Diabetes mellitus (DM) is a metabolic disease with
hyperglycemia, which is attributed to the defect of insulin
secretion or the impairment of its biological function (1). DM poses a severe threat to human
health, and often results in injuries to a number of target organs,
such as retinopathy, nephropathy, and peripheral neuropathy
(2). As with other organs, the lungs
are also a target organ of DM; the lung function of patients with
DM has been reported to be markedly decreased, and pulmonary
complications have been attested in patients with DM (3,4).
Tuberculosis is an ancient disease caused by infection with
Mycobacterium tuberculosis (5). As a major public health concern,
pulmonary tuberculosis (PTB) and DM pose a huge social burden in
China (6). It has been reported that
PTB-DM-associated comorbidity is high, and that the global
prevalence rate of PTB-DM comorbidity is estimated to be 13.73%
(7). Although the research
progresses of epidemiology, pathogenesis, and treatment management
for PTB-DM comorbidity have been summarized, the association
between PTB and DM has not been completely described. Therefore,
based on recent studies, the aim of the present review was to
discuss the epidemiology, pathogenesis, and therapeutic management
of patients with PTB-DM.
DM leads to an increased risk of developing PTB; an
early finding has suggested a link between PTB and DM, and an
increased prevalence of DM has been observed in patients with PTB
(8). The prevalence of PTB-DM
comorbidity is high among the Chinese elderly population (9). A study from Korea found that the
prevalence of PTB was significantly higher in patients with DM than
in the general population (10);
similar results were also demonstrated in Bangladesh (11). In Mexico, the prevalence of DM was
shown to have a significant influence on PTB morbidity, and the
incidence of PTB was increased by 82.64% in patients with DM, while
the incidence of PTB was decreased by 26.77% in patients without DM
(12). A recent study suggested a
positive association between DM morbidity and PTB in families with
tuberculosis (13). Stevenson et
al (14) found that DM was a
significant risk factor for the incidence of PTB, and that the
proportion of incident smear-positive tuberculosis was 20.2% due to
DM. The prevalence of DM was 18% in patients with PTB, while the
prevalence of DM was 8% in patients with suspected PTB (15). In a prospective study, Wang et
al (16) demonstrated that PTB
