Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a
devastating disease with a 5-year survival rate of <5% (1,2). Novel
complementary treatments are warranted since current treatments are
often inefficient and are associated with severe side-effects
(3). Data from epidemiological
studies have suggested positive therapeutic effects using cardiac
glycosides in the treatment of various types of cancer, and may
thus prove to be promising as a complementary treatment to current
PDAC therapeutics (4-7).
Cardiac glycosides are natural compounds most well
known for their cardiovascular effects. Digitoxin is one of the
most extensively used cardiac glycosides and it inhibits the
membrane receptor Na+/K+-ATPase by binding to
its α-subunit (8). The
Na+/K+-ATPase consists of three parts, the
β-subunit β1-3, the α-subunit with its four isoforms α1-4
(ATP1A1-4), and accessory proteins FXYD 1-7(9). The α- and β-subunits are differentially
expressed depending on the type of tissue (10). ATP1A1 is expressed in almost all
tissues (11), ATP1A2 is mainly
expressed in the heart, brain and skeletal muscles, ATP1A3 mostly
in nervous and muscle tissue, and ATP1A4 is only expressed in
spermatozoa (12,13). Binding experiments with various
cardiac glycosides have demonstrated that ATP1A2 and ATP1A3 have a
higher affinity to digitoxin than ATP1A1 (9,10,14). A
higher affinity indicates a more effective blockage by digitoxin.
The values for digitoxin binding constants, dissociation constant
(KD; inverse to affinity), are 38 nM for ATP1A1
and 14 nM for ATP1A3(9).
The blockage of the
Na+/K+-ATPase pumping function leads to an
increase in the intracellular concentration of sodium ions which,
in turn, forces the Na+/Ca2+-exchanger (NCX)
to transport Na+ out of the cell and Ca2+
into the cell (15). As a result,
the intracellular Ca2+ concentrations increase (16). In non-excitable cells under basal
conditions, the intracellular concentrations of Ca2+ are
maintained at a low homeostatic level, with a balance of influx and
efflux of Ca2+ (17).
Maintaining a low intracellular concentration of calcium
(Ca2+) is essential for the majority of eukaryotic
cells, since a high persistent intracellular concentrations of
Ca2+ is harmful and trigger apoptosis (18).
A deregulation of the α-subunits is often observed
in cancer cells, with an upregulation of ATP1A3 and the
downregulation of ATP1A1, such as in colorectal cancer and renal
cell carcinoma (13,19). Cancer cells have been shown to be
more sensitive to digitoxin than normal cells, where nanomolar
concentrations of digitoxin induce the apoptosis of cancer cells
(8,20-22).
It was thus hypothesized that the increased sensitivity of cancer
cells is dependent on the altered expression of the α-subunits of
Na+/K+-ATPase.
To investigate this hypothesis, the present study we
used three well-characterized commercially available pancreatic
cancer cell lines (AsPC-1, Panc-1 and CFPAC-1). The basal gene
expression of the three α-subunit isoforms of
Na+/K+-ATPase, ATP1A1, ATP1A2
and ATP1A3, as well as the protein levels of ATP1A1 and
ATP1A3 were measured. The effects of digitoxin treatment at 1-100
nM (therapeutic concentrations, 10-40 nM) were evaluated by
examining the changes in the expression of ATP1A1 of ATP1A3,
intracellular Ca2+ levels and cell viability.
Materials and methods
Cells and cell culture
The present study used three pancreatic cancer cell
lines, AsPC-1 (CRL-1682), Panc-1 (CRL-1469) and CFPAC-1 (CRL-1918)
(ATCC; LGC Standards GmbH). The AsPC-1 cells were grown in
RPMI-1640 medium supplemented with 1% HEPES and 1% sodium pyruvate,
the Panc-1 cells in Dulbecco's modified Eagle's medium (DMEM) with
1% L-glutamine, and the CFPAC-1 cells in DMEM. Media were also
supplemented with 10% FBS and 1% penicillin-streptomycin (PEST).
All cell culture media and reagents were purchased from
MilliporeSigma. All incubations were performed at 37˚C in 5%
CO2. PCR was used to test the cell lines for mycoplasma
infection (using the LookOut® Mycoplasma PCR Detection
kit; cat. no. MP0035, MilliporeSigma).
Seeding and treatment with
digitoxin
For digitoxin (cat. no. D5878, MilliporeSigma)
treatment, 5,000 cells were seeded in 100 µl complete growth medium
in 96-well plates and incubated at 3˚C with 5% CO2 for
20 h to a sub-confluent monolayer. Following 20 h of incubation,
new media (100 µl) containing digitoxin was added to the cells. The
control cells only received new media. The cells were further
incubated at 37˚C with 5% CO2 for 48 h. The
concentrations of digitoxin used in all experiments were as
follows: 0 nM (controls), and 1, 10, 25, 40 and 100 nM (human
therapeutic range, 10-40 nM).
Cell viability assay
The analysis of cell viability was performed using
the colorimetric method CellTiter 96® AQueous One
Solution Cell Proliferation assay (MTS) assay (Promega
Corporation). Following 48 h of incubation at 37˚C with 5%
CO2 with digitoxin, 20 µl MTS tetrazolium were added to
each well and incubated for 1 h at 37˚C with 5% CO2.
