Introduction
Age-related macular degeneration (AMD) is an eye
disease whose incidence rate increases with age and leads to
decreased central vision (1). AMD is
the most common cause of legal blindness among the population aged
>50 years in the western world (2). In the USA, the number of cases of legal
blindness caused by AMD are greater than those caused by glaucoma,
cataract and diabetic retinopathy in combination (3). AMD may have a severe impact on the
quality of life of affected individuals. It is associated with an
increased risk of functional disabilities, negative effects on
daily activities, an increased risk of depression and a higher risk
of developing cognitive impairments in older adults (4-6).
AMD has a variety of classification criteria. It is
traditionally classified into the early and late stage based on
color fundus images (7). Early-stage
AMD is characterized by large drusen, retinal pseudo-drusen and
pigmentary abnormalities. By contrast, late-stage AMD is divided
into neovascular AMD (nAMD) and geographic atrophy (GA). Although
AMD is the leading cause of legal blindness, the treatment methods
available for late-stage AMD, particularly nAMD, are limited. The
primary treatment for nAMD is based on the inhibition of vascular
endothelial growth factor (VEGF) (8). Although several complement inhibitors
are undergoing therapeutic clinical trials (ClinicalTrials.gov Identifiers: NCT05230537,
NCT03364153 and NCT04465955), there are currently no effective
therapeutic methods available for GA (9). In addition, there is also a lack of
effective treatment strategies for preventing and delaying the
progression of early- to late-stage AMD (7). Hence, the development of novel
therapeutic agents for AMD is mandatory.
β-Adrenergic receptor (β-AR) blockers (BBs) are
medications widely used in the treatment of heart diseases, such as
hypertension, arrhythmias and heart failure. Previous preclinical
studies have demonstrated a protective role of BBs against
neovascularization. For example, propranolol treatment has been
shown to reduce 50% of neovascularization in laser-induced
choroidal neovascularization by reducing the release of VEGF
(10). Reduced corneal
neovascularization with downregulated levels of VEGF and cytokines
was also observed following treatment with timolol in a murine
corneal suture model (11). Hence,
several clinical research studies have explored the possible
association between BBs and AMD (12-19).
However, some conflicting results have been reported. The studies
by Klein et al (17) and
Yeung et al (19) reported an
increased risk of developing nAMD in patients treated with BBs
compared to those not treated, while other studies, such as those
by Traband et al (12),
Kolomeyer et al (13), Thomas
et al (15), Song et
al (16) and Davis et al
(18) found have no association
between the use of BBs and nAMD development. Montero et al
(14) suggested a beneficial effect
of BBs against nAMD. Moreover, the majority of studies have focused
on nAMD, and not on GA and early AMD. According to their
selectivity for β-AR, the BBs used in clinical practice are divided
into two main categories: Selective and non-selective BBs (20). The majority of research focuses on
non-selective BBs. The association between the development of AMD
and the use of selective BBs has not been reported to date, at
least to the best of our knowledge. Thus, the present study
investigated the association between different types of BBs and the
risk of developing different stages of AMD using the data from the
National Health and Nutrition Examination Survey (NHANES).
Patients and methods
Data source and ethics approval
All data used in the present study were obtained
from the NHANES, which is a cross-sectional survey administered by
the Centers for Disease Control and Prevention's National Centre
for Health Statistics (NCHS) since 1999. It reflects the national
status of health and nutrition in the USA. Since the present study
employed de-identified information from the NHANES database
approved by the institutional review board of the NCHS, the Ethics
Committee of the Second Affiliated Hospital of Wenzhou Medical
University (Wenzhou, China) granted the study an exemption from
ethical review.
Participants enrolled
As the retinal examination was only available in two
NHANES cycles (2005-2006 and 2007-2008), participants were selected
