1. Introduction
Laparoscopy is a minimally invasive technique with
the characteristics of easy operation, a small incision, rapid
healing and satisfactory efficacy. It has been widely applied to
the treatment of gynecological diseases (1). Physiologically, gastrointestinal
motility results from muscularis mucosa contraction, segmentation
and peristalsis (rhythmic contractions) (2). The rapid recovery of gastrointestinal
motility often predicts satisfactory clinical outcomes following
laparoscopies, and various rehabilitation interventions have been
employed to prompt and restore gastrointestinal motility
post-surgery (3).
2. Factors influencing gastrointestinal
motility recovery following gynecological laparoscopies
Immediate anal exhaust following gynecological
laparoscopies (GLs) is almost impossible due to the absence of
intestinal smooth muscle contraction. Rhythmic motion slowly occurs
at 3 to 8 h post-surgery, beginning from the proximal small
intestine to the rectum and colon. Post-operative exhaust often
represents the recovery of gastrointestinal transit (4). However, the post-operative anal
evacuation time may vary among individuals; it is usually between
24-56 h in patients receiving GLs without other specific
treatments, with an average of 31 h (5). However, in >80% of this patient
group, the recovery gastrointestinal transit is delayed when they
develop symptoms, such as abdominal distension, and decreases in
peristalsis, anal exhaust and bowel episodes (6), though their peristalsis recovery time
is shorter than that of patients undergoing conventional laparotomy
(7,8).
The gastrointestinal tract is more sensitive to
surgical stress than other parts of the body, the recovery of which
can be disrupted or even impaired by surgical trauma,
post-operative pain, anesthetics and analgesics, carbon dioxide
pneumoperitoneum and other factors (9), leading to severe gastrointestinal
motility disorders in some patients (10,11).
Specifically, surgical trauma may cause post-operative
gastrointestinal dysfunction. The study conducted by Magrina
demonstrated that mortality rates following injury to the bowel
during laparoscopic procedures ranged from 2.5 to 5% (12). Post-operative pain leads to the
transient dysfunction of the enteric nervous system, inhibiting
gastrointestinal transit by the binding of abundant norepinephrine
released from sympathetic postganglionic neurons to receptors on
smooth muscle cells (13,14) and can also lead to intestinal
paralysis (15). As regards
anesthetics and analgesics, opioids are the most effective and
commonly used analgesics peri-operatively; however, they can induce
delayed gastric emptying and intestinal transit, resulting in fluid
and electrolyte disruption. The intraoperative use of anesthetics
and analgesics may also inhibit the recovery of post-operative
gastrointestinal motility. Carbon dioxide pneumoperitoneum has been
widely utilized in laparoscopies to create operating and viewing
space (16). However, carbon dioxide
insufflation into the abdomen has also been reported to disrupt
tissue or organ functions, such as breathing (17), digestive (18) and urinary functions (19). Following GL procedures, a small
amount of carbon dioxide can pass through the peritoneum, and can
be absorbed into the circulation and converted into carbonic acid,
causing hypercapnia; this triggers the release of catecholamines to
activate cholinergic neurons in the enteric nervous system
(20) to induce gastrointestinal
symptoms, such as nausea and vomiting (21).
The delayed recovery of gastrointestinal motility
can significantly increase challenges in post-operative recovery
and can aggravate patients' discomfort (22-25).
This can occur in addition to another risk factor, intestinal
dilation, that impairs wound healing and induces intestinal
paralysis, nausea and vomiting, or severe complications such as
arrhythmia and multiple organ dysfunction syndrome (26,27).
Both of these conditions may impair the effectiveness of nursing
care or rehabilitation, leading to longer hospital stays and higher
medical expenditures.
3. Gastrointestinal motility recovery
following gynecological laparoscopies
A consensus on the management of gastrointestinal
motility disorders post-GLs has not yet been reached, and the
efficacy of relevant interventions reported to date is
unsatisfactory (28). In the
majority of cases, commonly prescribed drugs against these symptoms
only achieve limited results, alongside pronounced adverse events
(29) and extra costs, which limit
their application in patients undergoing GLs (30). Traditional Chinese medicine (TCM) has
been shown to exert preventive and therapeutic effects on
post-operative gastrointestinal motility disorders. However, the
efficacy of TCM is accumulative; treatment based on syndrome
differentiation and acupuncture with needle manipulation are highly
demanding tasks for novices, and moxibustion may cause burns
(31,32). Therefore, non-drug treatments that
are easy to use, cost-effective, non-invasive and highly repeatable
for distinct operators are required to promote gastrointestinal
motility recovery following GLs and to improve the quality of life
of patients, promote early post-operative recovery and enhance the
quality of nursing care (33).