Viable cells metabolize tetrazolium to formazan, which was measured
at 490 nm (FLUOstar Omega, BMG Labtech). The obtained value is
directly proportional to the number of viable cells in the cell
culture.
Intracellular Ca2+
assay
Fluo-4 assay (Ca2+; Abcam) was used to
determine the intracellular Ca2+concentrations following
treatment with digitoxin. The cells were seeded and treated for 48
h with digitoxin at 37˚C with 5% CO2 in triplicate.
Following treatment, 100 µl of Fluo-4 AM dye-loading solution were
added to each well and incubated for 1 h. The fluorescence
intensity was measured at Ex/Em 485/520 (FLUOstar Omega, BMG
Labtech).
RNA-extraction and reverse
transcription-quantitative PCR
The cells were seeded at a density of
1.5x105 in 2.4 ml medium in six-well plates and
incubated at 37˚C in 5% CO2 to a sub-confluent
monolayer. After 20 h, the old medium was removed and new medium
with digitoxin was added. The control cells only received new
media. Following 48 h of treatment, RNA was extracted using a
RNeasy Mini kit (cat. no. 74104, Qiagen GmbH), according to the
supplier's protocol. A total 1 µg RNA from each sample was used for
cDNA synthesis using High-Capacity cDNA Reverse Transcription Kit
Reagent (25˚C for 10 min, 37˚C for 120 min, 85˚C for 5 min)
according to the manufacturer's manual (Thermo Fisher Scientific,
Inc.).
Complementary DNA (cDNA) corresponding to 5 ng RNA
was used in each qPCR reaction performed in duplicate for the
following TaqMan target transcripts: ATP1A1 (Hs00167556_m1),
ATP1A2 (Hs00265131_m1) and ATP1A3 (Hs00958036_m1)
using TaqMan™ Gene Expression Master Mix (4369016, Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: initial denaturation at 95˚C for 10
min, followed by 40 cycles of 95˚C for 15 sec and 60˚C for 1 min.
Quantitative gene expression data were normalized to the expression
level of the human reference gene, phosphomannomutase 1
(PMM1; Hs00963626_m1). The ΔCq values was used to analyze
the relative expression between the genes ATP1A1 and
ATP1A3 for each cell line (23). All qPCR reactions were performed on a
Pikoreal qPCR System (Thermo Fisher Scientific, Inc.).
Protein extraction and western blot
analysis
The cells were seeded at a quantity of
5x105 cells per 75 cm2 flask and incubated
for 20 h in 37˚C in 5% CO2. Thereafter, the old medium
was removed and new medium with digitoxin was added. The controls
only received new, complete medium. Following 48 h of treatment,
protein extraction was performed and the cells were lysed in lysis
buffer (cat. no. FNN0011, Thermo Fisher Scientific, Inc.)
supplemented with phenylmethylsulphonyl fluoride (PMSF; cat. no.
36978, Thermo Fisher Scientific, Inc.) and protein inhibitor
cocktail (cat. no. P2714, MilliporeSigma). The protein
concentration was determined using the Pierce™ BCA Protein Assay
kit (Thermo Fisher Scientific, Inc.).
From each sample, 10 µg of total protein were
separated by sodium dodecyl sulfate-polyacrylamide electrophoresis
in 8-16%, stain free gel (Bio-Rad Laboratories Inc.), Proteins were
later blotted onto PVDF membranes, blocked in Tris-buffered saline
with 0.1% Tween-20 (TBST) with 5% milk in 20˚C for 1 h, incubated
with either ATP1A1 and ATP1A3 primary antibodies (1:1,000; cat.
nos. MA1-16731 and MA3-915, Invitrogen; Thermo Fisher Scientific,
Inc.) for 1 h in room temperature. After washing with TBST three
times, the membranes were further incubated with the secondary
antibody, Alexa Fluor Plus 555 (1:2,500; cat. no. A32727,
Invitrogen; Thermo Fisher Scientific, Inc.). The protein expression
was measured using the ChemiDoc System (Bio-Rad Laboratories Inc.),
densitometric analysis of single and total protein expression was
performed using Image Lab Software ver. 6.1 (Bio-Rad Laboratories,
Inc.). Protein expression was normalized to the total protein for
each sample, total protein normalization (Fig. S1, Fig.
S2, Fig. S3 and Fig. S4).