from these two cycles. Since BBs are mainly used in patients with
hypertension, all the hypertensive participants selected were
>40 years of age. Participants who responded ‘Yes’ to the
question ‘have you ever been told by a doctor or other health
professional that you had hypertension, also called high blood
pressure?’ and those with an average systolic blood pressure ≥140
mmHg or average diastolic blood pressure ≥90 mmHg in the
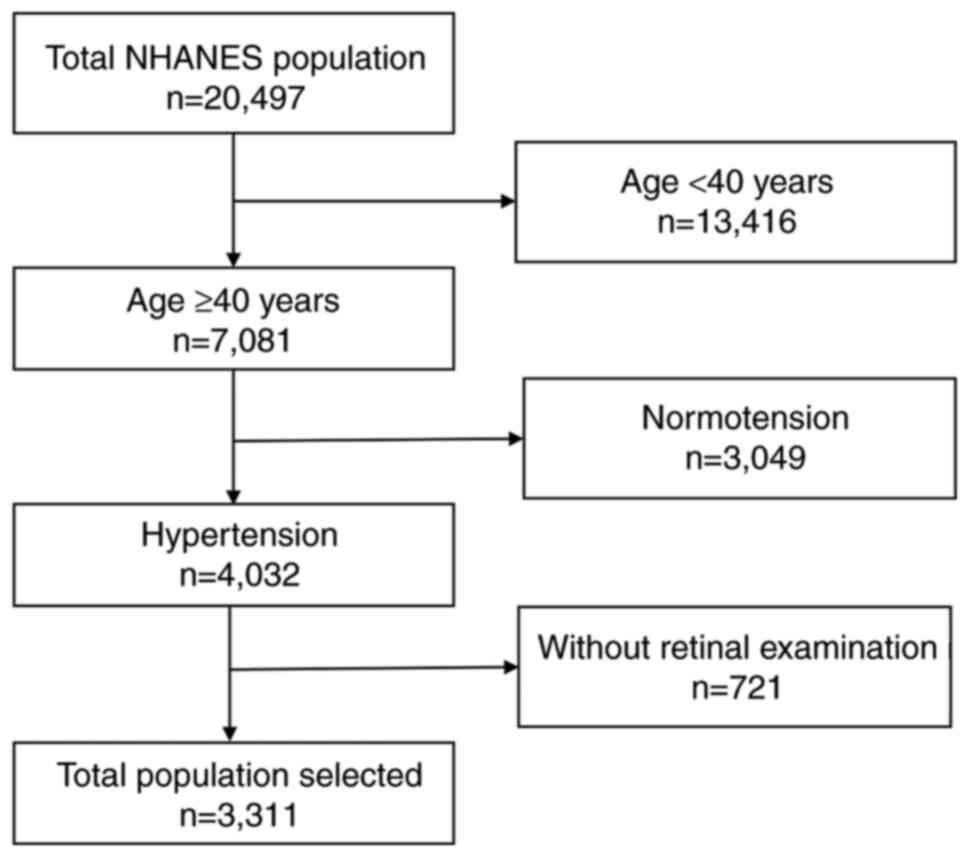
examination were defined as being hypertensive. In total, 20,497
participants were included from the two NHANES cycles; 13,416
participants were excluded as they were aged <40 years, among
whom 4,032 had hypertension. A total of 3,023 participants were
finally enrolled in the present study, for whom a complete retinal
examination had been performed. A flowchart of the process used for
the inclusion of participants is presented in Fig. 1.
Classification of AMD
In the NHANES database, AMD was classified into three
stages as follows: No AMD, early-stage AMD and late-stage AMD. The
classification criteria were the following: Any large (≥125 µm)
drusen, or retinal pseudodrusen or pigmentary abnormalities in the
retinal examination were defined as early-stage AMD; any GA or
exudative neovascularization in the retinal examination was defined
as late-stage AMD. Those without any signs of early- or late-stage
AMD in the retinal examination were considered as having no AMD. If
both eyes were affected by AMD, data from the eye with the more
severe stage of AMD were used.
Use of BBs and treatment duration
The use of BBs was identified according to the
self-reported prescription medications questionnaire (https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/questionnaires.aspx?BeginYear=2005).
Non-selective BBs included propranolol, carvedilol, nadolol,
sotalol, pindolol, labetalol, penbutolol and timolol. Selective BBs
included nebivolol, metoprolol, atenolol, bisoprolol, acebutolol,
and betaxolol. The duration of the use of BBs was also obtained
from the questionnaire, which was divided into four quartiles as
follows: First quartile, ≤2 years; second quartile, 2-4 years;
third quartile, 4-6 years; fourth quartile, >6 years). The
long-term use of BBs in participants was defined as a BB treatment
duration of >6 years.
Other variables examined
Other variables included demographic
characteristics, a history of comorbidities, health-related
behaviors and the use of anti-hypertensive drugs. Data regarding
age, sex, race, economic status and education level were collected
under the category of demographic characteristics. A history of
stroke, heart diseases, cancer or malignancy, diabetes mellitus,
thyroid issues, glaucoma and diabetic retinopathy were collected
with the history of comorbidities category. Health-related
behaviors provided information about smoking and alcohol
consumption. The use of anti-hypertensive drugs included the use of
BBs and treatment duration, renin-angiotensin system inhibitors
(RASIs), calcium channel blockers (CCBs) and diuretics.
Participants with coronary heart disease, heart attack, angina
pectoris or congestive heart failure were defined as having a
history of heart disease. Participants with anemia or chronic
bronchitis were defined as having a history of lung disease.
Participants with glaucoma were identified by cup-to-disc ratios
>0.6 in one eye. Participants with diabetes mellitus were
identified using the following criteria: i) Fasting plasma glucose
levels ≥126 mg/ml; ii) 2-h plasma glucose levels ≥200 mg/dl; iii)
HbA1c ≥6.5%; and iv) answering ‘Yes’ to the question of ‘have you
ever been told by a doctor or health professional that you have
diabetes or sugar diabetes?’. Participants with diabetic
retinopathy were identified by any signs of retinopathy (>14) on
fundus images and by a diagnosis of diabetes mellitus. Dara
regarding body mass index (BMI) and waist circumference had been
measured during a physical examination at the time of the survey.
Data on triglycerides (TGs), red blood cells (RBCs), white blood
cells (WBCs), high-density lipoprotein (HDL) and platelet (PLT)
levels of each participant had been obtained through a laboratory
examination.
Statistical analyses
Data were analyzed using a survey package in R
software (version 4.1.3; http://r-survey.r-forge.r-project.org/survey/) with
sampling weight following the complex sample design of NHANES.
