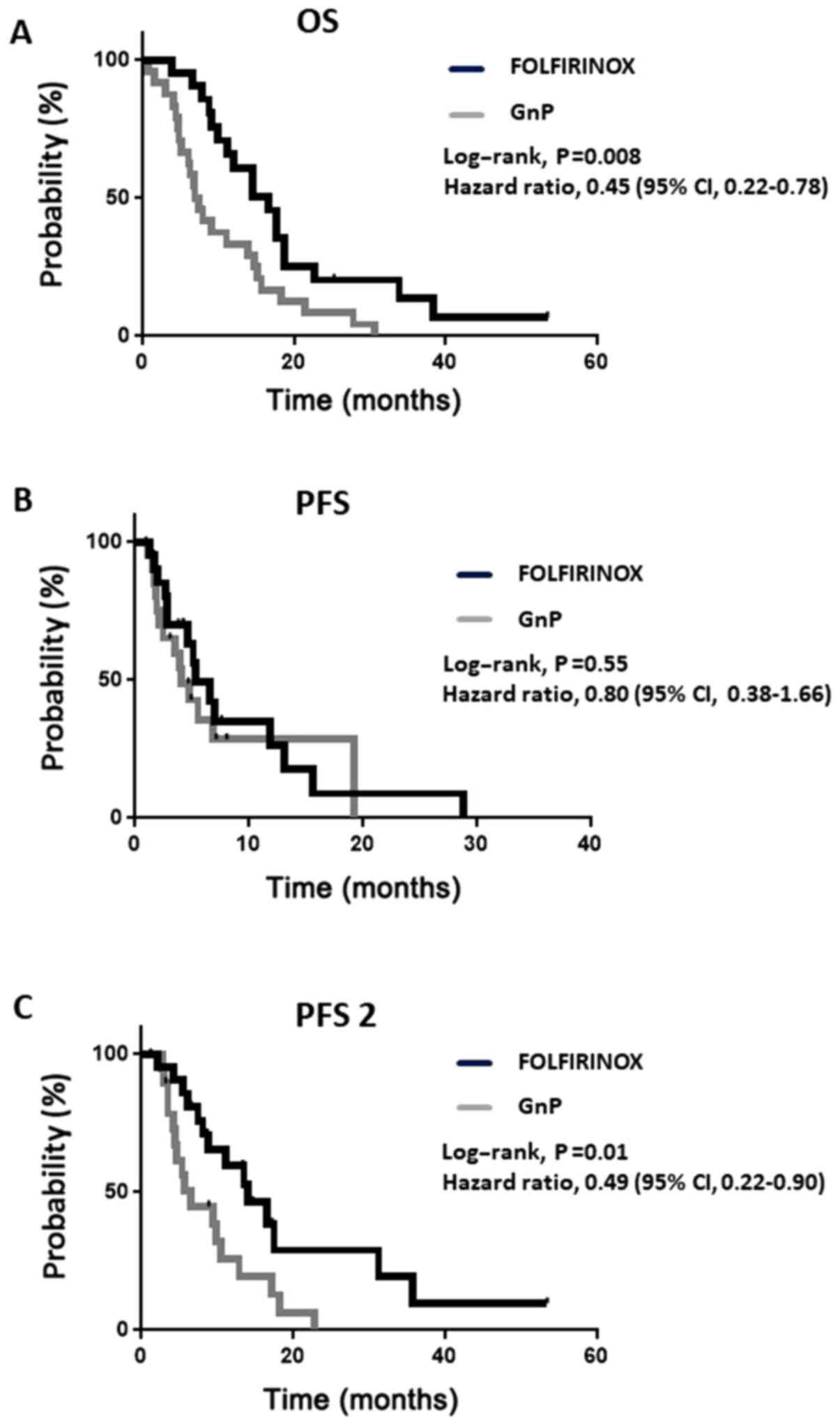
(Table II). However, the median OS
was longer in the FOLFIRINOX group than in the GnP group (16.7 vs.