also increased the risk of developing DM, and that the prevalence
of DM in patients with PTB was higher than that in patients without
PTB. The prevalence of PTB and DM comorbidity has been summarized
in various studies (Table I). These
studies suggest a noteworthy association between PTB and DM as
regards epidemiology.
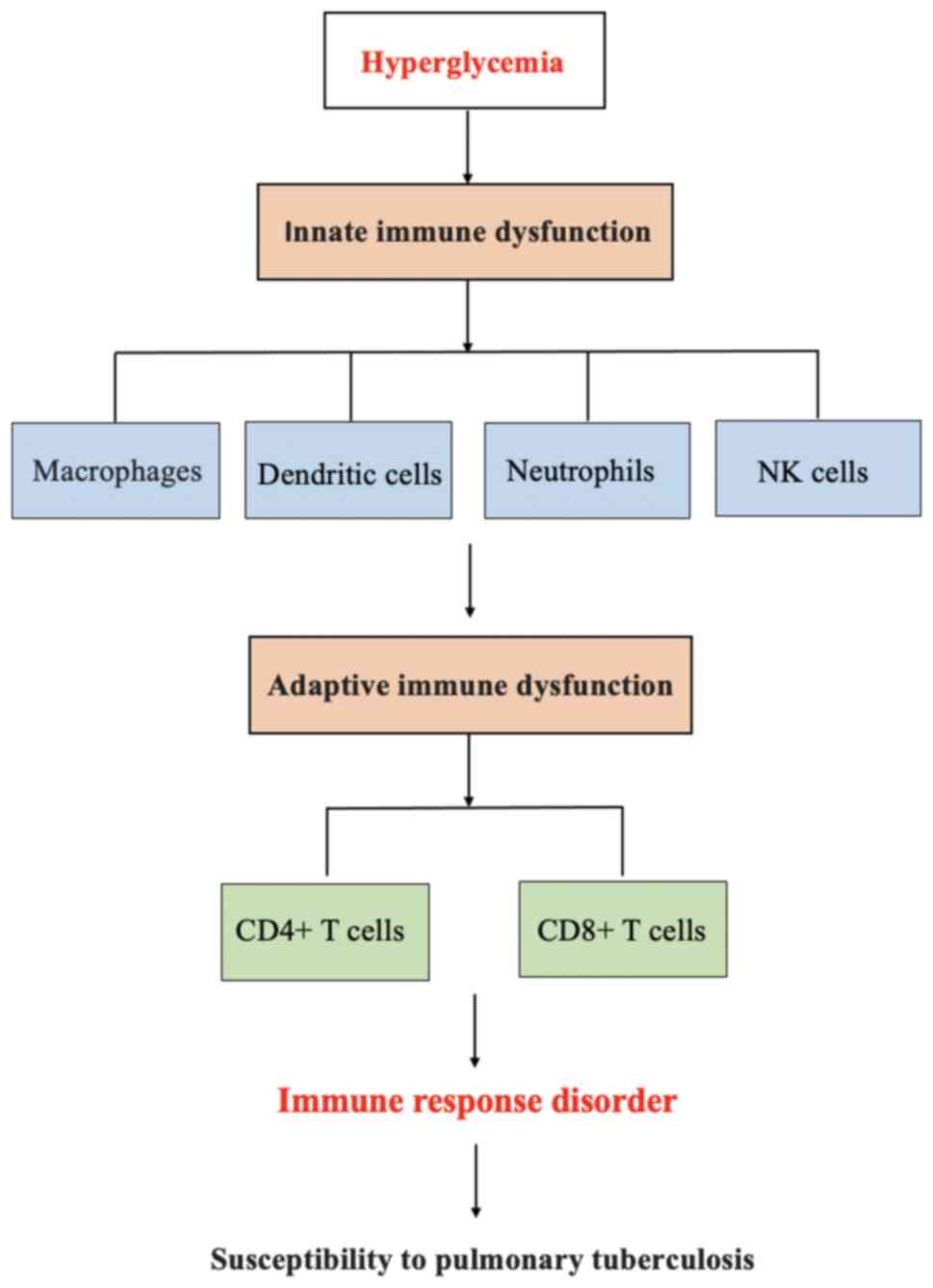
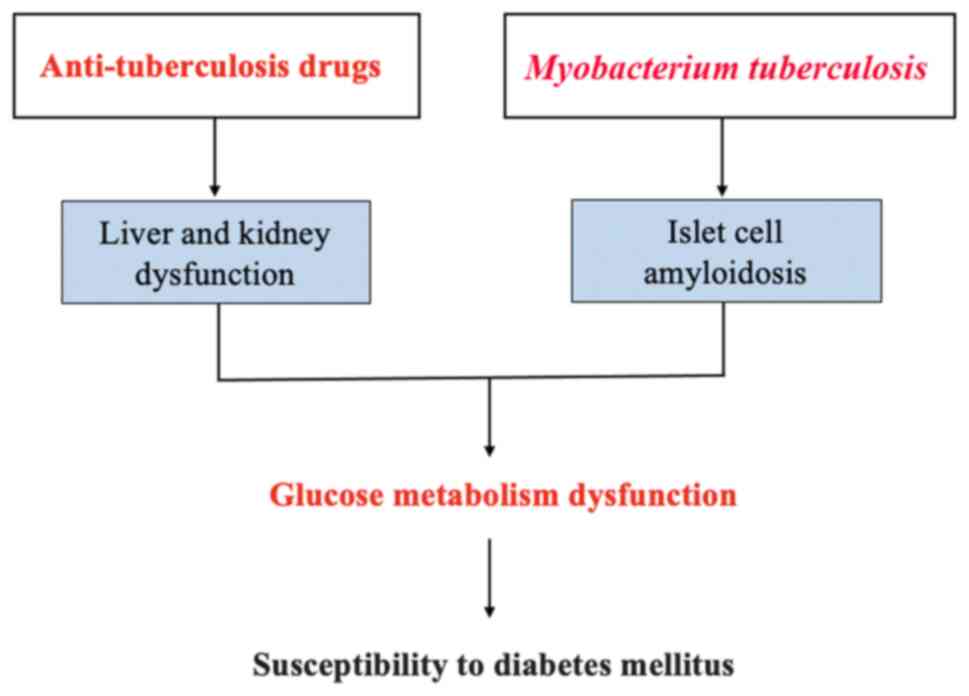
DM increases the risk of PTB by disrupting innate
and adaptive immunity responses. In turn, PTB weakens islet cell
function by causing islet amyloidosis, and anti-tuberculosis drugs
impair blood glucose homeostasis by affecting liver and kidney
functions in patients with PTB, which increases the risk of
developing DM in patients with PTB. The mechanisms of the
interaction between PTB and DM are presented in Figs. 1 and 2.
Macrophages are primary immune cells in the innate
immune response of PTB. An increased susceptibility to PTB occurs
due to a delayed innate immune response to the alveolar macrophages
in Mycobacterium tuberculosis infection in DM (17). A recent study found that
hyperglycemia affected the phagocytosis ability of macrophages by
the change of the expression pattern of recognition receptors in
PTB (18). In anti-tuberculosis
immunity, the phagocytosis ability of alveolar macrophages is
significantly decreased when human alveolar macrophages are
directly exposed to hyperglycemia (19). In a previous study on mouse with
diet-induced diabetes infected with Mycobacterium bovis, the
reduced uptake, killing, and production of inflammatory cytokines
in alveolar and peritoneal macrophages were observed, compared with
macrophages from non-diabetic mice (20). The expression of the macrophage
marker, CD14, and its receptors with collagen structure is reduced
in the alveolar macrophages of diabetic mice, promoting the
susceptibility of diabetic hosts to tuberculosis (21). In a study on diabetic mice with
Mycobacterium fortuitum infection, the mycobacterium load
was found to be significantly increased in the liver, spleen, and
lungs compared with controls, and the uptake of mycolic acid coated
beads was significantly reduced in macrophages isolated from
diabetic mice, indicating the decreased bacterial internalization,
killing, and cytokine responses of macrophages isolated from
diabetic mice (22). Evidently,
these studies suggest that the macrophage-mediated innate immune
response is a key pathological mechanism in PTB-DM comorbidity.