Continuous variables are presented as weight-adjusted mean ±
standard error, and qualitative variables as weight-adjusted
proportion ± standard error. ANOVA was used for the comparisons of
means among multiple groups followed by Tukey's post hoc test.
Survey-weighted univariate logistic regression was used to examine
the association between different types of BBs and the various
stages of AMD. A generalized additive model and natural cubic
spline were used to explore the non-linear association between BB
treatment duration and the risk of developing AMD. A multivariate
model adjusted for age, sex, race, stroke history, heart disease
history, thyroid disease history, glaucoma, RBCs and HDL was
applied. The results are presented as odds ratios (ORs) with 95%
confidence intervals (95% CIs). The correlation between the use of
BBs and the prevalence of AMD was calculated using Spearman's
correlation analysis. The correlation between the use of
non-selective BBs and the prevalence of AMD was also calculated
using Spearman's correlation analysis. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of the participants
enrolled
In total, 3,311 participants were enrolled in the
present study. The participants with AMD tended to be older, of
Caucasian or African-American origin, were married, had higher BMI
and HDL levels, had low levels of RBCs, and had a history of heart
disease, stroke and thyroid disease (Table I). In addition, a significant
difference was found in the use of RASIs and BBs between
participants with AMD and those with no AMD (Table I).
 | Table ICharacteristics of the participants
with AMD. |
Table I
Characteristics of the participants
with AMD.
| | AMD | |
|---|
| Characteristic | No AMD
(n=2,998) | Early-stage AMD
(n=272) | Late-stage AMD
(n=41) | P-value |
|---|
| Demographics | | | | |
|
Age
(years) | 59.15±0.41 | 70.10±1.27 | 78.21±1.23 | <0.001 |
|
Sex, female
(%) | 50.71±1.64 | 55.90±6.40 | 81.85±8.95 | 0.01 |
|
Race
(%) | | | | <0.001 |
|
Caucasian | 76.34±2.61 | 88.83±3.32 | 97.94±2.14 | |
|
African-American | 11.44±1.78 | 3.70±1.76 | 0.00±0.00 | |
|
Other | 12.22±1.49 | 7.47±2.46 | 2.06±2.14 | |
| Married or living
with a partner (%) | 69.75±1.54 | 57.28±6.88 | 30.40±7.79 | <0.001 |
| Education
(high-school and above) (%) | 80.36±1.08 | 72.13±7.15 | 66.58±12.28 | 0.981 |
| Income at or above
poverty | 86.28±1.22 | 79.42±3.00 | 80.81±8.46 | 0.539 |
| Health-related
behaviors | | | | |
| Smoking ≥100
cigarettes in whole lifetime (%) | 16.61±2.08 | 15.87±4.28 | 10.11±10.19 | 0.395 |
| Alcohol consumption
in whole lifetime, ≥12 drinks (%) | 53.76±1.61 | 63.41±3.03 | 37.46±6.74 | 0.266 |
| Examinations | | | | |
|
BMI
(kg/m2) | 30.42±0.19 | 29.76±0.74 | 27.22±1.29 | 0.001 |
|
Waist
circumference (cm) | 104.14±0.47 | 104.68±1.68 | 99.03±3.35 | 0.658 |
| Laboratory
tests | | | | |
|
RBCs
(106 cells/µl) | 4.73±0.02 | 4.63±0.05 | 4.34±0.04 | <0.001 |
|
WBCs
(103 cells/µl) | 6.96±0.09 | 7.11±0.25 | 7.12±0.27 | 0.669 |
|
PLT
(103 cells/µl) | 268.45±2.14 | 258.60±8.87 | 245.96±11.32 | 0.079 |
|
HDL
(mg/dl) | 53.71±0.52 | 57.84±2.05 | 65.87±2.49 | 0.002 |
|
TG
(mg/dl) | 160.44±3.09 | 146.52±12.60 | 123.43±18.49 | 0.072 |
| Disease
history | | | | |
|
Heart
diseases (%) | 12.84±1.24 | 21.08±5.29 | 34.29±8.04 | 0.019 |
|
Stroke
(%) | 5.24±0.60 | 16.22±4.23 | 32.28±18.40 | <0.001 |
|
Lung
diseases (%) | 16.56±1.44 | 12.56±3.11 | 16.92±7.62 | 0.111 |
|
Cancer or
malignancies (%) | 14.71±1.08 | 18.86±3.59 | 28.83±7.23 | 0.231 |
|
Thyroid
issues (%) | 14.78±1.34 | 23.64±4.03 | 36.27±11.58 | <0.001 |
|
Diabetes
mellitus (%) | 17.58±1.18 | 15.90±3.06 | 26.89±7.79 | 0.106 |
|
Glaucoma
(%) | 3.48±0.62 | 5.76±3.27 | 9.71±6.96 | 0.191 |
|
Diabetic
retinopathy (%) | 13.75±1.09 | 17.88±4.49 | 0.00±0.00 | 0.944 |
| Use of
anti-hypertensive drugs | | | | |
|
RASIs
(%) | 46.00±1.12 | 47.86±5.85 | 59.80±9.05 | <0.001 |
|
ACEI | 28.84±1.10 | 27.77±3.86 | 52.58±10.12 | 0.213 |
|
ARB | 18.12±1.33 | 20.10±4.35 | 17.07±6.09 | 0.431 |
|
BBs (%) | 24.77±1.61 | 35.96±4.69 | 12.94±7.04 | <0.001 |
|
Non-selective
BBs | 4.42±0.74 | 5.82±2.67 | 0.00±0.00 | 0.424 |
|
Selective
BBs | 20.42±1.38 | 30.55±3.97 | 12.94±7.04 | <0.001 |
|
CCB (%) | 17.00±1.27 | 17.09±3.07 | 34.66±10.02 | 0.018 |
|
Diuretics
(%) | 31.02±1.82 | 40.80±6.52 | 64.67±8.19 | 0.043 |
Use of BBs and the risk of AMD in the
hypertensive population
The association between the use of BBs and the risk
of developing AMD was explored among all the participants. A
significant correlation was found between the use of BBs and AMD
(Rho=0.06, P<0.05) (Fig. 2). BB
treatment increased the risk of developing AMD in the hypertensive
population (OR, 1.49; 95% CI, 1.21-1.84; P<0.001) (Table II). When the BBs were categorized
into non-selective and selective BBs, a significant association was
found between the selective BBs and AMD (OR, 1.59; 95% CI,
1.29-1.97; P<0.001) (Table II).