7.2 months; hazard ratio for mortality, 0.45; 95% CI, 0.22-0.78;
P<0.01) (Fig. 1A). In the PFS
analysis, 29 patients received first-line therapy until progressive
disease (PD) or mortality, including 67% of the patients in the
FOLFIRINOX group and 62% in the GnP group (Table III). In contrast to OS, no
significant difference was observed in PFS between the 2 groups
(Fig. 1B).
 | Table IIChemotherapy response. |
Table II
Chemotherapy response.
| Clinical
response | FOLFIRINOX, n=21
(%) | GnP, n=24 (%) |
|---|
| Complete
response | 0 (0) | 0 (0) |
| Partial response | 4(19) | 5(21) |
| Stable disease | 13(62) | 14(58) |
| Progressive
disease | 3(14) | 5(21) |
| Not assessable | 1(5) | 0 (0) |
| Disease control
rate | 85% | 79% |
 | Table IIISelection of therapy following
first-line therapy. |
Table III
Selection of therapy following
first-line therapy.
| | First-line
chemotherapy | |
|---|
| Treatment | FOLFIRINOX, n=21
(%) | GnP, n=24 (%) | P-value |
|---|
| First-line treatment
interruption for | | | |
|
Progression | 14(67) | 15(62) | NSa |
|
Toxicity | 7(33) | 9(38) | |
| Second-line
chemotherapy | | | |
|
GEM +
Nab-PTX/FOLFIRINOX | 14(67) | 3(12) |
<0.001a |
|
Other | 7(33) | 17(71) | |
| Best supportive
care | 0 | 4(17) | |
To validate the discrepancy between OS and PFS, PFS
2 was investigated, i.e., the time from the initiation of treatment
to second PD or mortality. PFS 2 was significantly longer in the
FOLFIRINOX group than in the GnP group (14.0 vs. 6.5 months, 95%
CI, 0.22-0.90; P<0.05) (Fig. 1C).
The rate of crossover between FOLFIRINOX and GnP was higher in the
FOLFIRINOX group (67%) than in the GnP group (12%) (Table III). All patients in the FOLFIRINOX
group began second-line therapy, whereas 17% (4 patients) form the
GnP group received best supportive care (BSC) instead of
second-line therapy (Table III),
and three of them had a PS of 2 at baseline. Various AEs caused the
cessation of first-line treatment between the 2 groups (Table IV). First-line therapy was
discontinued in 16 patients (7 and 9 patients in the FOLFIRINOX and
GnP groups, respectively) owing to AEs, and 4 patients had
pneumonitis in the latter group.
 | Table IVAdverse events leading to the
interruption of first-line therapy. |
Table IV
Adverse events leading to the
interruption of first-line therapy.
| Adverse events | FOLFIRINOX,
n=7 | GEM + Nab-PTX,
n=9 |
|---|
| Pneumonitis | 1 | 4 |
| Neutropenia | 1 | 0 |
| Infusion related
reaction | 1 | 0 |
| Phlebitis | 1 | 0 |
| Paroxysmal atrial
tachycardia | 1 | 0 |
| Gastric
hemorrhage | 1 | 0 |
| Diarrhea | 1 | 0 |
| Cholangitis | 0 | 2 |
| Peripheral sensory
neuropathy | 0 | 2 |
| Rash
maculopapular | 0 | 1 |