4. Low-frequency electrical stimulation
Low-frequency electrical stimulation (LFES) is a
suitable non-invasive technique that delivers low-frequency pulsed
currents to the muscles and nerves through skin surface electrodes
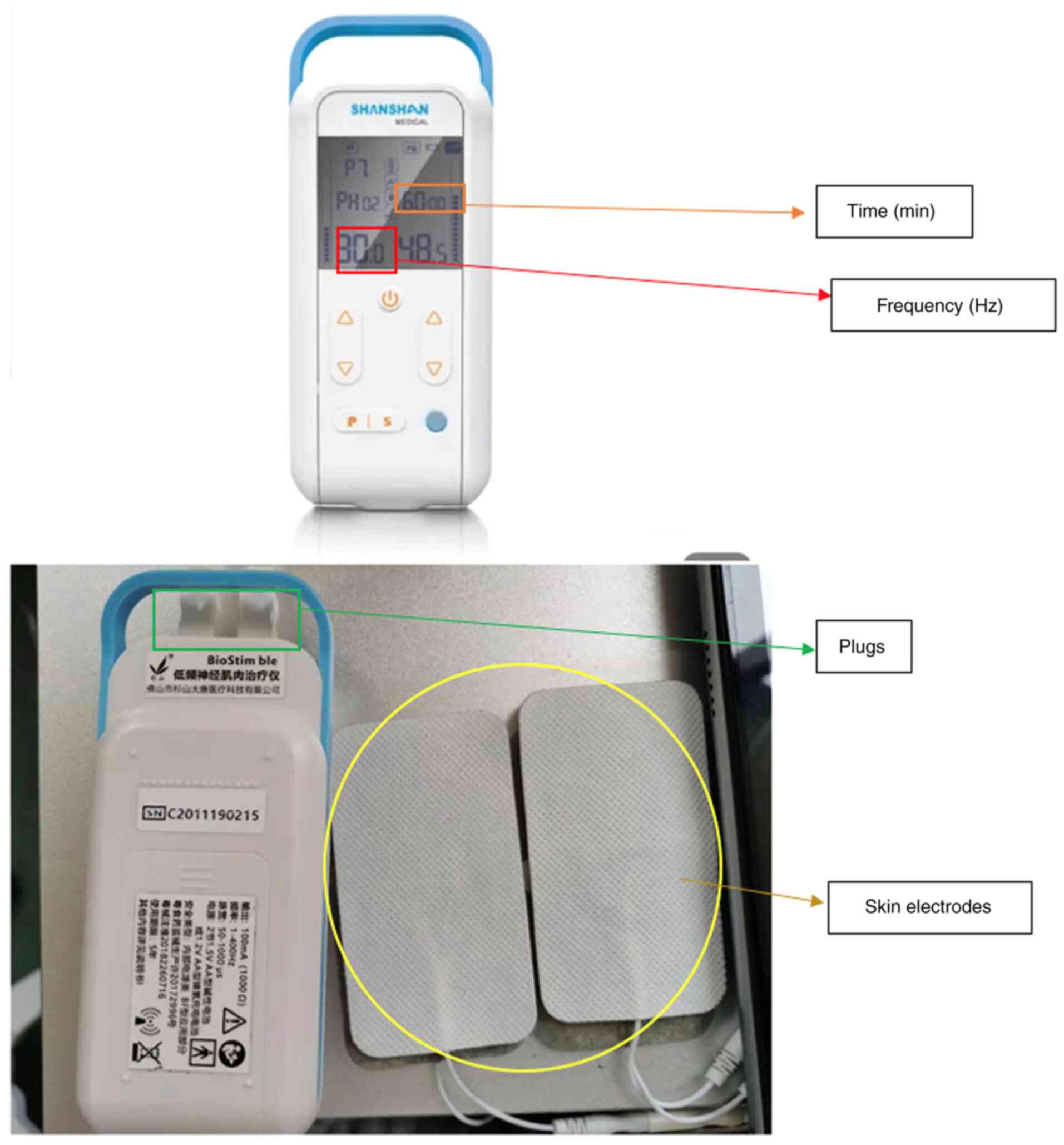
(34). The device is a portable
apparatus and easy to operate for novices (Fig. 1). It is placed on acupoints, in a
manner similar to acupuncture, but is not inserted into the skin.