Dendritic cells exist in the lung parenchyma,
bronchoalveolar fluid, and nasal mucosa of humans, rats, and mice
(23). It has been suggested that
infection with Mycobacterium tuberculosis can induce
dendritic cells to mature and migrate into the draining lymph nodes
(24,25). The results from a clinical study
revealed that the frequency of plasmacytoid and myeloid dendritic
cells in PTB-DM comorbidity was decreased at baseline and at 2
months of anti-tuberculosis treatment compared to PTB without DM,
and that a significantly increased frequency was observed in
plasmacytoid and myeloid dendritic cells when the antituberculosis
treatments were successful completed, suggesting that DM may alter
the frequency of innate subset distribution of dendritic cells in
PTB-DM comorbidity (26). Kumar
et al (27) found that
coincidence DM resulted in a significant reduction in the frequency
of plasmacytoid and myeloid dendritic cells in PTB, and that DM
altered the distribution of dendritic cells in patients with active
and latent tuberculosis.
Neutrophils are another innate cell type that plays
a crucial role in the pathogenesis of PTB. Patients with PTB-DM
have an elevated peripheral neutrophil count compared to PTB
patients without DM (28).
Similarly, the neutrophil count of patients with DM infected with
Mycobacterium tuberculosis is higher than that in patients
without DM, and neutrophils exhibit a reduced phagocytic capacity
for Mycobacterium tuberculosis, suggesting an impaired
immune function of neutrophils for the tuberculosis in patients
with DM (29). Eruslanov et
al (30) reported that the
accumulation of neutrophils contributed to the development of
tuberculosis, indicating that the neutrophils may function as a
‘Trojan horse’ for mycobacterium.
Natural killer (NK) cells are effector cells in an
innate immunity response, and play an important role in the host
defense against mycobacterial infection (31). Zahran et al (32) suggested that the natural killer cell
count could be used as a marker to assess disease activity in
patients with PTB, and that the peripheral natural killer cell
count was a prognostic indicator for patients with active PTB. Of
note, the natural killer cells in both peripheral blood and
bronchoalveolar lavage have been reported to be significantly
increased in patients with PTB-DM compared with PTB patients
without DM (33). An increased
frequency of tuberculosis antigen-stimulated natural killer cells
expressing cytokines has also been observed in PTB-DM (34). Another study demonstrated that
natural killer cells promoted the pathological immune response in
the infection of Mycobacterium tuberculosis in diabetic mice
via NK-CD11c+ cell interactions (35).
The IFN-γ response of peripheral blood mononuclear
cells is decreased in PTB-DM (53).
In diabetic mice infected with Mycobacterium tuberculosis,
Yamashiro et al (54) found
the production of IFN-γ was significantly lower than that in
non-diabetic mice, and that the decreased number of Th1-related
cytokines leaded to an impaired host defense against infection with
Mycobacterium tuberculosis. In a study performed by
Meenakshi et al (55), the
production of IFN-γ was markedly decreased following
Mycobacterium tuberculosis stimulation in patients with
PTB-DM. Moreover, Stalenhoef et al (41) observed decreased levels of
non-specific IFN-γ in patients with DM without PTB. However, Gan
et al (56) suggested that no
significant difference was observed in the T-cell IFN-γ responses
to Mycobacterium tuberculosis-specific antigens between
PTB-DM and PTB-non-DM. Thus, further investigations are required to
confirm the IFN-γ response in PTB-DM.
In a previous study, the pancreatic endocrine
function was investigated in 51 patients with primary active PTB
before and after glucagon stimulation, the results revealed that
relative insulin deficiency resulted in persistent hyperglycemia in
these patients, and that the delayed concentration peaks of
immunoreactive insulin and C peptide were observed in these
patients (57). Compared with
patients with PTB or DM alone, infection with Mycobacterium
tuberculosis evidently sustains hyperglycemia in patients with
DM (58). The prevention of
Mycobacterium tuberculosis infection effective reduces
weight loss, hyperglycemia, and insulin resistance during DM
progression, manifesting that these parameters of glucose
metabolism may be affected by Mycobacterium tuberculosis
(59). Liver and kidney function
play indispensable role in maintaining glucose metabolism
homeostasis (60,61). Patients with PTB may be subjected to
glucose metabolism dysfunction due to the impairment of liver and
kidney function during anti-tuberculosis therapy. It has been
reported that isoniazid can cause liver damage in anti-tuberculosis
treatment (62). Isoniazid,
rifampicin, and pyrazinamide cause hepatotoxicity at a probability
ranging from 1 to 57% (63).
Rifampicin re-administration may lead to an acute kidney injury
(64). In addition, the specific
amyloidosis of the pancreas is demonstrated in patients with
tuberculous infection, while intense islet cell amyloidosis is also
commonly observed in DM (65). Of
note, islet cell amyloidosis, as a by-product of systemic
tubercular infection, is dissolved by rifampicin; thus, it is
suggested that infection with Mycobacterium tuberculosis may
increase the risk of developing DM by causing the islet cell
amyloidosis in patients with PTB (65). However, further studies are warranted
to elucidate the mechanisms through which PTB increases the risk of
developing DM.
DM is associated with the prognosis in patients with
PTB, and DM has an adverse effect on the outcomes of PTB treatments
(66). In patients newly diagnosed
with PTB with DM or PDM, DM and hyperglycemia have been found to be
associated with an increased bacterial burden of Mycobacterium
tuberculosis and the risk of PTB transmission (15), and a poor blood glucose control has
been demonstrated to increase the risk of PTB in patients with DM
(67,68). Gil-Santana et al (69) suggested that DM was associated with
the severity of PTB. An association between blood glucose levels
and the computed tomography severity score has been found in
patients with PTB-DM (70).