Statistical analysis
The used assays are based on absorbance and
fluorescence in microplate format and RT-qPCR. Biological and
technical replicates were three or more, (two technical replicates
for RT-qPCR ). Statistical analysis was performed using IBM SPSS
Statistics 27 software (IBM Corp.) and one-way analysis of variance
(ANOVA) followed by the Bonferroni correction, to confirm
significant differences between treated vs. untreated cells (i.e.,
control) in each assay. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Digitoxin within the therapeutic range
exerts an effect on cell viability and intracellular
Ca2+ concentrations in Panc-1 and CFPAC-1 cells, but not
in AsPC-1 cells
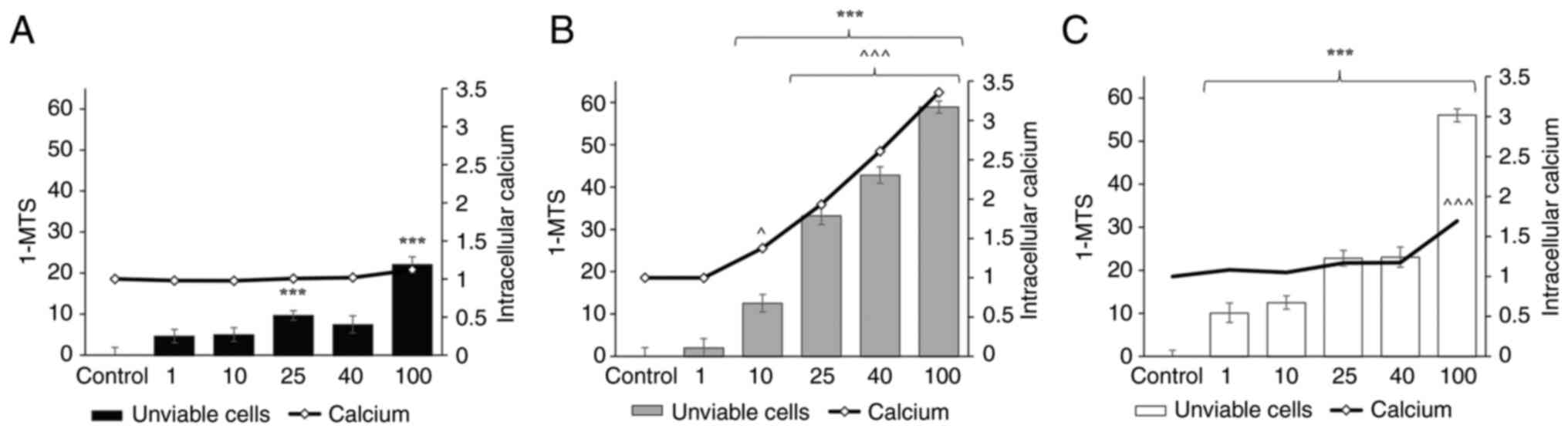
The AsPC-1 cell line was less affected by digitoxin
compared to the Panc-1 and CFPAC-1 cells, with only a significant
effect observed on cell viability in the AsPC-1 treated with 25 and
100 nM digitoxin (Fig. 1A).
Digitoxin at concentrations ranging from 10-100 nM decreased the
viability of the Panc-1 cells following 48 h of treatment and also
that of the CFPAC-1 cells at concentrations ranging from 1-100 nM.
For the Panc-1 cells treated with digitoxin at the concentration of
10 nM, the number of viable cells decreased (number of unviable
cells, 12.6±1.15%, P<0.001) compared to the control cells.
Within the therapeutic range in humans (25-40 nM), the viable cell
number was decreased even further (number of unviable cells at 40
nM: Panc-1 cells, 43.8±1.15%, P<0.001; CFPAC-1 cells,
23.0±1.16%; P<0.001) compared to the controls (Fig. 1B and C).
The intracellular Ca2+ concentrations
increased in a concentration-dependent manner following 48 h of
treatment with digitoxin in the Panc-1 cells. Digitoxin at a
concentration of 25 nM led to a 2-fold increase (±0.04, P<0.001)
in intracellular Ca2+ levels in the Panc-1 cells. The
digitoxin concentrations of 40 and 100 nM increased the
intracellular Ca2+ levels 2.6-fold (±0.04, P<0.001)
and 3.4-fold (±0.04, P<0.001), respectively in Panc-1 cells
(Fig. 1B). In the Panc-1 cells, a
marked increase in intracellular Ca2+ levels were
observed, while the CFPAC-1 cells only exhibited a significant
increase in intracellular Ca2+ levels following
treatment with digitoxin at 100 nM (±0.03, P<0.001) (Fig. 1C). In the AsPC-1 cells, the
concentration of intracellular Ca2+ was not markedly
altered following treatment with digitoxin (Fig. 1A).