Thus, LFES can also exert a therapeutic effect similar to that of
acupuncture, but circumvents adverse events, such as subcutaneous
hematoma, accidental needle-sticks and fainting during acupuncture
treatment (35); it is therefore
more acceptable for patients. This treatment has been utilized in
departments of obstetrics and gynecology, cardiovascular diseases,
rehabilitation, TCM and surgery (36).
Mechanisms of LFES
Bioelectricity is the basis for nerve conduction,
and bioelectrical signals delineate the activity of neurons and
muscle cells (37). Applying a
current of designated magnitude to the organ of interest or central
nerves or peripheral plexuses innervating it can stimulate muscle
contractions (38), thus aiding in
the restoration of peristalsis (39). Currently, the mechanisms primarily
responsible for LFES treatment, although not fully explored, have
been ascertained as follows: Electrical stimulation to block or
enhance neuronal electrical activity to restore intestinal
function; electrical stimulation on muscularis mucosae with
distinct frequencies of currents to induce the contraction or
relaxation of intestinal muscularis mucosae directly; long-term,
chronic electrical stimulation to alter tissue structure (40,41).
LFES has exhibited marked potential in preventing
post-operative ileus and gastrointestinal motility disorders
(42,43), including esophageal motility
disorders, gastroesophageal reflux disease, functional dyspepsia,
chronic intestinal pseudo-obstruction, post-operative intestinal
obstruction and irritable bowel syndrome with diarrhea or
constipation (44-47).
The delivery of LFES with skin electrodes placed on the sites
corresponding to the gastrointestinal segment with abnormal
motility patterns (48) can trigger
the contractions of gastrointestinal smooth muscle cells and can
thus stimulate peristalsis, gastric emptying, intestinal transit
and absorption (49). Furthermore,
the activation of submucosal and myenteric plexuses by LFES
facilitates gastrointestinal fluid secretion, and blood and
lymphatic circulation in the gastrointestinal tract post- (50). Overall, LFES promotes the early
recovery of gastrointestinal function, post-operative exhaust and
defecation, and also alleviates abdominal distension and pain by
stimulating gastrointestinal peristalsis (51).
Efficacy of LFES
Electrical stimulation was first reported as a
treatment by Bilgutay et al (52) in the 1960s, who employed intestinal
electrical stimulation with a tube electrode introduced into the
stomach, which markedly ameliorated post-operative intestinal
obstruction. However, this procedure remains largely unrepeatable
due to methodological complexity. Electrical stimulation was
systematically studied in the 1970s, for example; Kelley and Code
(53) proposed the electrical
stimulation of the canine stomach and small intestine to alter
their electromyographic patterns and motility. This technique has
evolved into special gastric electrical stimulation, or
Enterra® Therapy (Medtronic), in the late 1990s to boost
gastric motility in gastroparesis (54). However, a later study proved its
critical role in inhibiting nausea and vomiting, rather than
increasing motility (55).
The electrical stimulation of acupoints refers to
the application of a pulsating electrical current to acupuncture
needles for acupoint stimulation. Electrical stimulation of
acupoints increase gastrointestinal motility by regulating vagus
nerve activation (56). It has been
widely used for various gastrointestinal conditions in China and
the West (57-59).
In the study conducted by Huang et al (60), 64 patients undergoing laparoscopic
colorectal resection were randomly divided into two groups, the
control group (group A) and the electrical stimulation group (group
B). Patients in the electrical stimulation group received
electrical stimulation of bilateral Zusanli (ST 36) at 30 min prior
to anesthesia to the end of surgery. The stimulatory effect of LFES
at ST-36 on gastric motility was associated with vagal activity.
The patients in the control group were not given the stimulation.
The post-operative anal exhaust time in group B was significantly
shorter than that of group A (60).
Low-frequency electrical stimulation can promote the recovery of
postoperative gastrointestinal function and reduce the pain
intensity 48 h after surgery, thus satisfying the need of early
postoperative analgesia (61).