Recently, a prospective cohort study indicated that patients with
PTB with DM had a poorer prognosis than those without DM (71). A poor blood glucose control has been
shown to be associated with the outcomes of PTB treatments, while
improved hyperglycemia can reduce the effects of DM in patients
with PTB (72). A meta-analysis
suggested that DM was associated with an increased risk of a poor
treatment response in patients with PTB, and that DM might increase
the risk of PTB resistance (73).
PTB patients with DM are more likely to have a failed response to
anti-tuberculosis therapy than those without DM (12). In addition, DM is a risk factor for
the positive rate of tuberculosis culture after receiving
anti-tuberculosis treatment, anti-tuberculosis treatment failure,
and mortality in patients with PTB (74). Evidently, blood glucose control is
associated with the outcomes of anti-tuberculosis treatment in
patients with PTB-DM.
Anti-tuberculosis medications are divided into
first- and second-line drugs; first-line anti-tuberculosis drugs
include rifampicin, isoniazid, pyrazine, ethambutol, and
streptomycin; these have a high efficiency and acceptable toxicity;
second-line anti-tuberculosis drugs, such as fluoroquinolones,
aminoglycosides, are used for multidrug resistance (75-77).
Mycobacterium tuberculosis has the ability to manipulate
innate and adaptive immune responses, which is known as the
tuberculous escape mechanism; thus, Mycobacterium
tuberculosis can avoid the intracellular killing and macrophage
phagocytosis by the escape mechanism; however, hyperglycemia
aggravates its escape mechanism, leading to an increased risk of
anti-tuberculosis treatment failure in patients with PTB-DM
(78). Poor blood glucose control
increases the risk of pyrazinamide treatment failure, and DM also
appears to influence the pharmacokinetic-pharmacodynamic
association between isoniazid and rifampicin (79). Babalik et al (80) demonstrated that the plasma
concentrations of isoniazid and rifampicin were reduced by ~50% in
patients with PTB-DM; their data indicate that hyperglycemia
significantly reduces the efficiency of anti-tuberculosis
drugs.
DM significantly increases the risk of resistance to
anti-tuberculosis medications (81).
The use of hypoglycemic drugs significantly increases the
effectiveness of anti-tuberculosis treatment in patients with
PTB-DM. For instance, metformin can be used as an effective
auxiliary anti-tuberculosis drug in patients with PTB-DM, as it can
improve sputum culture transformation following 2 months of therapy
(82); secondly, metformin use has
been reported to be significantly associated with reduced mortality
during antituberculosis treatment in patients with PTB-DM (83). Therefore, clinically, additional
attention needs to be paid to the treatment and management of
patients with PTB-DM; a satisfactory blood glucose control
increases the efficacy of anti-tuberculosis treatment in these
patients.
The present review provided an update on PTB-DM from
the perspectives of epidemiology, pathogenesis, and treatment;
however, there are still a number of issues that need to be further
addressed in the future. First, the majority of epidemiological
studies are based on a cross-sectional design; thus, further
prospective cohort studies with large sample sizes are required to
clarify a causality between PTB and DM. Second, islet cell
dysfunction plays a key role in the pathogenesis of DM; current
studies mainly focus on the effects of hyperglycemia on the
susceptibility to Mycobacterium tuberculosis; however, few
studies have explored the mechanisms through which Mycobacterium
tuberculosis infection affects islet cell function in patients
with PTB-DM.
In conclusion, the present review provides insight
for future studies on the link between PTB and DM, particularly as
regards the mechanisms of their interaction. A better understanding
of the mechanisms of the association between DM and PTB may help to
formulate effective treatment strategies with which to reduce the
double burden of PTB-DM.
Not applicable.
Funding: No funding was received.
Not applicable.
YFP conceived and designed the study. YFP performed
the literature search and wrote the manuscript. YFP has read and
approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The author declares that he has no competing
interests.
|
1
|
International Diabetes Federation. IDF
Diabetes Atlas, 10th edition. International Diabetes Federation,
Brussels, 2021.
|
|
2
|
Zheng Y, Ley SH and Hu FB: Global
aetiology and epidemiology of type 2 diabetes mellitus and its
complications. Nat Rev Endocrinol. 14:88–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kaparianos A, Argyropoulou E, Sampsonas F,
Karkoulias K, Tsiamita M and Spiropoulos K: Pulmonary complications
in diabetes mellitus. Chron Respir Dis. 5:101–108. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ali MO: Pulmonary complications in
diabetes mellitus. Mymensingh Med J. 23:603–605. 2014.PubMed/NCBI
|
|
5
|
Natarajan A, Beena PM, Devnikar AV and
Mali S: A systemic review on tuberculosis. Indian J Tuberc.
67:295–311. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zheng C, Hu M and Gao F: Diabetes and
pulmonary tuberculosis: A global overview with special focus on the
situation in Asian countries with high TB-DM burden. Glob Health
Action. 10:1–11. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li M, Chen T, Hua Z, Yan H, Wang D, Li Z,
Kang Y, Zhu N and Li C: Global, regional, and national prevalence
of diabetes mellitus in patients with pulmonary tuberculosis: A
systematic review and meta-analysis. Diabetol Metab Syndr.