Expression of ATP1A3 is high in Panc-1
and CFPAC-1 cells, but not in AsPC-1 cells
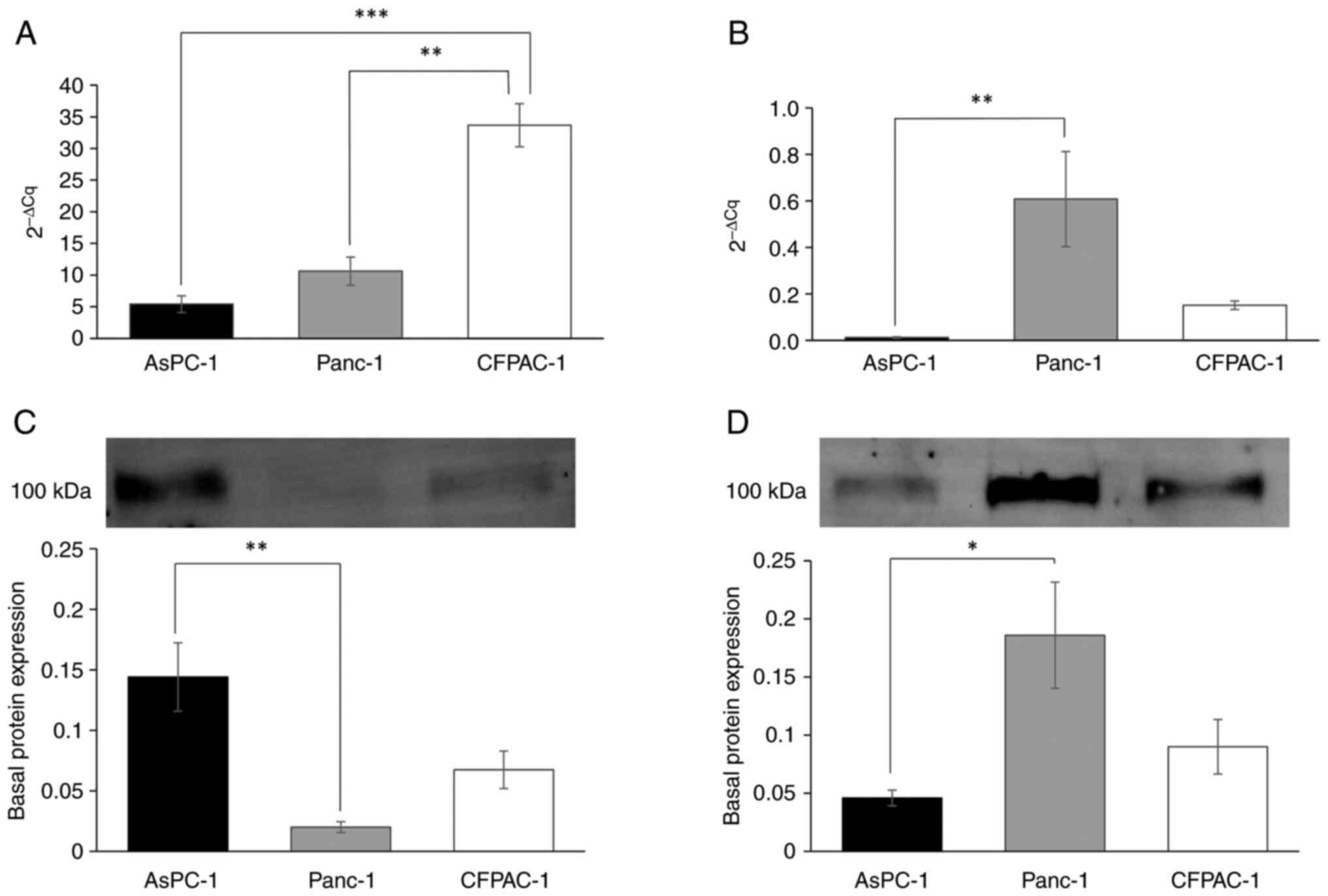
The basal transcriptional expression of
ATP1A1 was low in AsPC-1 and Panc-1 cells, and 6-fold higher
in the CFPAC-1 cells compared to the AsPC-1 cells (Fig. 2A). The ATP1A3 subunit in
Panc-1 cells was transcriptionally expressed >50-fold higher
(P<0.001) compared to the AsPC-1 cells, and 4-fold times higher
than the CFPAC-1 cells (Fig. 2B). At
the protein level, the AsPC-1 cells exhibited a significant ~7-fold
higher expression of ATP1A1 compared to the Panc-1 cells (±0.01,
P=0.002, AsPC-1 cells) (Fig. 2C).
The Panc-1 cells exhibited a significantly higher expression
(4-fold) of ATP1A3 compared to the AsPC-1 cells (±0.01, P=0.022),
while the CFPAC-1 cells had a 2-fold higher protein expression of
ATP1A3 compared to the AsPC-1 cells (Fig. 2D).
Digitoxin treatment affects the gene
and protein expression of ATP1A1 and ATP1A3 in Panc-1 cells
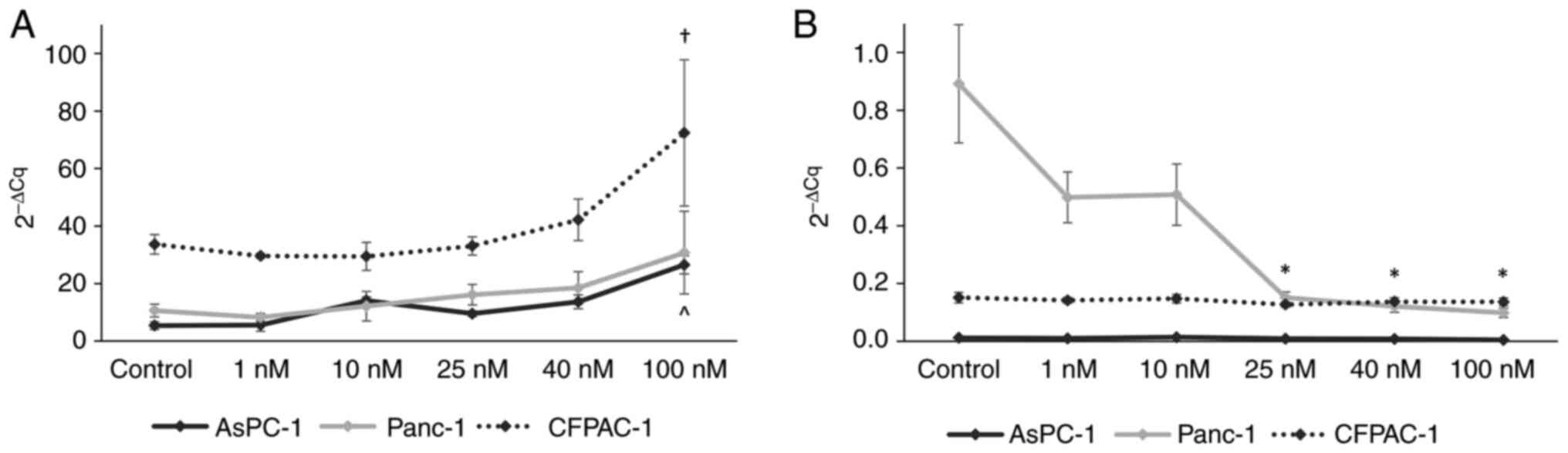
A one-way between-groups ANOVA was conducted to
explore the effects of digitoxin treatment on ATP1A1 and
ATP1A3 gene expression levels. Significant differences were
found for ATP1A expression in the AsPC-1 cells [F(5,
12)=14.71, P<0.001] and CFPAC-1 cells [F(5, 11)=7.16, P=0.003].