Over the past 20 years, electrical stimulation has
been shown to be effective in normalizing gastric dysrhythmia,
accelerating gastric emptying and improving nausea and vomiting
(62). Clinical data from previous
studies describe gastrointestinal motility following electrical
stimulation. Zhang et al (63) performed ST36-LFES in 42 patients
prior to abdominal surgery and found that symptoms associated with
post-operative gastrointestinal motility disorders were markedly
ameliorated, probably by increasing vagal activity and inhibiting
sympathetic activity.
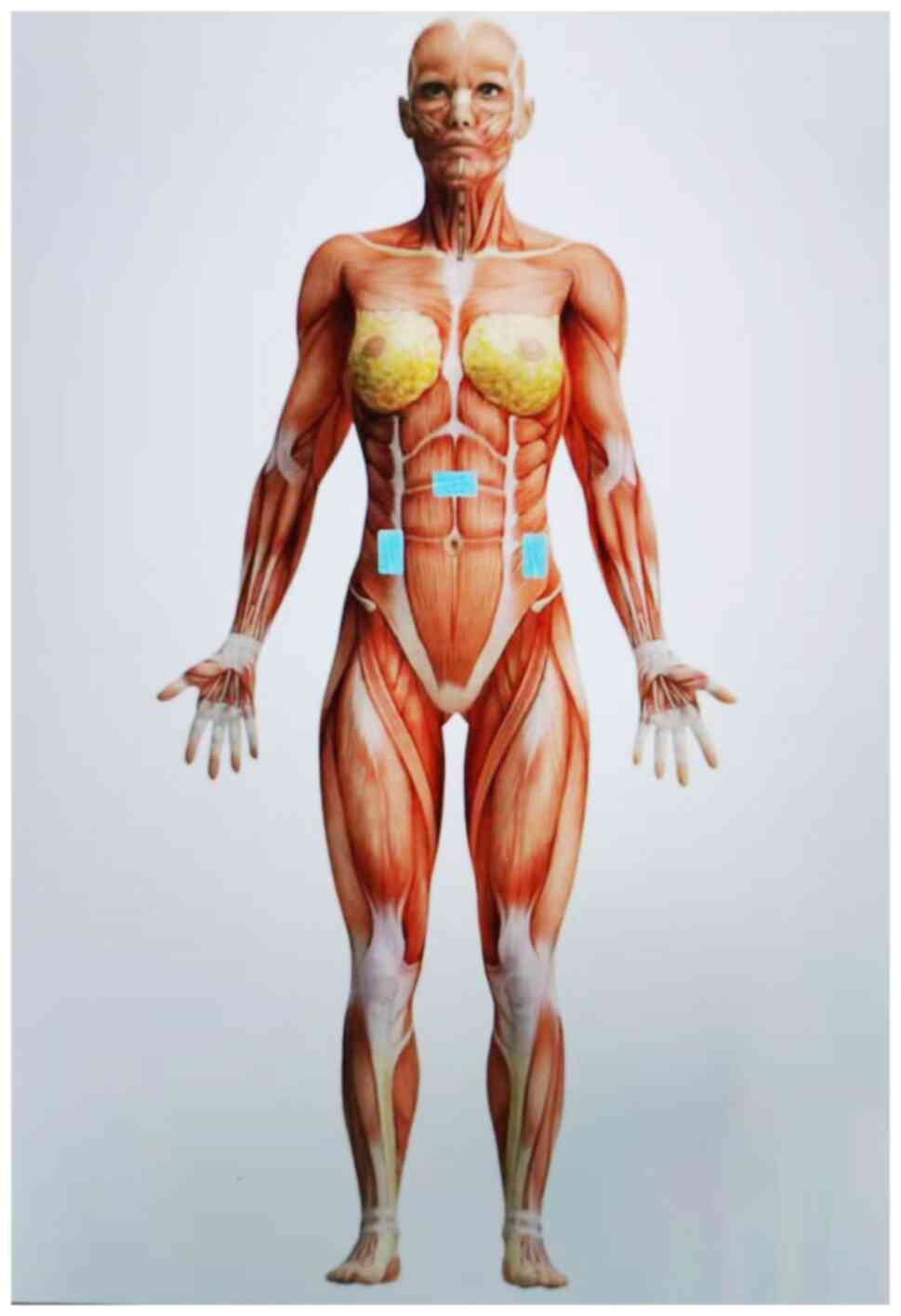
Early LFES on the gastrointestinal tract post-GLs
can restore autonomic nerve function, boost local blood circulation
and accelerate the recovery of gastrointestinal motility to
forestall abdominal distension and pain. This is achieved by simply
placing skin electrodes on the abdominal sites corresponding to the
ascending, transverse, or descending colon and employing a
frequency of 30 Hz (Fig. 2). It is a
more convenient and efficient tool for routine nursing care
compared with transcutaneous electrical acupoint stimulation and
electrical pulse stimulation, without locating acupoints or
adjusting for stimulation frequency during the treatment. It has
been universally accepted by patients due to its safety and
cost-effectiveness, without affecting other therapies administered
simultaneously or limiting daily life activities.