13(127)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mugusi F, Swai AB, Alberti KG and McLarty
DG: Increased prevalence of diabetes mellitus in patients with
pulmonary tuberculosis in Tanzania. Tubercle. 71:271–276.
1990.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wu Q, Wang M, Zhang Y, Wang W, Ye TF, Liu
K and Chen SH: Epidemiological characteristics and their
influencing factors among pulmonary tuberculosis patients with and
without diabetes mellitus: A survey study from drug resistance
surveillance in east china. Front Public Health.
9(777000)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee EH, Lee JM, Kang YA, Leem AY, Kim EY,
Jung JY, Park MS, Kim YS, Kim SK, Chang J and Kim SY: Prevalence
and impact of diabetes mellitus among patients with active
pulmonary tuberculosis in South Korea. Lung. 195:209–215.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rahim Z, Momi MS, Saha SK, Zaman K, Uddin
KN, Jamil SN, Nahar N, Khan AK, Cooreman EA, Ahmed M, et al:
Pulmonary tuberculosis in patients with diabetes mellitus in
Bangladesh. Int J Tuberc Lung Dis. 16:1132–1133. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Delgado-Sánchez G, García-García L,
Castellanos-Joya M, Cruz-Hervert P, Ferreyra-Reyes L,
Ferreira-Guerrero E, Hernández A, Ortega-Baeza VM, Montero-Campos
R, Sulca JA, et al: Association of pulmonary tuberculosis and
diabetes in Mexico: Analysis of the national tuberculosis registry
2000-2012. PLoS One. 10(e0129312)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Guo S, Lei S, Li J, Li L, Chen H and
Chongsuvivatwong V: Gradient association between pulmonary
tuberculosis and diabetes mellitus among households with a
tuberculosis case: A contact tracing-based study. Sci Rep.
12(1854)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Stevenson CR, Forouhi NG, Roglic G,
Williams BG, Lauer JA, Dye C and Unwin N: Diabetes and
tuberculosis: The impact of the diabetes epidemic on tuberculosis
incidence. BMC Public Health. 7(234)2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mave V, Meshram S, Lokhande R, Kadam D,
Dharmshale S, Bharadwaj R, Kagal A, Pradhan N, Deshmukh S, Atre S,
et al: Prevalence of dysglycemia and clinical presentation of
pulmonary tuberculosis in Western India. Int J Tuberc Lung Dis.
21:1280–1287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Q, Ma A, Han X, Zhao S, Cai J, Ma Y,
Zhao J, Wang Y, Dong H, Zhao Z, et al: Prevalence of type 2
diabetes among newly detected pulmonary tuberculosis patients in
China: A community based cohort study. PLoS One.
8(e82660)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vallerskog T, Martens GW and Kornfeld H:
Diabetic mice display a delayed adaptive immune response to
Mycobacterium tuberculosis. J Immunol. 184:6275–6282.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Panda S, Seelan DM, Faisal S, Arora A,
Luthra K, Palanichamy JK, Mohan A, Vikram NK, Gupta NK,
Ramakrishnan L and Singh A: Chronic hyperglycemia drives
alterations in macrophage effector function in pulmonary
tuberculosis. Eur J Immunol. 52:1595–1609. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vance J, Santos A, Sadofsky L, Morice A
and Cervantes J: Effect of high glucose on human alveolar
macrophage phenotype and phagocytosis of mycobacteria. Lung.
197:89–94. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alim MA, Sikder S, Sathkumara H, Kupz A,
Rush CM, Govan BL and Ketheesan N: Dysregulation of key cytokines
may contribute to increased susceptibility of diabetic mice to
Mycobacterium bovis BCG infection. Tuberculosis (Edinb).
115:113–120. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Martinez N, Ketheesan N, West K,
Vallerskog T and Kornfeld H: Impaired recognition of mycobacterium
tuberculosis by alveolar macrophages from diabetic mice. J Infect
Dis. 214:1629–1637. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Alim MA, Sikder S, Bridson TL, Rush CM,
Govan BL and Ketheesan N: Anti-mycobacterial function of
macrophages is impaired in a diet induced model of type 2 diabetes.
Tuberculosis (Edinb). 102:47–54. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sertl K, Takemura T, Tschachler E, Ferrans
VJ, Kaliner MA and Shevach EM: Dendritic cells with
antigen-presenting capability reside in airway epithelium, lung
parenchyma, and visceral pleura. J Exp Med. 163:436–451.
1986.PubMed/NCBI View Article : Google Scholar
|
|
24
|
McWilliam AS, Marsh AM and Holt PG:
Inflammatory infiltration of the upper airway epithelium during
Sendai virus infection: Involvement of epithelial dendritic cells.
J Virol. 71:226–236. 1997.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Holt PG, Stumbles PA and McWilliam AS:
Functional studies on dendritic cells in the respiratory tract and
related mucosal tissues. J Leukoc Biol. 66:272–275. 1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kumar NP, Moideen K, Sivakumar S, Menon
PA, Viswanathan V, Kornfeld H and Babu S: Modulation of dendritic
cell and monocyte subsets in tuberculosis-diabetes co-morbidity
upon standard tuberculosis treatment. Tuberculosis (Edinb).