By contrast, no correlation was found between the use of
non-selective BBs and AMD (Rho=0.07, P>0.05) (Fig. 3). However, no association was found
between the use of BBs and AMD after adjusting for age, race,
stroke history, heart disease history, thyroid disease history,
glaucoma, RBCs and HDL (Table
II).
 | Table IIAssociation between AMD and the use
of BBs. |
Table II
Association between AMD and the use
of BBs.
| | No adjusted | Multivariate
adjusteda |
|---|
| Parameter | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| BB use | 1.49
(1.21-1.84) | <0.001 | 0.94
(0.71-1.25) | 0.66 |
| BB category | | | | |
|
Non-selective
BBs | 0.86
(0.48-1.57) | 0.62 | 0.69
(0.37-1.33) | 0.26 |
|
Selective
BBs | 1.59
(1.29-1.97) | <0.001 | 1.01
(0.77-1.33) | 0.92 |
Use of BBs and the risk of early- and
late-stage AMD in the hypertensive population
As the use of BBs did not have a significant effect
on the risk of developing AMD following multivariate adjustment,
the present study further explored whether the use of BBs was
related to the different stages of AMD. Of note, there was no
significant association between the use of BBs and the risk of
early-stage AMD (Table III).
Furthermore, no association was found after classifying the BBs
into non-selective and selective BBs in the adjusted model.
(Table III). However, the BBs
exerted a beneficial effect (OR, 0.34; 95% CI, 0.13-0.92; P=0.04)
against late-stage AMD in the multivariate adjusted model (Table III). The protective effect for
late-stage AMD was observed in the non-selective BBs (OR, 0.20; 95%
CI, 0.07-0.61; P<0.001) (Table
III). By contrast, the selective BBs were not found to be
significantly associated with late-stage AMD (OR, 0.46; 95% CI,
0.18-1.15; P=0.09) (Table
III).
 | Table IIIAssociation between different stages
of AMD and various category of BBs. |
Table III
Association between different stages
of AMD and various category of BBs.
| | Early-stage
AMDa | Late-stage
AMDa |
|---|
| Parameter | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| BB use | 1.13
(0.85-1.5) | 0.39 | 0.34
(0.13-0.92) | 0.04 |
| BB category | | | | |
|
Non-selective
BBs | 0.86
(0.45-1.64) | 0.638 | 0.20
(0.07-0.61) | <0.001 |
|
Selective
BBs | 1.17
(0.89-1.53) | 0.244 | 0.46
(0.18-1.15) | 0.09 |
BB treatment duration and risk of
AMD
The aforementioned results indicated the protective
effect of BBs against late-stage AMD. However, the potential
cumulative effects of time were not previously considered in the
literature, at least to the best of our knowledge. Hence, in the
present study, the association between the use of BBs and the risk
of developing AMD was further investigated. It was found that BB
treatment duration had no association with the risk of developing
AMD (OR, 0.98; 95% CI, 0.94-1.02; P=0.231; R2=0.114)
(Table IV). In addition, no liner
association was found between BB treatment duration and AMD using
line regression analysis (OR, 0.998; 95% CI, 0.996-1.001; P=0.275;
R2=0.061) (Table IV). A
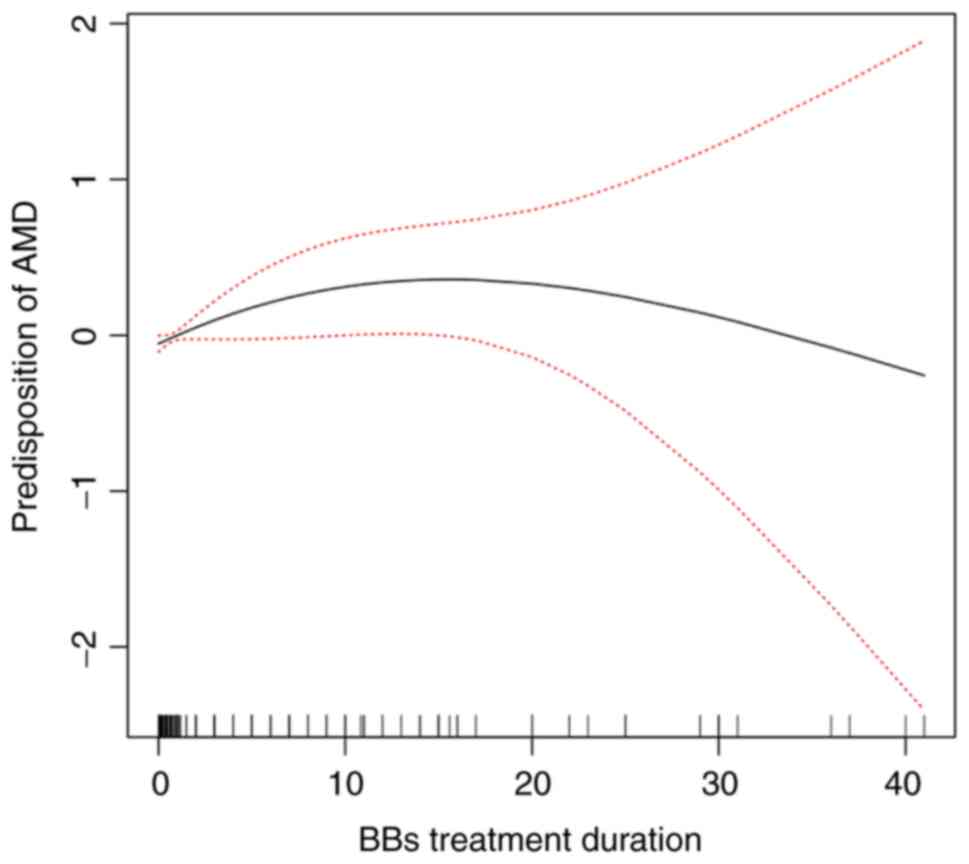
generalized additive model and natural cubic spline were introduced
to examine the non-linear association. Thought the smoothing