5. Conclusion and future perspectives
LFES promotes gastrointestinal motility post-GLs
probably through elevating plasma ghrelin and motilin and
parasympathetic activity. It is a non-invasive and
non-pharmacological intervention recommended for early nursing care
or rehabilitation post-surgery. Nonetheless, future clinical
studies on adults, particularly placebo-controlled studies, are
required to validate its efficacy and safety. A database for
electrophysiological properties in patients undergoing LFES
treatment is conducive to offering sufficient data for
bioinformatics or clinical studies and establishing guidelines of
LFES for the management of gynecological diseases.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Research Center for
Medical and Health Science and Technology Development of the
National Health Commission (grant no. HDSL202004003).
Availability of data and materials
Not applicable.
Authors' contributions
YW conceived and the study and drafted the
manuscript. XT, LvG and LiG critically reviewed the manuscript. All
authors have read and approved the final version to be published.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saha R, Shrestha NS, Thapa M, Shrestha J,
Bajracharya J and Karki SC: Experiences of gynecological
laparoscopic surgeries in a teaching hospital. J Nepal Health Res
Counc. 11:49–52. 2013.PubMed/NCBI
|
|
2
|
Camilleri M: Gastrointestinal motility
disorders in neurologic disease. J Clin Invest.
131(e143771)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Greenwood-Van Meerveld B, Johnson AC and
Grundy D: Gastrointestinal physiology and function. Handb Exp
Pharmacol. 239:1–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stakenborg N, Gomez-Pinilla PJ and
Boeckxstaens GE: Postoperative Ileus: Pathophysiology, current
therapeutic approaches. Handb Exp Pharmacol. 239:39–57.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Turkay U, Yavuz A, Hortu I, Terzi H and
Kale A: The impact of chewing gum on postoperative bowel activity
and postoperative pain after total laparoscopic hysterectomy. J
Obstet Gynaecol. 40:705–709. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kalogera E, Glaser GE, Kumar A, Dowdy SC
and Langstraat CL: Enhanced recovery after minimally invasive
gynecologic procedures with bowel surgery: A systematic review. J
Minim Invasive Gynecol. 26:288–298. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Levy L and Tsaltas J: Recent advances in
benign gynecological laparoscopic surgery. Fac Rev. 10(60)2021.
|
|
8
|
Aarts JW, Nieboer TE, Johnson N, Tavender
E, Garry R, Mol BW and Kluivers KB: Surgical approach to
hysterectomy for benign gynaecological disease. Cochrane Database
Syst Rev. 2015(CD003677)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Weibel S, Schaefer MS, Raj D, Rucker G,
Pace NL, Schlesinger T, Meybohm P, Kienbaum P, Eberhart LHJ and
Kranke P: Drugs for preventing postoperative nausea and vomiting in
adults after general anaesthesia: An abridged Cochrane network
meta-analysis. Anaesthesia. 76:962–973. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ahn EJ, Choi GJ, Kang H, Baek CW, Jung YH
and Woo YC: Comparison of ramosetron with palonosetron for
prevention of postoperative nausea and vomiting in patients
receiving opioid-based intravenous patient-controlled analgesia
after gynecological laparoscopy. Biomed Res Int.
2017(9341738)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mazzotta E, Villalobos-Hernandez EC,
Fiorda-Diaz J, Harzman A and Christofi FL: Postoperative ileus and
postoperative gastrointestinal tract dysfunction: Pathogenic
mechanisms and novel treatment strategies beyond colorectal
enhanced recovery after surgery protocols. Front Pharmacol.