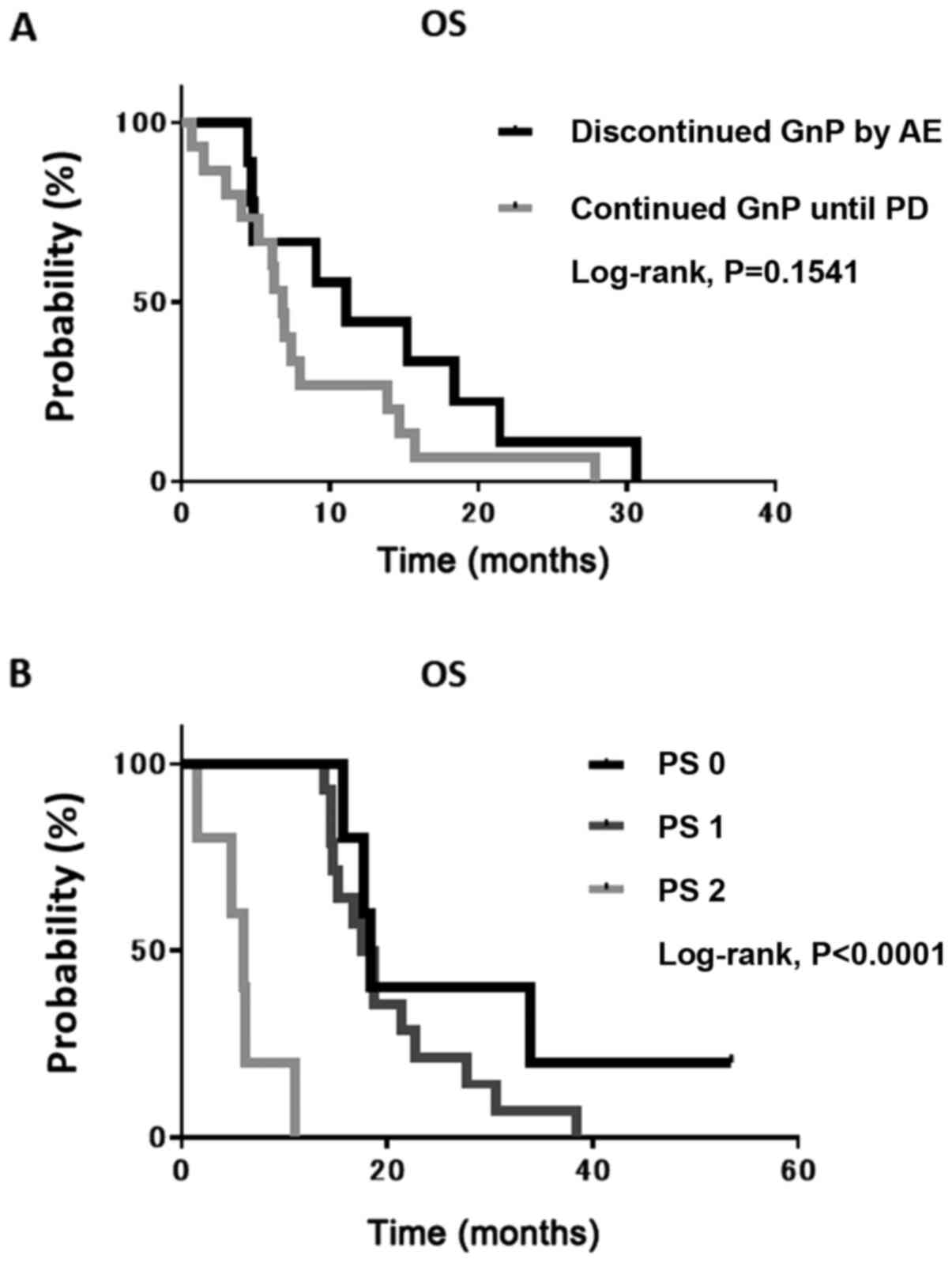
Subgroup analyses in the GnP
group
Considering that the response rate and PFS did not
differ significantly between the 2 groups, it was conceivable that
the discontinuation of GnP treatment owing to AEs predicted an
unfavorable survival as the AE conflicted with the exclusion
criteria of the second-line therapy. To examine this hypothesis,
the median OS of patients who continued GnP until PD and in those
who discontinued it following AE was determined. However, no
significant difference was found between them (Fig. 2A). Thereafter, the present study
focused on patients with a lower PS as most switched from
first-line therapy to BSC. The median OS of patients with a PS of 2
was significantly shorter than that of patients with either a PS of
0 or 1 (Fig. 2B).
Discussion
The present study demonstrated that FOLFIRINOX
noticeably improved the prognosis of patients with inoperable
pancreatic cancer compared with GnP when either of the two
therapies was used as a first-line therapy. This result was
accounted for by a shorter PFS 2 and more patients with a PS of 2
receiving no second-line therapy, but BSC in the GnP group than in
the FOLFIRINOX group. In 41 patients receiving second-line therapy,
14 out of the 21 patients switched from FOLFIRINOX to GnP, whereas
only 3 out of 20 patients switched from GnP to FOLIRINOX (Table III). The crossover of the two
therapies was far less common in the GnP group than in the
FOLFIRINOX group. This is likely to have contributed to the
shortening of PFS 2, as some studies have demonstrated that there
were no significant differences in median OS between patients with
inoperable pancreatic cancer treated with FOLFIRINOX followed by
GnP and vice versa (11,12).
In the present study, the low crossover rate in the
GnP group was due to AEs, including pneumonitis, cholangitis, and
peripheral sensory neuropathy, in the presence of which treatment
with irinotecan and oxaliplatin could not be continued. A previous
meta-analysis by Pusceddu et al (13) suggested that neutropenia and febrile
neutropenia were significantly higher in the FOLFIRINOX arm than in
the GnP arm as first-line therapy. In the present study, first
cycle treatment with G-CSF and the modified regimen without
fluorouracil through bolus intravenous infusion curtailed
neutropenia, which enabled patients in the FOLFIRINOX group to
continue the therapy. A previous retrospective study by Williet
et al (8) reported that the
number of patients treated with GnP following FOLFIRINOX failure
was higher than that of patients treated with the reverse sequence,
as the former was more feasible than the latter, corresponding to
the findings of the present study.