101:191–200. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kumar NP, Moideen K, Dhakshinraj SD,
Banurekha VV, Nair D, Dolla C, Kumaran P and Babu S: Profiling
leucocyte subsets in tuberculosis-diabetes co-morbidity.
Immunology. 146:243–250. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Andrade BB, Kumar NP, Sridhar R, Banurekha
VV, Jawahar MS, Nutman TB, Sher A and Babu S: Heightened plasma
levels of heme oxygenase-1 and tissue inhibitor of
metalloproteinase-4 as well as elevated peripheral neutrophil
counts are associated with TB-diabetes comorbidity. Chest.
145:1244–1254. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Raposo-García S, Guerra-Laso JM,
García-García S, Juan-García J, López-Fidalgo E, Diez-Tascón C,
Nebreda-Mayoral T, López-Medrano R and Rivero-Lezcano OM:
Immunological response to Mycobacterium tuberculosis infection in
blood from type 2 diabetes patients. Immunol Lett. 186:41–45.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Eruslanov EB, Lyadova IV, Kondratieva TK,
Majorov KB, Scheglov IV, Orlova MO and Apt AS: Neutrophil responses
to Mycobacterium tuberculosis infection in genetically susceptible
and resistant mice. Infect Immun. 73:1744–1753. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Vankayalapati R and Barnes PF: Innate and
adaptive immune responses to human Mycobacterium tuberculosis
infection. Tuberculosis. 89 (Suppl 1):S77–S80. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zahran WA, Ghonaim MM, Koura BA, El-Banna
H, Ali SM and El-Sheikh N: Human natural killer T cells (NKT), NK
and T cells in pulmonary tuberculosis: Potential indicators for
disease activity and prognosis. Egypt J Immunol. 13:67–78.
2006.PubMed/NCBI
|
|
33
|
Zhang Q, Xiao HP, Cui HY and Sugawara I:
Significant increase in natural-killer T cells in patients with
tuberculosis complicated by type 2 diabetes mellitus. J Int Med
Res. 39:105–111. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kumar NP, Sridhar R, Nair D, Banurekha VV,
Nutman TB and Babu S: Type 2 diabetes mellitus is associated with
altered CD8(+) T and natural killer cell function in pulmonary
tuberculosis. Immunology. 144:677–686. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cheekatla SS, Tripathi D,
Venkatasubramanian S, Nathella PK, Paidipally P, Ishibashi M, Welch
E, Tvinnereim AR, Ikebe M, Valluri VL, et al: NK-CD11c+ cell
crosstalk in diabetes enhances IL-6-mediated inflammation during
mycobacterium tuberculosis infection. PLoS Pathog.
12(e1005972)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Prezzemolo T, Guggino G, La Manna MP, Di
Liberto D, Dieli F and Caccamo N: Functional signatures of human
CD4 and CD8 T cell responses to mycobacterium tuberculosis. Front
Immunol. 5(180)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
St Paul M and Ohashi PS: The roles of
CD8+ T cell subsets in antitumor immunity. Trends Cell
Biol. 30:695–704. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mayer-Barber KD and Barber DL: Innate and
adaptive cellular immune responses to mycobacterium tuberculosis
infection. Cold Spring Harb Perspect Med. 5(a018424)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ponnana M, Pydi S and Gaddam S:
Enumeration of lymphocyte subsets during follow-up in the pulmonary
tuberculosis patients with co morbid diabetes mellitus. Clin Chim
Acta. 510:566–572. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kumar NP, Sridhar R, Banurekha VV, Jawahar
MS, Nutman TB and Babu S: Expansion of pathogen-specific T-helper 1
and T-helper 17 cells in pulmonary tuberculosis with coincident
type 2 diabetes mellitus. J Infect Dis. 208:739–7348.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Stalenhoef JE, Alisjahbana B, Nelwan EJ,
van der Ven-Jongekrijg J, Ottenhoff TH, van der Meer JW, Nelwan RH,
Netea MG and van Crevel R: The role of interferon-gamma in the
increased tuberculosis risk in type 2 diabetes mellitus. Eur J Clin
Microbiol Infect Dis. 27:97–103. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fernández RDV, Díaz A, Bongiovanni B,
Gallucci G, Bértola D, Gardeñez W, Lioi S, Bertolin Y, Galliano R,
Bay ML, et al: Evidence for a more disrupted immune-endocrine
relation and cortisol immunologic influences in the context of
tuberculosis and type 2 diabetes comorbidity. Front Endocrinol
(Lausanne). 11(126)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sun Q, Zhang Q, Xiao H, Cui H and Su B:
Significance of the frequency of CD4+CD25+CD127- T-cells in
patients with pulmonary tuberculosis and diabetes mellitus.
Respirology. 17:876–882. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kathamuthu GR, Kumar NP, Moideen K, Dolla
C, Kumaran P and Babu S: Multi-dimensionality immunophenotyping
analyses of MAIT cells expressing Th1/Th17 cytokines and cytotoxic
markers in latent tuberculosis diabetes comorbidity. Pathogens.