11(583422)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Magrina JF: Complications of laparoscopic
surgery. Clin Obstet Gynecol. 45:469–480. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Foong D, Zhou J, Zarrouk A, Ho V and
O'Connor MD: Understanding the biology of human interstitial cells
of cajal in gastrointestinal motility. Int J Mol Sci.
21(4540)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Beck K, Friebe A and Voussen B: Nitrergic
signaling via interstitial cells of Cajal and smooth muscle cells
influences circular smooth muscle contractility in murine colon.
Neurogastroenterol Motil. 30(e13300)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Koo KC, Yoon YE, Chung BH, Hong SJ and Rha
KH: Analgesic opioid dose is an important indicator of
postoperative ileus following radical cystectomy with ileal
conduit: Experience in the robotic surgery era. Yonsei Med J.
55:1359–1365. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nesek-Adam V, Mrsic V, Smiljanic A,
Oberhofer D and Grizelj-Stojcic E: Pathophysiologic effects of
CO2-pneumoperitoneum in laparoscopic surgery. Acta Med
Croatica. 61:165–170. 2007.PubMed/NCBI
|
|
17
|
Kabakchiev C, Valverde A, Singh A and
Beaufrere H: Cardiovascular and respiratory effects of carbon
dioxide pneumoperitoneum in the domestic rabbit (Oryctolagus
cuniculus). Can J Vet Res. 84:108–114. 2020.PubMed/NCBI
|
|
18
|
Nishiwaki S, Araki H, Hayashi M, Takada J,
Iwashita M, Tagami A, Hatakeyama H, Hayashi T, Maeda T and Saito K:
Inhibitory effects of carbon dioxide insufflation on
pneumoperitoneum and bowel distension after percutaneous endoscopic
gastrostomy. World J Gastroenterol. 18:3565–3570. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Marton Filho MA, Alves RL, Nascimento PDJ,
Tarquinio GDS, Mega PF and Pinheiro Módolo NS: Effects of
pneumoperitoneum on kidney injury biomarkers: A randomized clinical
trial. PLoS One. 16(e0247088)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cho JS, Kim HI, Lee KY, An JY, Bai SJ, Cho
JY and Yoo YC: Effect of intraoperative dexmedetomidine infusion on
postoperative bowel movements in patients undergoing laparoscopic
gastrectomy: A prospective, randomized, placebo-controlled study.
Medicine (Baltimore). 94(e959)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Goel A, Gupta S, Bhagat TS and Garg P:
Comparative analysis of hemodynamic changes and shoulder tip pain
under standard pressure versus low-pressure pneumoperitoneum in
laparoscopic cholecystectomy. Euroasian J Hepatogastroenterol.
9:5–8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wei W, Bai W, Yang Y, Li Y, Teng X, Wan Y
and Zhu J: Pulmonary protection of transcutaneous electrical
acupoint stimulation in gynecological laparoscopic surgery: A
randomized controlled trial. Exp Ther Med. 19:511–518.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lian M, Zhao X, Wang H, Chen L and Li S:
Respiratory dynamics and dead space to tidal volume ratio of
volume-controlled versus pressure-controlled ventilation during
prolonged gynecological laparoscopic surgery. Surg Endosc.
31:3605–3613. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sao CH, Chan-Tiopianco M, Chung KC, Chen
YJ, Horng HC, Lee WL and Wang PH: Pain after laparoscopic surgery:
Focus on shoulder-tip pain after gynecological laparoscopic
surgery. J Chin Med Assoc. 82:819–826. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Atkinson TM, Giraud GD, Togioka BM, Jones
DB and Cigarroa JE: Cardiovascular and ventilatory consequences of
laparoscopic surgery. Circulation. 135:700–710. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nathan N: Management of postoperative
nausea and vomiting: The 4th Consensus Guidelines. Anesth Analg.
131(410)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gan TJ, Diemunsch P, Habib AS, Kovac A,
Kranke P, Meyer TA, Watcha M, Chung F, Angus S, Apfel CC, et al:
Consensus guidelines for the management of postoperative nausea and
vomiting. Anesth Analg. 118:85–113. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bragg D, El-Sharkawy AM, Psaltis E,
Maxwell-Armstrong CA and Lobo DN: Postoperative ileus: Recent
developments in pathophysiology and management. Clin Nutr.