splines curve, the predisposition to AMD exhibited a trend to first
increase, and to then decrease with the increasing treatment
duration of BBs (Fig. 4). Thus, the
BB treatment duration was we divided into four quartiles as
follows: ≤2 years, 2-4 years, 4-6 years, and >6 years. Compared
to the patients not on BB treatment, a decreased risk of developing
AMD was only found in the last quartile of BB treatment duration
(OR, 0.65; 95% CI, 0.43-0.98; P=0.04; R2=0.493)
(Table V). The other groups did not
exhibit a significant difference compared with the patients not on
BB treatment (Table V). Similar
results were found when examining the association between BB
treatment duration and late-stage AMD. Only the fourth quartile of
BB treatment duration exhibited a significant association with the
risk of late-stage AMD compared with the BB non-users (OR, 0.13;
95% CI, 0.03-0.63; P=0.01) (Table
V). Furthermore, with the increasing duration of BB treatment,
a significant decrease in the magnitude of associations with the
risk of late-stage AMD was observed (P for trend=0.048) (Table V). The other quartiles did not
exhibit a significant association with the BB non-users (Table V). By contrast, for the early stage
of AMD, no significant difference was found in BB treatment
duration compared with the BB non-users (Table V).
 | Table IVAssociation between AMD and BB
treatment duration in the different modelsa. |
Table IV
Association between AMD and BB
treatment duration in the different modelsa.
| Model | OR (95% CI) | P-value | R2 |
|---|
| Logistic
regression | 0.98
(0.94-1.02) | 0.231 | 0.114 |
| Linear
regression | 0.998
(0.996-1.001) | 0.275 | 0.061 |
 | Table VAssociation between AMD and BB
treatment duration in the generalized additive model. |
Table V
Association between AMD and BB
treatment duration in the generalized additive model.
| | AMDa | Early-stage
AMDa | Late-stage
AMDa |
|---|
| BB duration | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| | Reference
(non-users) | | Reference
(non-users) | | Reference
(non-users) | |
| <2 years | 0.93
(0.59-1.47) | 0.76 | 1.06
(0.69-1.64) | 0.77 | 0.47
(0.12-1.84) | 0.26 |
| 2-4 years | 1.73 (0.87,
3.40) | 0.11 | 2.03
(0.97-4.24) | 0.06 | 0.40
(0.04-3.90) | 0.40 |
| 4-6 years | 1.08 (0.57,
2.02) | 0.81 | 1.27
(0.72-2.24) | 0.39 | 0.38
(0.04-3.64) | 0.38 |
| >6 years | 0.65 (0.43,
0.98) | 0.04 | 0.82
(0.54-1.27) | 0.36 | 0.13
(0.03-0.63) | 0.01 |
| P for trend | 0.94 (0.87,
1.03) | 0.21 | 1.00
(0.92-1.09) | 0.96 | 0.46
(0.40-0.99) | 0.048 |
Long-term use of RASIs and different
subtypes of AMD
The aforementioned results suggested that a BB
treatment duration >6 years may decrease the risk of developing
AMD. Therefore, the long-term use of BBs was defined as a BB
treatment duration >6 years the present study. Since the
previous assessments only focused on nAMD without considering GA
and early-stage AMD, the long-term use of BBs was investigated in
order to assess its influence on the different subtypes of AMD. Two
major subtypes of early-stage AMD were mainly considered in the
present study, including pigmentary abnormalities and soft drusen.