Irinotecan is metabolized and excreted into the
gastrointestinal tract through a biliary route following
intravenous administration (14).
Thus, clinicians tend to avoid the use of irinotecan in patients
with biliary obstruction who need biliary stent placement. In fact,
the results of the present study demonstrated that more patients
with a biliary stent received GnP as first-line therapy than
FOLFIRINOX. However, a previous study by Kang et al
(15) demonstrated that FOLFIRINOX
markedly prolonged median stent patency and OS compared with
gemcitabine-based chemotherapy, including GnP in patients with
pancreatic cancer with stent insertion. FOLFIRINOX as a first-line
therapy may improve the prognosis of patients if biliary
obstruction and subsequent jaundice are prevented with the biliary
stent beforehand.
In recent years, metastatic sites and gene mutations
in patients with pancreatic cancer have attracted increasing
attention owing to the effectiveness of first-line chemotherapy.
Peritoneal carcinomatosis is a poor prognostic factor for patients
with pancreatic cancer treated with FOLFIRINOX (16). Moreover, Nab-paclitaxel is expected
to have potential in treating peritoneal metastasis due to its
pharmacological action, which maintains a high drug concentration
in peritoneal lesions (17). Thus,
further studies are required to examine the efficacy of GnP in
treating pancreatic cancer with peritoneal metastasis. By contrast,
Kondo et al (18) suggested
that homologous recombination repair (HRR)-related gene mutations
predicted a favorable prognosis for 17 patients with pancreatic
cancer who received oxaliplatin-based chemotherapy. Thus, a
prospective trial to investigate the effect of HRR-related gene
mutations on the efficacy of FOLFIRINOX is warranted.
In practice, there are differences in the
characteristics of patients with inoperable pancreatic cancer that
meet the criteria for FOLFIRINOX and GnP. Peixoto et al
(19) reported that 25 and 45% of
patients met the FOLFIRINOX and GnP criteria, respectively, which
were in accordance with the pivotal phase III trials (5,6). A
common reason for FOLFIRINOX ineligibility was a PS ≥2,
corresponding to the finding of the present study that the majority
of patients with a PS of 2 received GnP. The prognosis of patients
with a PS of 2 resulted in a shorter OS in the GnP group.
In conclusion, the present study reflected
real-world data regarding the selection of first-line therapies for
patients with inoperable pancreatic cancer. Although patients with
a PS of 2 are more likely to be assigned GnP treatment than
FOLFIRINOX treatment, the results presented herein revealed that
GnP was not established as an effective and feasible treatment for
such patients. Additionally, it was found that biliary stent
placement impaired the chance of FOLFIRINOX treatment despite the
release of obstructive jaundice. These findings may help clinicians
select FOLFIRINOX treatment for patients who exhibit good
tolerance, while encouraging the development of first-line therapy
for pancreatic cancer patients with a worse PS.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AT designed the study. SS, NY, CS, YT, AN, KY, ST
and KO followed-up on the patients. HS and AT collected the data,
performed the statistical analyses, and wrote the manuscript. SY
supervised the study, was involved in the study design and edited
the manuscript. HS and KO confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Board
of Kanazawa University (trial number 2019-178). The present study
was a retrospective study using anonymized patient data; thus, no
patient consent was required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Oettle H, Post S, Neuhaus P, Gellert K,
Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C,
et al: Adjuvant chemotherapy with gemcitabine vs observation in
patients undergoing curative-intent resection of pancreatic cancer:
A randomized controlled trial. JAMA. 297:267–277. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Golan T, Hammel P, Reni M, Van Cutsem E,
Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, et
al: Maintenance olaparib for germline BRCA-mutated metastatic
pancreatic cancer. N Engl J Med. 381:317–327. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Doebele RC, Drilon A, Paz-Ares L, Siena S,
Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, et al:
trial investigators: Entrectinib in patients with advanced or
metastatic NTRK fusion-positive solid tumours: Integrated analysis
of three phase 1-2 trials. Lancet Oncol. 21:271–282.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: Groupe Tumeurs Digestives of Unicancer;
PRODIGE Intergroup: FOLFIRINOX versus gemcitabine for metastatic
pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang-Gillam A, Li CP, Bodoky G, Dean A,
Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, et
al: NAPOLI-1 Study Group: Nanoliposomal irinotecan with
fluorouracil and folinic acid in metastatic pancreatic cancer after
previous gemcitabine-based therapy (NAPOLI-1): A global,
randomised, open-label, phase 3 trial. Lancet. 387:545–557.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Williet N, Saint A, Pointet AL, Tougeron
D, Pernot S, Pozet A, Bechade D, Trouilloud I, Lourenco N,
Hautefeuille V, et al: Folfirinox versus
gemcitabine/nab-paclitaxel as first-line therapy in patients with
metastatic pancreatic cancer: A comparative propensity score study.