34:367–376. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Traut U, Brugger L, Kunz R, Pauli-Magnus
C, Haug K, Bucher HC and Koller MT: Systemic prokinetic
pharmacologic treatment for postoperative adynamic ileus following
abdominal surgery in adults. Cochrane Database Syst Rev: CD004930,
2008 doi: 10.1002/14651858.CD004930.pub3.
|
|
30
|
Scott MJ, Baldini G, Fearon KC, Feldheiser
A, Feldman LS, Gan TJ, Ljungqvist O, Lobo DN, Rockall TA, Schricker
T and Carli F: Enhanced recovery after surgery (ERAS) for
gastrointestinal surgery, part 1: Pathophysiological
considerations. Acta Anaesthesiol Scand. 59:1212–1231.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gao X, Zhang Y, Zhang Y, Ku Y and Guo Y:
Electroacupuncture for gastrointestinal function recovery after
gynecological surgery: A systematic review and meta-analysis. Evid
Based Complement Alternat Med. 2021(8329366)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cheong KB, Zhang J and Huang Y:
Effectiveness of acupuncture in postoperative ileus: A systematic
review and meta-analysis. J Tradit Chin Med. 36:271–282.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang YP, Herndon CC and Lu CL:
Non-pharmacological approach in the management of functional
dyspepsia. J Neurogastroenterol Motil. 26:6–15. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Li JJ, Zhao WS, Shao XM, Yang AM, Zhang FF
and Fang JQ: Effect of transcutaneous electrical acupoint
stimulation on post-surgical gastrointestinal function, autonomic
nerve activities and plasma brain-gut peptide levels in patients
undergoing gastrointestinal surgery. Zhen Ci Yan Jiu. 41:240–246.
2016.PubMed/NCBI(In Chinese).
|
|
35
|
He D, Wang FZ, Zhang Z, Huang F, Chen JJ
and Li B: Effect of low-frequency electrical acupoint stimulation
on gastrointestinal motility function following radical gastrectomy
in patients with gastric cancer. Zhen Ci Yan Jiu. 45:51–56.
2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
36
|
Hirata A and Reilly JP: Special section:
Recent progress in low-frequency dosimetry modeling: From induction
to electrostimulation. Phys Med Biol. 61:E1–E2. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Korkmazov M: Bioresonance. Main principles
of bioresonance and electromagnetic therapy. Vestn Otorinolaringol.
59–61. 2008.PubMed/NCBI(In Russian).
|
|
38
|
Mansourian M and Shanei A: Evaluation of
pulsed electromagnetic field effects: A systematic review and
meta-analysis on highlights of two decades of research in vitro
studies. Biomed Res Int. 2021(6647497)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen Y, Cai Q, Pan J, Zhang D, Wang J,
Guan R, Tian W, Lei H, Niu Y, Guo Y, et al: Role and mechanism of
micro-energy treatment in regenerative medicine. Transl Androl
Urol. 9:690–701. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Metz CN and Pavlov VA: Vagus nerve
cholinergic circuitry to the liver and the gastrointestinal tract
in the neuroimmune communicatome. Am J Physiol Gastrointest Liver
Physiol. 315:G651–GG8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Elshiwi AM, Hamada HA, Mosaad D, Ragab
IMA, Koura GM and Alrawaili SM: Effect of pulsed electromagnetic
field on nonspecific low back pain patients: A randomized
controlled trial. Braz J Phys Ther. 23:244–249. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yin J and Chen JD: Mechanisms and
potential applications of intestinal electrical stimulation. Dig
Dis Sci. 55:1208–1220. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Payne SC, Furness JB and Stebbing MJ:
Bioelectric neuromodulation for gastrointestinal disorders:
Effectiveness and mechanisms. Nat Rev Gastroenterol Hepatol.