However, there was no association between the long-term use of BBs
and the two subtypes of early-stage AMD (Table VI). In late-stage AMD, the long-term
use of BBs was a protective factor for GA (OR, 0.07; 95% CI,
0.02-0.28; P<0.001) (Table VI).
However, it was considered that the result of the long-term use of
BBs for GA was not reliable as the number of GA cases was very
small. There was also no significant association between the
long-term use of BBs and nAMD (Table
VI).
 | Table VIAssociation between AMD subtypes and
long-term BB treatmenta. |
Table VI
Association between AMD subtypes and
long-term BB treatmenta.
| | OR (95% CI) | P-value |
|---|
| Early-stage AMD
manifestations | | |
|
Pigmentary
abnormalities | 1.05
(0.72-1.52) | 0.79 |
|
Any soft
drusen | 0.81
(0.56-1.15) | 0.22 |
| Late-stage AMD
subtypes | | |
|
Exudative
AMD | 0.35
(0.05-2.48) | 0.27 |
|
Geographic
atrophy | 0.07
(0.02-0.28) | <0.001 |
Discussion
In the present study, although there was
insufficient evidence for the exact association between the use of
BBs and AMD, a decreased association was found between the use of
BBs and late-stage AMD among hypertensive participants from NHANES.
The use of BBs, particularly long-term BB treatment, was found to
exert a protective effect against late-stage AMD. Even though a
significant protective effect of the long-term use of BBs against
GA was found, due to the limited number of number cases of GA in
the NHANES database, the outcome cannot be considered reliable.
However, this result may provide the basis for the future clinical
use of BBs and may guide future treatment strategies patients with
AMD.
Several experimental studies have reported that β-AR
plays a critical role in the development and progression of AMD,
and suggest that BBs may be prophylactic drugs for nAMD. For
example, propranolol was found to reduce retinal neovascularization
and vascular leakage and was considered to downregulate retinal
VEGF and insulin-like growth factor 1 expression (21). Carvedilol has also been demonstrated
to modulate the expression of VEGF and hypoxia-inducible factor-1α
induced by hypoxia (22). Dal Monte
et al (23) also found that
β-AR activation increased the expression of VEGF by increasing
nitric oxide (NO) production, while β-AR blockers exerted the
opposite effect by decreasing NO levels. BBs can reduce
neovascularization. In addition, they can also improve the survival
of retinal neurons. Betaxolol has been shown to exert
neuroprotective effects in the retina by decreasing the expression
of neuronal nitric oxide synthase (24). Betaxolol also reduces the death of
neurons, reducing the calcium ion influx and sodium ion influx
(25,26). In summary, BB treatment has been
shown to exert therapeutic effects against neovascularization,
which is the main pathophysiological mechanism of nAMD, and against
the death of retinal neurons, which is the dominant mechanism of GA
(27,28).
Although preclinical studies have indicated that BBs
may be an effective treatment for AMD, clinical research on AMD and
BBs has not yielded ideal results. A positive outcome was reported
in the retrospective study by Montero et al (14). They found that the need for
bevacizumab injections was decreased in patients with nAMD treated
with oral systemic BBs compared to the BB non-users (14). However, that study was limited by
small sample size. As hypertension is a risk factor for AMD, using
participants not treated with BBs as the control group may possibly
introduce confounding bias. A retrospective study involving the
database of large national USA insurers found a opposite outcome
(12). A comparator medication class
with similar diseases was selected to address the bias. The effects
of injections of anti-VEGF agents in hypertensive patients with BBs
did not differ from those on hypertensive patients with CCBs
(12). The aforementioned studies
focused on the injection incidence. In comparison, other clinical
studies have paid attention to the association between the risk of
developing AMD and the use of BBs, and found negative results. For
example, Davis et al (18)
found no difference in the use of BBs between patients with GA and
wet AMD. Thomas et al (15)
also found there was no significant association between the use of
BB and choroidal neovascularization in nAMD. However, the Beaver
Dam Eye Study (BDES) revealed opposite results (17). BB treatment was associated with an
increased 5-year incidence of exudative AMD over a 20-year period.
That study also had limitations, such as not considering BB
treatment duration. Furthermore, two longitudinal studies on BB
treatment duration and AMD were conducted to explore the
association between BB treatment duration and nAMD. Yeung et
al (19) found that the
continuous use of BBs was associated with a higher risk of nAMD
compared with non-users. By contrast, Kolomeyer et al
(13) reported that patients using
BBs were significantly less likely to develop nAMD at 90 and 180
days than patients using CCBs. The aforementioned studies
concentrated on nAMD, while the study by Song et al
(16) focused on GA; they found no
significant association between BBs and nAMD.