11(87)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kathamuthu GR, Kumar NP, Moideen K, Menon
PA and Babu S: Decreased frequencies of Gamma/Delta T cells
expressing Th1/Th17 cytokine, cytotoxic, and immune markers in
latent tuberculosis-diabetes/pre-diabetes comorbidity. Front Cell
Infect Microbiol. 11(756854)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wei R, Li P, Xue Y, Liu Y, Gong W and Zhao
W: Impact of diabetes mellitus on the immunity of tuberculosis
patients: A retrospective, cross-sectional study. Risk Manag
Healthc Policy. 15:611–627. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kumar NP, Moideen K, George PJ, Dolla C,
Kumaran P and Babu S: Impaired cytokine but enhanced cytotoxic
marker expression in mycobacterium tuberculosis-induced CD8+ T
cells in individuals with type 2 diabetes and latent mycobacterium
tuberculosis infection. J Infect Dis. 213:866–870. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang X, Ma A, Han X, Chan L, Liang H,
Litifu A and Xue F: T cell profile was altered in pulmonary
tuberculosis patients with type 2 diabetes. Med Sci Monit.
24:636–642. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kumar NP, Moideen K, Viswanathan V,
Kornfeld H and Babu S: Effect of standard tuberculosis treatment on
naive, memory and regulatory T-cell homeostasis in
tuberculosis-diabetes co-morbidity. Immunology. 149:87–97.
2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kumar S, Lakhiwal R, Singh CP, Bhandiwad
C, Sharma N, Singhal V and Chakranarayan A: Study of correlation of
CD4, CD8 count with tuberculous pneumonia and non tuberculous
bacterial pneumonia in type 2 diabetes mellitu. J Assoc Physicians
India. 70:11–12. 2022.PubMed/NCBI
|
|
51
|
Kumar NP, Moideen K, Dolla C, Kumaran P
and Babu S: Prediabetes is associated with the modulation of
antigen-specific Th1/Tc1 and Th17/Tc17 responses in latent
Mycobacterium tuberculosis infection. PLoS One.
12(e0178000)2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mily A, Sarker P, Taznin I, Hossain D, Haq
MA, Kamal SMM, Agerberth B, Brighenti S and Raqib R: Slow
radiological improvement and persistent low-grade inflammation
after chemotherapy in tuberculosis patients with type 2 diabetes.
BMC Infect Dis. 20(933)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Guo Q, Zhang J, Li G, Liu S, Xiao G, Bi J,
Li F, Zhang S, Ou M, He X, et al: Elevated antigen-specific IFN-γ
responses in bronchoalveolar lavage fluid impervious to clinical
comorbidities improve the pulmonary tuberculosis diagnosis.
Tuberculosis (Edinb). 122(101942)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yamashiro S, Kawakami K, Uezu K, Kinjo T,
Miyagi K, Nakamura K and Saito A: Lower expression of Th1-related
cytokines and inducible nitric oxide synthase in mice with
streptozotocin-induced diabetes mellitus infected with
Mycobacterium tuberculosis. Clin Exp Immunol. 139:57–64.
2005.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Meenakshi P, Ramya S, Lavanya J,
Vijayalakshmi V and Sumanlatha G: Effect of IFN-γ, IL-12 and IL-10
cytokine production and mRNA expression in tuberculosis patients
with diabetes mellitus and their household contacts. Cytokine.
81:127–136. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Gan SH, KhinMar KW, Barkham TM, Koh CK,
Shen L, Wang YT and Chee CB: Interferon-γ responses to
Mycobacterium tuberculosis-specific antigens in diabetes mellitus.
Eur Respir J. 44:805–808. 2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Karachunskiĭ MA, Balabolkin MI and
Beglarian NR: Changes in carbohydrate metabolism in patients with
tuberculosis. Vestn Ross Akad Med Nauk. 7:18–21. 1995.PubMed/NCBI(In Russian).
|
|
58
|
Chen H, Su L, Bao J, Zhang K, Li Y and Mao
E: The impact of pulmonary tuberculosis on immunological and
metabolic features of diabetic patients. Front Immunol.
13(973991)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Segura-Cerda CA, Marquina-Castillo B,
Lozano-Ordaz V, Mata-Espinosa D, Barrios-Payán JA, López-Torres MO,
Aceves-Sánchez MJ, Bielefeldt-Ohmann H, Hernández-Pando R and
Flores-Valdez MA: BCG and BCGΔBCG1419c protect type 2 diabetic mice
against tuberculosis via different participation of T and B
lymphocytes, dendritic cells and pro-inflammatory cytokines. NPJ
Vaccines. 5(21)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Adeva-Andany MM, Pérez-Felpete N,
Fernández-Fernández C, Donapetry-García C and Pazos-García C: Liver
glucose metabolism in humans. Biosci Rep. 36(e00416)2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Legouis D, Faivre A, Cippà PE and de
Seigneux S: Renal gluconeogenesis: An underestimated role of the
kidney in systemic glucose metabolism. Nephrol Dial Transplant.
37:1417–1425. 2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Gray EL and Goldberg HF: Baseline abnormal
liver function tests are more important than age in the development
of isoniazid-induced hepatoxicity for patients receiving preventive
therapy for latent tuberculosis infection. Intern Med J.