No effects on ATP1A1 gene expression were observed in the
Panc-1 cells; instead a change in the expression of the
ATP1A3 gene was observed with digitoxin treatment. A
decrease in ATP1A3 expression with the increasing digitoxin
concentration was confirmed in the Panc-1 cells [F(5, 12)=5.51,
P=0.007]. In order to analyze the effects of each digitoxin
concentration on ATP1A1 and ATP1A3 expression
separately, post hoc comparisons using the Bonferroni correction
were made. The analysis revealed a significant difference in
ATP1A1 expression in the AsPC-1 cells with digitoxin
treatment at 100 nM (±0.172, P<0.001) (Fig. 3A). In the CFPAC-1 cells, treatment
with digitoxin at 100 nM led to a significant increase in
ATP1A1 gene expression (±3.474, P=0.011) (Fig. 3A). In the Panc-1 cells, no
significant effects on ATP1A1 gene expression were observed
for the individual digitoxin concentrations; however, there was a
significant decrease in ATP1A3 gene expression with various
digitoxin concentrations (25 nM: ±0.059, P=0.034; 40 nM: ±0.059,
P=0.024; 100 nM: ±0.059, P=0.018) (Fig.
3B). ATP1A3 expression was not markedly altered by
digitoxin either in the AsPC-1 or in the CFPAC-1 cells (Fig. 3B).
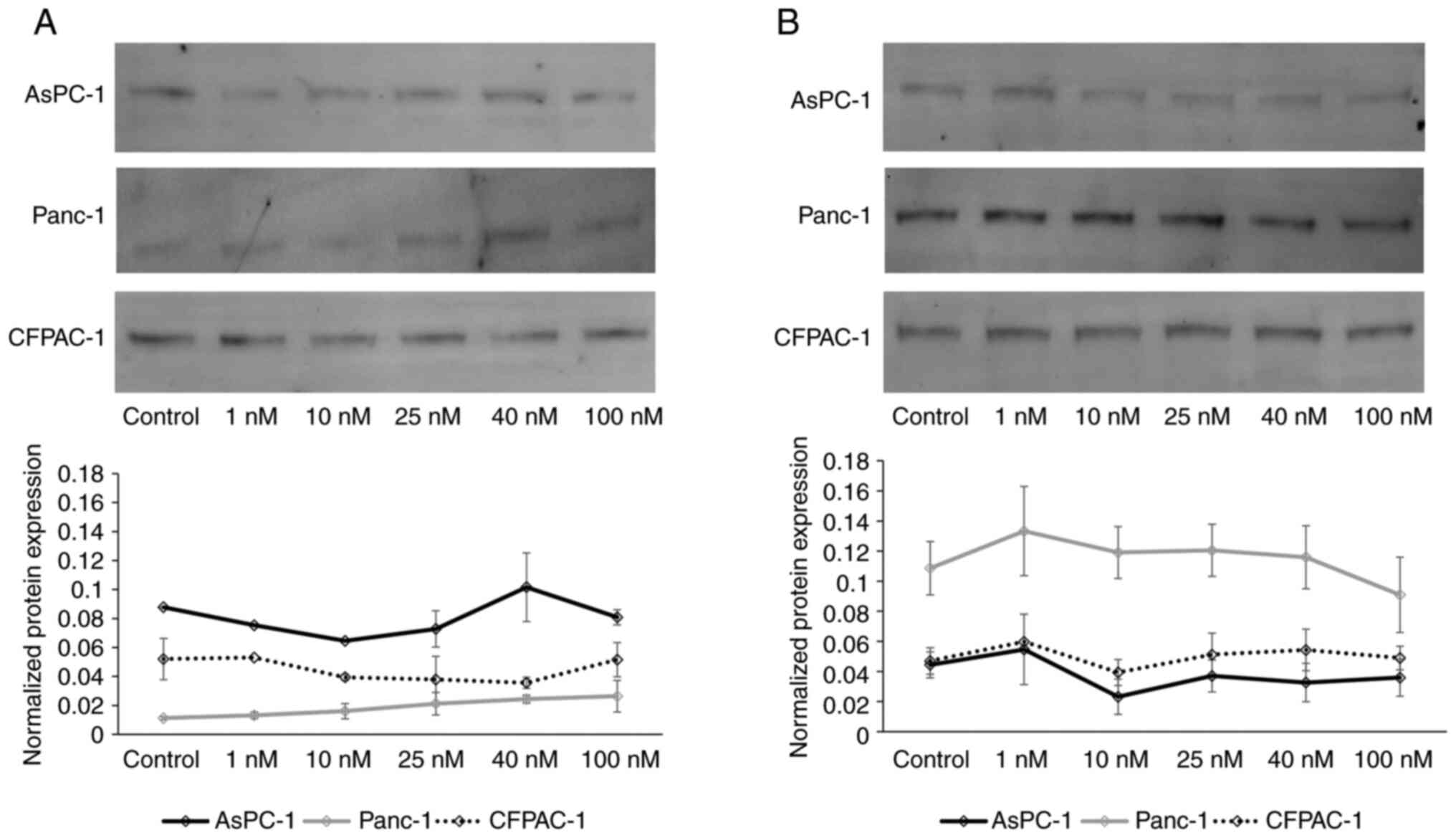
Western blot analysis of ATP1A1 and ATP1A3 was
performed following treatment with digitoxin to evaluate the
effects at the protein level (Fig.