Several researchers have examined the association
between BBs and AMD. Although several of the outcomes were
negative, further studies are required to fully elucidate the
association. In the present study, the use of BBs was not found to
be significantly associated with early-stage AMD and nAMD compared
with non-users, reflecting the conclusions of some studies
(12,13,15,18).
However, long-term treatment with non-selective BBs had a
protective effect against late-stage AMD. Since β-AR in the retina
has an age-related overexpression and a super-sensitivity effect,
it is possible that continuous BB treatment exerts a protective
effect against AMD (29). However,
the positive effect identified in GA in the present study was
different from the study of Song et al (16), which found the use of BBs had no
association with the incidence of GA. There were some explanations
accounting for this difference. On the one hand, the participants
enrolled were different. In the present study, hypertensive
patients not treated with BBs were set as the controls, while other
studies did not consider hypertension (14,15,16,18). On
the other hand, the BB treatment duration may differ, as it was not
considered in other studies (12-18).
Thus, a prolonged BB therapeutic duration may result in a different
outcome.
The present study has certain strengths. Firstly,
hypertensive participants were enrolled to avoid confounding bias.
In addition, a number of confounding factors aside from
hypertension were adjusted for in the multivariate analysis.
Secondly, the effect of different categories of BBs was
investigated, while the majority of previous studies (12-16).
only focused on non-selective BBs. Non-selective BBs block β1-AR
and β2-AR, while selective BBs mainly inhibit β1-AR. However, all
the subtypes of β-AR are expressed in retinal cells (30). The further examination of selective
BBs in AMD is thus warranted. Herein, the association between the
use of selective BBs and AMD was explored, revealing no significant
association. Moreover, the association between the use of BBs and
early-stage AMD was not explored in other studies (12-16,18,19).
BDES reported no significant difference in the use of BBs and
early-stage AMD. The results of the present study are in accordance
with this outcome. Thirdly, the present study concentrated on the
duration of BB treatment. Although short-term BB treatment
(duration, <6 years) had no effect on AMD, there was a
significant trend for decreasing the magnitude of associations of
late-stage AMD with the increasing treatment duration of BBs.
However, there are limitations to the present study
which should be mentioned. Firstly, all the data used were derived
from NHANES, which is a cross-sectional study. The inherent flaws
of cross-sectional design studies are unavoidable. Secondly, the
use of BBs before or after AMD cannot be confirmed. Thirdly, the
interactions among different anti-hypertensive drugs were not
considered, which could lead to an overestimation of the protective
effects of BBs.
In conclusion, the present study demonstrates that
although the use of BBs did not affect early-stage AMD, the
long-term use of BBs is a protective factor against the risk of AMD
among hypertensive patients. However, the outcomes obtained need to
be further validated in completely randomized or multi-center
clinical trials involving the use of BBs and GA.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WF, DL and GS were involved in data collection. JL,
YL, MC and HZ were involved in data analysis. JL and YL prepared
the original draft of the manuscript. HZ was involved in the
writing, critical reviewing and editing of the manuscript. YL and
HZ confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
As the present study employed de-identified
information from the NHANES database approved by the institutional
review board of the NCHS, the Ethics Committee of the Second
Affiliated Hospital of Wenzhou Medical University granted the study
an exemption from ethical review.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Friedman DS, O'Colmain BJ, Muñoz B, Tomany
SC, McCarty C, de Jong PT, Nemesure B, Mitchell P and Kempen J: Eye
Diseases Prevalence Research Group. Prevalence of age-related
macular degeneration in the United States. Arch Ophthalmol.
122:564–572. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wong WL, Su X, Li X, Cheung CMG, Klein R,
Cheng CY and Wong TY: Global prevalence of age-related macular
degeneration and disease burden projection for 2020 and 2040: A
systematic review and meta-analysis. Lancet Glob Health.
2:e106–e116. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Klein R and Klein BE: The prevalence of
age-related eye diseases and visual impairment in aging: Current
estimates. Invest Ophthalmol Vis Sci. 54:ORSF5–ORSF13.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brody BL, Gamst AC, Williams RA, Smith AR,
Lau PW, Dolnak D, Rapaport MH, Kaplan RM and Brown SI: Depression,
visual acuity, comorbidity, and disability associated with
age-related macular degeneration. Ophthalmology. 108:1893–1901.
2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gusar I, Čanović S, Ljubičić M, Šare S,
Perić S, Caktaš IL, Kovačević P, Paštar Z and Konjevoda S:
Religiousness, anxiety and depression in patients with glaucoma,
age-related macular degeneration and diabetic retinopathy.
Psychiatr Danub. 33 (Suppl 4):S965–S973. 2021.PubMed/NCBI
|
|
6
|
Macnamara A, Chen C, Schinazi VR,
Saredakis D and Loetscher T: Simulating macular degeneration to
investigate activities of daily living: A systematic review. Front
Neurosci. 15(663062)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mitchell P, Liew G, Gopinath B and Wong
TY: Age-related macular degeneration. Lancet. 392:1147–1159.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ricci F, Bandello F, Navarra P, Staurenghi
G, Stumpp M and Zarbin M: Neovascular age-related macular
degeneration: Therapeutic management and new-upcoming approaches.