Therap Adv Gastroenterol. 12(1756284819878660)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Matsumoto T, Kurioka Y, Okazaki U, Matsuo
Y, Kimura S, Miura K, Tsuduki T, Takagi S, Takatani M and Morishita
H: FOLFIRINOX for advanced pancreatic cancer patients after
Nab-paclitaxel plus gemcitabine failure. Pancreas. 49:574–578.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee JC, Woo SM, Shin DW, Kim J, Yang SY,
Kim MJ, Kim JW, Kim JW, Lee WJ, Cha HS, et al: Comparison of
FOLFIRINOX and gemcitabine plus Nab-paclitaxel for treatment of
metastatic pancreatic cancer: using Korean pancreatic cancer
(K-PaC) registry. Am J Clin Oncol. 43:654–659. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vogl UM, Andalibi H, Klaus A, Vormittag L,
Schima W, Heinrich B, Kafka A, Winkler T and Öhler L:
Nab-paclitaxel and gemcitabine or FOLFIRINOX as first-line
treatment in patients with unresectable adenocarcinoma of the
pancreas: Does sequence matter? BMC Cancer. 19(28)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pusceddu S, Ghidini M, Torchio M, Corti F,
Tomasello G, Niger M, Prinzi N, Nichetti F, Coinu A, Di Bartolomeo
M, et al: Comparative effectiveness of gemcitabine plus
Nab-paclitaxel and FOLFIRINOX in the first-line setting of
metastatic pancreatic cancer: a systematic review and
meta-analysis. Cancers (Basel). 11(E484)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Itoh T, Takemoto I, Itagaki S, Sasaki K,
Hirano T and Iseki K: Biliary excretion of irinotecan and its
metabolites. J Pharm Pharm Sci. 7:13–18. 2004.PubMed/NCBI
|
|
15
|
Kang J, Lee SH, Choi JH, Paik WH, Ahn DW,
Jeong JB, Ryu JK and Kim YT: Folfirinox chemotherapy prolongs stent
patency in patients with malignant biliary obstruction due to
unresectable pancreatic cancer. Hepatobiliary Pancreat Dis Int.
19:590–595. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bonnet E, Mastier C, Lardy-Cléaud A,
Rochefort P, Sarabi M, Guibert P, Cattey-Javouhey A, Desseigne F
and de La Fouchardière C: FOLFIRINOX in patients with peritoneal
carcinomatosis from pancreatic adenocarcinoma: A retrospective
study. Curr Oncol. 26:e466–e472. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kinoshita J, Fushida S, Tsukada T, Oyama
K, Watanabe T, Shoji M, Okamoto K, Nakanuma S, Sakai S, Makino I,
et al: Comparative study of the antitumor activity of
Nab-paclitaxel and intraperitoneal solvent-based paclitaxel
regarding peritoneal metastasis in gastric cancer. Oncol Rep.
32:89–96. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kondo T, Kanai M, Kou T, Sakuma T,
Mochizuki H, Kamada M, Nakatsui M, Uza N, Kodama Y, Masui T, et al:
Association between homologous recombination repair gene mutations
and response to oxaliplatin in pancreatic cancer. Oncotarget.
9:19817–19825. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Peixoto RD, Ho M, Renouf DJ, Lim HJ, Gill
S, Ruan JY and Cheung WY: Eligibility of metastatic pancreatic
cancer patients for first-line palliative intent nab-paclitaxel
plus gemcitabine versus FOLFIRINOX. Am J Clin Oncol. 40:507–511.
2017.PubMed/NCBI View Article : Google Scholar
|