4). A one-way between-groups ANOVA was conducted to explore the
effects of digitoxin treatment on ATP1A1 and ATP1A3 protein
expression. The results revealed slightly increased protein levels
of ATP1A1, and decreased protein levels of ATP1A3 in the Panc-1
cells following treatment, although with no significant differences
(Fig. 4).
Discussion
The cardiac glycoside, digitoxin, is an extensively
used drug in the treatment of cardiovascular diseases (15), and when used within the therapeutic
range of 25-40 nM, no major side-effects have been observed in
normal cells (24,25). The present study investigated a
potentially novel use of this already established drug for the
treatment of pancreatic cancer, a disease which remains very
difficult to cure with current treatments (26,27).
Previous studies have demonstrated a reduction in cell viability in
several cancer cell lines following treatment with cardiac
glycosides (20,27), and the results from the present study
with digitoxin treatment in the pancreatic cancer cell lines,
AsPC-1, Panc-1 and CFPAC-1, confirm that digitoxin may be useful as
a potential treatment for subgroups of patients with pancreatic
cancer.
The hypothesis behind the present study relies on
the fact that the membrane receptor
Na+/K+-ATPase isoform ATP1A3 has a higher
affinity for digitoxin than the ATP1A1 isoform, which suggests that
a higher expression of ATP1A3 compared to ATP1A1 would provide a
more effective blockage of the Na+/K+-ATPase
by digitoxin (14). A change in
expression of the α-subunits ATP1A1 and ATP1A3 is observed in
certain tumors compared to normal tissue. In a study on human
colorectal carcinoma, colorectal cancer tissue was compared with
normal mucosa, and it was found that ATP1A1 was
transcriptionally downregulated in cancer tissues and that
ATP1A3 expression was upregulated (13). Banerjee et al (28) also demonstrated a decreasing
expression of ATP1A1 in primary tumors and metastases compared to
the normal tissue. The altered expression of these two isoforms is
probably the reason for an increased sensitivity to digitoxin
treatment, considering the higher affinity of digitoxin to the
ATP1A3 isoform (9). In another
study, an overall downregulation in
Na+/K+-ATPase activity was observed in
colorectal cancer cells following digitoxin treatment (13), and an unregulated increase in
intracellular Ca2+ levels induced the apoptosis of HeLa
cells (29).
In the present study, the data suggested a close
association between the fraction of unviable cells and
intracellular Ca2+ concentrations in all three
pancreatic cancer cell lines. However, there was a major difference
in the response to digitoxin within the therapeutic range (25-40
nM) between the different cell lines, where the Panc-1 cells
exhibited a marked response, with a notable decrease in viability
and a marked increase in the intracellular Ca2+ levels,
while AsPC-1 cell viability was only slightly affected by digitoxin
at these concentrations.
There is a notable difference in characteristics
between the cell lines in the present study with regards to their
basal levels of ATP1A1 and ATP1A3 gene and protein
expression. Since the Panc-1 cells had a very high basal
expression of ATP1A3, and a lower expression of ATP1A1 compared to
the other two cell lines, it was hypothesized that the blockage of
Na+/K+-ATPase by digitoxin in this cell line
was dependent on the relative high expression of ATP1A3. The more
effective blockage of ATP1A3 by digitoxin led to a notable increase
of the intracellular Ca2+concentrations in the Panc-1
cells. The CFPAC-1 cells with a relatively higher expression of
ATP1A1 and a lower expression of ATP1A3 compared to the Panc-1
cells exhibited only a significant increase in intracellular
Ca2+ levels with the supratherapeutic concentration of
digitoxin (100 nM). No change in the intracellular
Ca2+concentrations was found in the AsPC-1 cells. The
AsPC-1 cell line had a high ATP1A1 expression and a low ATP1A3
expression, which further underlines the importance of the
expression levels of these subunits.
The Panc-1 cells exhibited a response to digitoxin
affecting the gene expression of the
Na+/K+-ATPase α-subunit ATP1A3, which
decreased in the cells treated with 25-100 nM digitoxin. This
effect is possibly a protective cell survival mechanism. In the
AsPC-1 and CFPAC-1 cells, only the gene expression of ATP1A1
was affected (increased expression) by treatment with 100 nM
digitoxin. Cells are heavily dependent on the function of
Na+/K+-ATPases for the intracellular ion
homeostasis, blocking these pumps with drugs or siRNA trigger the
cells to produce more Na+/K+-ATPases to
compensate (28).