Int J Mol Sci. 21(8242)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Park YG, Park YS and Kim IB: Complement
system and potential therapeutics in age-related macular
degeneration. Int J Mol Sci. 22(6851)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lavine JA, Sang Y, Wang S, Ip MS and
Sheibani N: Attenuation of choroidal neovascularization by
β(2)-adrenoreceptor antagonism. JAMA Ophthalmol. 131:376–382.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cho YK, Shin EY, Uehara H and Ambati B:
The effect of 0.5% timolol maleate on Corneal(Lymph)Angiogenesis in
a murine suture model. J Ocul Pharmacol Ther. 34:403–409.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Traband A, Shaffer JA and VanderBeek BL:
Systemic beta-blockers in neovascular age-related macular
degeneration. Retina. 37:41–46. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kolomeyer AM, Maguire MG, Pan W and
VanderBeek BL: Systemic beta-blockers and risk of progression to
neovascular age-related macular degeneration. Retina. 39:918–925.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Montero JA, Ruiz-Moreno JM, Sanchis-Merino
E and Perez-Martin S: Systemic beta-blockers may reduce the need
for repeated intravitreal injections in patients with wet
age-related macular degeneration treated by bevacizumab. Retina.
33:508–512. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Thomas AS, Redd T and Hwang T: Effect of
systemic beta-blockers, ace inhibitors, and angiotensin receptor
blockers on development of choroidal neovascularization in patients
with age-related macular degeneration. Retina. 35:1964–1968.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Song D, Hua P, VanderBeek BL, Dunaief JL,
Grunwald JE, Daniel E, Maguire MG, Martin DF and Ying GS: CATT
Research Group. Systemic medication use and the incidence and
growth of geographic atrophy in the comparison of age-related
macular degeneration treatments trials. Retina. 41:1455–1462.
2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Klein R, Myers CE and Klein BEK:
Vasodilators, blood pressure-lowering medications, and age-related
macular degeneration: The beaver dam eye study. Ophthalmology.
121:1604–1611. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Davis A, Cohen SM, Pautler SE,
Billiris-Findlay K and Eichenbaum DA: Beta blocker use and
age-related macular degeneration. Acta Ophthalmol. 90:e162–e163.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yeung L, Huang TS, Lin YH, Hsu KH,
Chien-Chieh Huang J and Sun CC: β-blockers and neovascular
age-related macular degeneration. Ophthalmology. 124:409–411.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ogrodowczyk M, Dettlaff K and Jelinska A:
Beta-blockers: Current state of knowledge and perspectives. Mini
Rev Med Chem. 16:40–54. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ristori C, Filippi L, Dal Monte M, Martini
D, Cammalleri M, Fortunato P, la Marca G, Fiorini P and Bagnoli P:
Role of the adrenergic system in a mouse model of oxygen-induced
retinopathy: Antiangiogenic effects of beta-adrenoreceptor
blockade. Invest Ophthalmol Vis Sci. 52:155–170. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shyu KG, Lu MJ, Chang H, Sun HY, Wang BW
and Kuan P: Carvedilol modulates the expression of
hypoxia-inducible factor-1alpha and vascular endothelial growth
factor in a rat model of volume-overload heart failure. J Card
Fail. 11:152–159. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dal Monte M, Filippi L and Bagnoli P:
Beta3-adrenergic receptors modulate vascular endothelial growth
factor release in response to hypoxia through the nitric oxide
pathway in mouse retinal explants. Naunyn Schmiedebergs Arch
Pharmacol. 386:269–278. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cheon EW, Park CH, Kang SS, Cho GJ, Yoo
JM, Song JK and Choi WS: Nitric oxide synthase expression in the
transient ischemic rat retina: neuroprotection of betaxolol.
Neurosci Lett. 330:265–269. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hirooka K, Kelly ME, Baldridge WH and
Barnes S: Suppressive actions of betaxolol on ionic currents in
retinal ganglion cells may explain its neuroprotective effects. Exp
Eye Res. 70:611–621. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chidlow G, Melena J and Osborne NN:
Betaxolol, a beta(1)-adrenoceptor antagonist, reduces Na(+) influx
into cortical synaptosomes by direct interaction with Na(+)
channels: Comparison with other beta-adrenoceptor antagonists. Br J
Pharmacol. 130:759–766. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zając-Pytrus HM, Pilecka A, Turno-Kręcicka
A, Adamiec-Mroczek J and Misiuk-Hojło M: The dry form of
age-related macular degeneration (AMD): The current concepts of
pathogenesis and prospects for treatment. Adv Clin Exp Med.
24:1099–1104. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ammar MJ, Hsu J, Chiang A, Ho AC and
Regillo CD: Age-related macular degeneration therapy: A review.
Curr Opin Ophthalmol. 31:215–221. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Smith CP, Sharma S and Steinle JJ:
Age-related changes in sympathetic neurotransmission in rat retina
and choroid. Exp Eye Res. 84:75–81. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Casini G, Dal Monte M, Fornaciari I,
Filippi L and Bagnoli P: The β-adrenergic system as a possible new
target for pharmacologic treatment of neovascular retinal diseases.
Prog Retin Eye Res. 42:103–129. 2014.PubMed/NCBI View Article : Google Scholar
|