16:89–105. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen JD, Yin J and Wei W: Electrical
therapies for gastrointestinal motility disorders. Expert Rev
Gastroenterol Hepatol. 11:407–418. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Perry KA, Pham TH, Spechler SJ, Hunter JG,
Melvin WS and Velanovich V: 2014 SSAT State-of-the-Art Conference:
Advances in diagnosis and management of gastroesophageal reflux
disease. J Gastrointest Surg. 19:458–466. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ji T, Li X, Lin L, Jiang L, Wang M, Zhou
X, Zhang R and Chen JDz: An alternative to current therapies of
functional dyspepsia: Self-administrated transcutaneous
electroacupuncture improves dyspeptic symptoms. Evid Based
Complement Alternat Med. 2014(832523)2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Dinning PG, Hunt L, Patton V, Zhang T,
Szczesniak M, Gebski V, Jones M, Stewart P, Lubowski DZ and Cook
IJ: Treatment efficacy of sacral nerve stimulation in slow transit
constipation: A two-phase, double-blind randomized controlled
crossover study. Am J Gastroenterol. 110:733–740. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Moore JS, Gibson PR and Burgell RE:
Neuromodulation via interferential electrical stimulation as a
novel therapy in gastrointestinal motility disorders. J
Neurogastroenterol Motil. 24:19–29. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
49
|
Miller L, Farajidavar A and Vegesna A: Use
of bioelectronics in the gastrointestinal tract. Cold Spring Harb
Perspect Med. 9(a034165)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Marti L, Galata C, Beutner U, Hetzer F,
Pipitone N, Wolff K, Borovicka J, Brunner W, Sulz MC and Maurus C:
Percutaneous tibial nerve stimulation (pTNS): Success rate and the
role of rectal capacity. Int J Colorectal Dis. 32:789–796.
2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bonaz B, Sinniger V and Pellissier S: The
vagus nerve in the neuro-immune axis: Implications in the pathology
of the gastrointestinal tract. Front Immunol.
8(1452)2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bilgutay AM, Wingrove R, Griffen WO,
Bonnabeau RC Jr and Lillehei CW: Gastro-intestinal pacing: A new
concept in the treatment of Ileus. Ann Surg. 158:338–348.
1963.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kelly KA and Code CF: Duodenal-gastric
reflux and slowed gastric emptying by electrical pacing of the
canine duodenal pacesetter potential. Gastroenterology. 72:429–433.
1977.PubMed/NCBI
|
|
54
|
Familoni BO, Abell TL, Nemoto D, Voeller G
and Johnson B: Efficacy of electrical stimulation at frequencies
higher than basal rate in canine stomach. Dig Dis Sci. 42:892–897.
1997.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yin J, Abell TD, McCallum RW and Chen JD:
Gastric neuromodulation with Enterra system for nausea and vomiting
in patients with gastroparesis. Neuromodulation. 15:224–231.
2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Li YQ, Zhu B, Rong PJ, Ben H and Li YH:
Neural mechanism of acupuncture-modulated gastric motility. World J
Gastroenterol. 13:709–716. 2007.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Huang Z, Zhang N, Xu F, Yin J, Dai N and
Chen JD: Ameliorating effect of transcutaneous electroacupuncture
on impaired gastric accommodation induced by cold meal in healthy
subjects. J Gastroenterol Hepatol. 31:561–566. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Pang T, Lu C, Wang K, Liang C, Yu Z, Zhu B
and Xu B: Electroacupuncture at ST25 inhibits cisapride-induced
gastric motility in an intensity-dependent manner. Evid Based
Complement Alternat Med. 2016(3457025)2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhang CX and Guo LK: Dalitong granule
combined with electroacupuncture in the treatment of functional
dyspepsia: A randomized controlled trial. Chin J Integr Med.
21:743–750. 2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Huang W, Long W, Xiao J, Zhao G and Yu T:
Effect of electrically stimulating acupoint, Zusanli (ST 36), on
patient's recovery after laparoscopic colorectal cancer resection:
A randomized controlled trial. J Tradit Chin Med. 39:433–439.
2019.PubMed/NCBI
|
|
61
|
Yang ZK, Wu ML, Xin JJ, He W, Su YS, Shi
H, Wang XY, Hu L, Jing XH and Litscher G: Manual acupuncture and
laser acupuncture for autonomic regulations in rats: Observation on
heart rate variability and gastric motility. Evid Based Complement
Alternat Med. 2013(276320)2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhang J and Chen JD: Systematic review:
Applications and future of gastric electrical stimulation. Aliment
Pharmacol Ther. 24:991–1002. 2006.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zhang B, Xu F, Hu P, Zhang M, Tong K, Ma
G, Xu Y, Zhu L and Chen JDZ: Needleless transcutaneous electrical
acustimulation: A pilot study evaluating improvement in
post-operative recovery. Am J Gastroenterol. 113:1026–1035.
2018.PubMed/NCBI View Article : Google Scholar
|