The sensitivity to digitoxin may be explained by
this difference in the expression of the
Na+/K+-ATPase α-subunit isoforms between the
cell lines. A high ATP1A3 and low ATP1A1 expression corresponded
with a high sensitivity to digitoxin treatment, considering the
decrease in cell viability. An increase in ATP1A1 expression and/or
a decrease in ATP1A3 expression may be a way for the cells to
rescue the blockage of the ATP1A3 subunits in the
Na+/K+-ATPase by digitoxin. These results are
correlative and further studies on the mechanisms behind the
difference in responses of the different ATP1A isoforms are
warranted.
In conclusion, the present study demonstrated the
potential of digitoxin as an anticancer agent for a subset of
pancreatic cancers. A very high anticancer efficacy of digitoxin
was observed in pancreatic cancer cells with a high expression of
the ATP1A3 subunit of the Na+/K+-ATPase,
compared to the cells that had a low expression of the ATP1A3
Na+/K+-ATPase subunit. Thus, this may be
useful as a marker for effective digitoxin treatment.
Supplementary Material
Basal protein expression of ATP1A1 and
ATP1A3. Protein standard (Precision Plus Protein™ All
Blue Prestained Protein Standards; cat. no. 1610373, Bio-Rad
Laboratories, Inc.). (A) Total protein for normalization of protein
expression of ATP1A1, (B) Total protein for normalization of
protein expression of ATP1A3. (C) Protein expression of ATP1A1, and
(D) protein expression of ATP1A3. ATP1A1 and ATP1A3,
Na+/K+-ATPase alpha subunits 1 and 3.
Protein expression of ATP1A1 and
ATP1A3 in AsPC-1 cells and the control with digitoxin treatment.
Protein standard (Precision Plus Protein™ All Blue
Prestained Protein Standards; cat. no. 1610373, Bio-Rad
Laboratories, Inc.). (A) Total protein for normalization of protein
expression of ATP1A1, (B) Total protein for normalization of
protein expression of ATP1A3, (C) protein expression of ATP1A1, and
(D) protein expression of ATP1A3. ATP1A1 and ATP1A3,
Na+/K+-ATPase alpha subunits 1 and 3.
Protein expression of ATP1A1 and
ATP1A3 in Panc-1 cells and the control and with digitoxin
treatment. Protein standard (Precision Plus Protein™ All
Blue Prestained Protein Standards; cat. no. 1610373, Bio-Rad
Laboratories, Inc.). (A) Total protein for normalization of protein
expression of ATP1A1, (B) Total protein for normalization of
protein expression of ATP1A3, (C) protein expression of ATP1A1, and
(D) protein expression of ATP1A3. ATP1A1 and ATP1A3,
Na+/K+-ATPase alpha subunits 1 and 3.
Protein expression of ATP1A1 and
ATP1A3 in CFPAC-1 cells and the control and with digitoxin
treatment. Protein standard (Precision Plus Protein™ All
Blue Prestained Protein Standards; cat. no. 1610373, Bio-Rad
Laboratories, Inc.). (A) Total protein for normalization of protein
expression of ATP1A1, (B) Total protein for normalization of
protein expression of ATP1A3, (C) protein expression of ATP1A1, and
(D) protein expression. Of ATP1A3. ATP1A1 and ATP1A3,
Na+/K+-ATPase alpha subunits 1 and 3.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL has performed the experiments, statistical
analysis and drafted the manuscript. HL and FS designed the study
and performed the data interpretation. FS contributed with
manuscript writing and critical editing. KE contributed to the
study methodology, manuscript writing and critical editing. HL and
FS confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ansari D, Gustafsson A and Andersson R:
Update on the management of pancreatic cancer: Surgery is not
enough. World J Gastroenterol. 21:3157–3165. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Becker AE, Hernandez YG, Frucht H and
Lucas AL: Pancreatic ductal adenocarcinoma: Risk factors,
screening, and early detection. World J Gastroenterol.
20:11182–11198. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pereira NP and Corrêa JR: Pancreatic
cancer: Treatment approaches and trends. J Cancer Metastasis Treat.
4(30)2018.
|
|
4
|
Frankel AE, Eskiocak U, Gill JG, Yuan S,
Ramesh V, Froehlich TW, Ahn C and Morrison SJ: Digoxin plus
trametinib therapy achieves disease control in BRAF wild-type
metastatic melanoma patients. Neoplasia. 19:255–260.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Coleman DT, Gray AL, Stephens CA, Scott ML
and Cardelli JA: Repurposed drug screen identifies cardiac
glycosides as inhibitors of TGF-β-induced cancer-associated
fibroblast differentiation. Oncotarget. 7:32200–32209.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kepp O, Menger L, Vacchelli E, Adjemian S,
Martins I, Ma Y, Sukkurwala AQ, Michaud M, Galluzzi L, Zitvogel L
and Kroemer G: Anticancer activity of cardiac glycosides: At the
frontier between cell-autonomous and immunological effects.
Oncoimmunology. 1:1640–1642. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Haux J: Digitoxin has specific properties
for potential use to treat cancer and inflammatory diseases.