46:281–287. 2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Becker MW, Schwambach KH, Lunardelli M and
Blatt CR: Overview of drug induced liver injury in Brazil: What is
the role of public health policy on the evidence? World J
Gastrointest Pharmacol Ther. 12:40–55. 2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Covic A, Golea O, Segall L, Meadipudi S,
Munteanu L, Nicolicioiu M, Tudorache V, Covic M and Goldsmith DJ: A
clinical description of rifampicin-induced acute renal failure in
170 consecutive cases. J Indian Med Assoc. 102:22–25.
2004.PubMed/NCBI
|
|
65
|
Broxmeyer L: Diabetes mellitus,
tuberculosis and the mycobacteria: Two millenia of enigma. Med
Hypotheses. 65:433–439. 2005.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Sahakyan S, Petrosyan V and Abrahamyan L:
Diabetes mellitus and treatment outcomes of pulmonary tuberculosis:
A cohort study. Int J Public Health. 65:37–43. 2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ahmed M, Omer I, Osman SM and Ahmed-Abakur
EH: Association between pulmonary tuberculosis and Type 2 diabetes
in Sudanese patients. Int J Mycobacteriol. 6:97–101.
2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wang Y, Dou M, Kou T, Liu Y, Lv W, Han L,
Wang N, Ma A, Kok FJ, Schouten EG and Wang Q: Risk of having
pulmonary tuberculosis in type 2 diabetes: A hospital-based matched
case-control study. Asia Pac J Clin Nutr. 30:303–310.
2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gil-Santana L, Almeida-Junior JL, Oliveira
CA, Hickson LS, Daltro C, Castro S, Kornfeld H, Netto EM and
Andrade BB: Diabetes is associated with worse clinical presentation
in tuberculosis patients from Brazil: A retrospective cohort study.
PLoS One. 11(e0146876)2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ren Y, Ren H, Tian Q, Li X and Liu Y: The
relationship between computed tomography appearance of pulmonary
tuberculosis and blood glucose levels in 763 diabetes mellitus
patients with pulmonary tuberculosis: A comparative study.
Endocrine. 76:584–592. 2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Buasroung P, Petnak T, Liwtanakitpipat P
and Kiertiburanakul S: Prevalence of diabetes mellitus in patients
with tuberculosis: A prospective cohort study. Int J Infect Dis.
116:374–379. 2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Chiang CY, Bai KJ, Lin HH, Chien ST, Lee
JJ, Enarson DA, Lee TI and Yu MC: The influence of diabetes,
glycemic control, and diabetes-related comorbidities on pulmonary
tuberculosis. PLoS One. 10(e0121698)2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Huangfu P, Ugarte-Gil C, Golub J, Pearson
F and Critchley J: The effects of diabetes on tuberculosis
treatment outcomes: An updated systematic review and meta-analysis.
Int J Tuberc Lung Dis. 23:783–796. 2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Ma Y, Huang ML, Li T, DU J, Shu W, Xie SH,
Wang HH, Zhu GF, Tan SY, Fu YY, et al: Role of diabetes mellitus on
treatment effects in drug-susceptible initial pulmonary
tuberculosis patients in China. Biomed Environ Sci. 30:671–675.
2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Brunton L, Chapner B and Knollmann B: In:
The Pharmacological Basis of Therapeutics-Goodman and Gillman.
Brunton L and Chapner B (eds). 12th edition. Mc Graw Hill Medical,
San Diego, CA, 2011.
|
|
76
|
Katzung BG, Mastres SB and Trevor AJ:
Basic and Clinical Pharmacology. 14th edition. Mc Graw Hill
Education, Singapore, 2018.
|
|
77
|
Parida SK, Axelsson-Robertson R, Rao MV,
Singh N, Master I, Lutckii A, Keshavjee S, Andersson J, Zumla A and
Maeurer M: Totally drug- resistant tuberculosis and adjunct
therapies. J Intern Med. 277:388–405. 2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Novita BD: Metformin: A review of its
potential as enhancer for anti tuberculosis efficacy in diabetes
mellitus-tuberculosis coinfection patients. Indian J Tuberc.
66:294–298. 2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Alfarisi O, Mave V, Gaikwad S,
Sahasrabudhe T, Ramachandran G, Kumar H, Gupte N, Kulkarni V,
Deshmukh S, Atre S, et al: Effect of diabetes mellitus on the
pharmacokinetics and pharmacodynamics of tuberculosis treatment.
Antimicrob Agents Chemother. 62:e01383–e01318. 2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Babalik A, Ulus IH, Bakirci N, Kuyucu T,
Arpag H, Dagyildizi L and Capaner E: Plasma concentrations of
isoniazid and rifampin are decreased in adult pulmonary
tuberculosis patients with diabetes mellitus. Antimicrob Agents
Chemother. 57:5740–5742. 2013.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Hsu AH, Lee JJ, Chiang CY, Li YH, Chen LK
and Lin CB: Diabetes is associated with drug-resistant tuberculosis
in Eastern Taiwan. Int J Tuberc Lung Dis. 17:354–356.
2013.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Lee YJ, Han SK, Park JH, Lee JK, Kim DK,
Chung HS and Heo EY: The effect of metformin on culture conversion
in tuberculosis patients with diabetes mellitus. Korean J Intern
Med. 33:933–940. 2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Degner NR, Wang JY, Golub JE and
Karakousis PC: Metformin use reverses the increased mortality
associated with diabetes mellitus during tuberculosis treatment.
Clin Infect Dis. 66:198–205. 2018.PubMed/NCBI View Article : Google Scholar
|