Research and Reviews on Healthcare: Open Access Journal 2:
2018.
|
|
8
|
Lopez-Lazaro M: Digitoxin as an anticancer
agent with selectivity for cancer cells: Possible mechanisms
involved. Expert Opin Ther Targets. 11:1043–1053. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Katz A, Lifshitz Y, Bab-Dinitz E,
Kapri-Pardes E, Goldshleger R, Tal DM and Karlish SJD: Selectivity
of digitalis glycosides for isoforms of human Na,K-ATPase. J Biol
Chem. 285:19582–19592. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Newman RA, Yang P, Pawlus AD and Block KI:
Cardiac glycosides as novel cancer therapeutic agents. Mol Interv.
8:36–49. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Mijatovic T and Kiss R: Cardiotonic
steroids-mediated Na+/K+-ATPase targeting could circumvent various
chemoresistance pathways. Planta Med. 79:189–198. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Clausen MV, Hilbers F and Poulsen H: The
structure and function of the Na,K-ATPase isoforms in health and
disease. Front Physiol. 8(371)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sakai H, Suzuki T, Maeda M, Takahashi Y,
Horikawa N, Minamimura T, Tsukada K and Takeguchi N: Up-regulation
of Na+,K+-ATPase α3-isoform and down-regulation of the α1-isoform
in human colorectal cancer. FEBS Lett. 563:151–154. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Noël F, Fagoo M and Godfraind T: A
comparison of the affinities of rat (Na+ + K+)-ATPase isozymes for
cardioactive steroids, role of lactone ring, sugar moiety and KCl
concentration. Biochem Pharmacol. 40:2611–2616. 1990.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Arispe N, Diaz JC, Simakova O and Pollard
HB: Heart failure drug digitoxin induces calcium uptake into cells
by forming transmembrane calcium channels. Proc Natl Acad Sci.
105:2610–2615. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Menger L, Vacchelli E, Kepp O, Eggermont
A, Tartour E, Zitvogel L, Kroemer G and Galluzzi L: Trial watch:
Cardiac glycosides and cancer therapy. Oncoimmunology.
2(e23082)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Contreras L, Drago I, Zampese E and Pozzan
T: Mitochondria: The calcium connection. Biochim Biophys Acta.
1797:607–618. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Brini M and Carafoli E: The plasma
membrane Ca²+ ATPase and the plasma membrane sodium calcium
exchanger cooperate in the regulation of cell calcium. Cold Spring
Harb Perspect Biol. 3(a004168)2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang D, Zhang P, Yang P, He Y, Wang X,
Yang Y, Zhu H, Xu N and Liang S: Downregulation of ATP1A1 promotes
cancer development in renal cell carcinoma. Clin Proteomics.
14(15)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Calderón-Montaño JM, Burgos-Morón E, Orta
ML, Mateos S and López-Lázaro M: A hydroalcoholic extract from the
leaves of Nerium oleander inhibits glycolysis and induces selective
killing of lung cancer cells. Planta Med. 79:1017–1023.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang QF, Dalgard CL, Eidelman O, Jozwik C,
Pollard BS, Srivastava M and Pollard HB: Digitoxin induces
apoptosis in cancer cells by inhibiting nuclear factor of activated
T-cells-driven c-MYC expression. J Carcinog. 12(8)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lindholm H, Ejeskär K and Szekeres F:
Digitoxin affects metabolism, ROS production and proliferation in
pancreatic cancer cells differently depending on the cell
phenotype. Int J Mol Sci. 23(8237)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
López-Lázaro M, Pastor N, Azrak SS, Ayuso
MJ, Cortés F and Austin CA: Digitoxin, at concentrations commonly
found in the plasma of cardiac patients, antagonizes etoposide and
idarubicin activity in K562 leukemia cells. Leuk Res. 30:895–898.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bavendiek U, Berliner D, Dávila LA, Schwab
J, Maier L, Philipp SA, Rieth A, Westenfeld R, Piorkowski C, Weber
K, et al: Rationale and design of the DIGIT-HF trial (DIGitoxin to
Improve ouTcomes in patients with advanced chronic Heart Failure):
A randomized, double-blind, placebo-controlled study. Eur J Heart
Fail. 21:676–684. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Orth M, Metzger P, Gerum S, Mayerle J,
Schneider G, Belka C, Schnurr M and Lauber K: Pancreatic ductal
adenocarcinoma: Biological hallmarks, current status, and future
perspectives of combined modality treatment approaches. Radiat
Oncol. 14(141)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Varbanov HP, Kuttler F, Banfi D, Turcatti
G and Dyson PJ: Repositioning approved drugs for the treatment of
problematic cancers using a screening approach. PLoS One.
12(e0171052)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Banerjee M, Cui X, Li Z, Yu H, Cai L, Jia
X, He D, Wang C, Gao T and Xie Z: Na/K-ATPase Y260
phosphorylation-mediated src regulation in control of aerobic
glycolysis and tumor growth. Sci Rep. 8(12322)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gan H, Qi M, Chan C, Leung P, Ye G, Lei Y,
Liu A, Xue F, Liu D, Ye W, et al: Digitoxin inhibits HeLa cell
growth through the induction of G2/M cell cycle arrest and
apoptosis in vitro and in vivo. Int J Oncol.
57:562–573. 2020.PubMed/NCBI View Article : Google Scholar
|