Introduction
Obstructive sleep apnea (OSA) is a common disorder
characterized by severe daytime sleepiness and repeated episodes of
upper airway obstruction while sleeping (1). Although it can affect women and
children, OSA is more frequently observed in elderly males. In
females, the incidence increases following menopause to the point
that post-menopausal rates are comparable to those of males
(2). OSA has been linked to
cardiovascular diseases, including coronary artery disease,
arterial hypertension, diabetes mellitus, metabolic syndrome and
cerebrovascular disease (3-5). It
is estimated that >80% of those suffering from OSA, ranging in
severity from moderate to severe, remain undiagnosed (6).
A full-night polysomnography (PSG) in a sleep clinic
is the gold standard for detecting OSA. However, this is not
advised as a typical screening approach as it is time-consuming and
costly (7).
Thus, a rapid and reliable screening procedure for
high-risk populations is still required. The selection of a
screening technique will depend on the capability to achieve a
specific goal: To include patients with OSA for proper sleep
testing, to identify those with more severe disease in order to
enable early diagnosis and treatment, and to exclude patients
without OSA or with mild OSA, whose assessment and treatment are
less urgent.
A number of clinical scores, including the STOP-BANG
questionnaire, Epworth Sleepiness Scale (ESS) and Berlin
questionnaire, are currently being used as screening tools. The ESS
is utilized for OSA, even though it was designed to assess the
severity of subjective daytime sleepiness. The ESS questionnaire
requests individuals to rate their likelihood of dozing off in
eight distinct settings, on a range from 0 to 3. The soporific
quality of these scenarios was the deciding factor in their
selection (8). A self-administered
questionnaire used to calculate the STOP-BANG score combines data
of a patient's complaints and clinical features. Body mass index
(BMI), age, neck circumference and sex are among the clinical
factors taken into account while evaluating the complaints, while
snoring, fatigue, observed apnea and elevated blood pressure are
also included (9). The Berlin
questionnaire requests individuals about snoring, obesity, daytime
sleepiness, fatigue and arterial hypertension, and it was initially
based on a sample of 744 individuals, of whom 13% were diagnosed
with a polygraphic recording in the home environment (10).
The Lausanne Neck circumference, Obesity, Snoring,
Age, Sex (NoSAS) score test, a simple, effective and practical tool
that identifies those at risk of OSA, has recently been proposed as
a screening tool (11). The NoSAS
score evaluates five factors: Neck circumference, Obesity, Snoring,
Age and Sex, and each factor assigns a certain number of points: 4
for a neck circumference >40 cm, 3 for a BMI of 25
kg/m2 to <30 kg/m2, or 5 for a BMI of ≥30
kg/m2, 2 for snoring, 4 for an age >55 years, and 2
for the male sex. The NoSAS score ranges between 0 and 17, with
scores of ≥8 indicating a high probability of OSA. This test was
shown to have a negative predictive value (NPV) of 90 and 98% in
two ethnically distinct cohorts; thereby, it facilitates the
identification of those at risk of the disease and the exclusion of
others without risk (11).
The EES, STOP-BANG and Berlin questionnaires have
all been previously validated as screening tools for OSA in Greek
patients (12-14).
Thus, the aim of the present study was to validate, for the first
time, to the best of our knowledge, the NoSAS score in the Greek
population and to compare its screening abilities for OSA with the
STOP-BANG questionnaire, the Berlin questionnaire and the ESS.
Patients and methods
Study design
The present study retrospectively analyzed
individuals who had previously undergone a full-night PSG between
October 1, 2018 and November 30, 2021 at the Sleep Clinic of the
Sismanogleio Hospital, Athens, Greece. The Institutional Review
Board and the Independent Department of Quality, Research, and
Continuing Education of Sismanogleio Hospital approved the research
protocol (5974/05.04.2021 and 8077/16/04/2021, respectively). All
patients provided written informed consent for inclusion in the
study. All participants were suspected of having OSA. The criteria
for inclusion were as follows: i) An age >18 years; ii) not
previously diagnosed or treated for OSA; iii) available
comprehensive anthropometric and demographic data regarding ESS,
and STOP-BANG and Berlin questionnaires; and iv) a sleep efficacy
≥60%. The exclusion criteria were the following: Individuals with
an active psychiatric disorder; a history of brain tumors; a
history of epilepsy; a history of benzodiazepine use; patients
unable to read and/or write; individuals with alternative
diagnoses, i.e., central sleep apnea and obesity/hypoventilation
syndrome; and all patients with PSG assessment with technical
errors during data collection.
In the present study, demographics such as age and
sex, anthropometric parameters such as height, weight, BMI and neck
circumference, scores of ESS, STOP-BANG questionnaire, Berlin
questionnaire, and PSG data such as the apnea-hypopnea index (AHI)
were obtained from the recorded data of the patients. All
questionnaires were completed at the same time and independently by
all patients. The comorbidities of all individuals were also noted.
The NoSAS score was determined with the use of the recorded data of
the participants.
Screening questionnaires
The ESS consists of eight questions with a
four-point Likert response scale (0-3) and a score range of 0-24. A
score of 10 on the ESS suggests a high risk of OSA and excessive
daytime sleepiness (8). A total of
eight yes/no items comprise the STOP-BANG questionnaire, four of
which are demographic (BANG: BMI, >35 kg/m2; age,
>50 years; neck circumference, >40 cm; male sex) and four of
which are subjective (STOP: snoring, fatigue, observed apnea and
elevated blood pressure). The overall score is between 0 and 8. The
patient is at a high risk for OSA if they respond ‘yes’ to three or
more questions (9). The 11 questions
of the Berlin questionnaire are divided into three groups: Five
questions concerning snoring are included in the first category,
three questions about daytime sleepiness and fatigue are included
in the second category, and information about BMI and the history
of hypertension is included in the third and final category. The
answers to these three categories were used to calculate the Berlin
questionnaire score as follows: The first and second categories
were deemed positive if the answers suggested frequent symptoms
(>3-4 times/week) on two or more survey items, and the third
category was determined as positive if there was a history of
arterial hypertension or a BMI of >30 kg/m2. The
participants were categorized as being at a high risk of having OSA
if they scored positively in two or more categories (10). Valid Greek language versions of the
aforementioned questionnaires were used (12-14).
The NoSAS score was first translated into Greek by a
professional translation company. The translated score was then
translated back into English by clinicians who were proficient in
the language. The clinicians determined on the final version of the
translated score. A NoSAS score ≥8 is suggestive of being at high
risk for OSA (11).
PSG
The diagnosis of OSA was made using a PSG.
Electromyography of the chin and the leg, electrooculography,
electroencephalography, oxygen saturation, electrocardiography,
abdominal and thoracic respiratory effort, body position and air
flow (nasal pressure transducer and oronasal thermistor), and
tracheal microphone were recorded using the Respironics Alice 6 LDx
Diagnostic Sleep System (Philips).
PSG data were evaluated by a physician who is a
sleep disorders specialist and who was blinded to the results of
the NoSAS questionnaire. The American Academy of Sleep Medicine
(AASM) criteria were used to score the sleep and respiratory events
(15). The AHI was determined by
calculating the number of apnea and hypopnea events per hour. OSA
was diagnosed based on AHI. The severity of OSA was classified as
follows: Mild (AHI, ≥5 and <15 events/h), moderate (AHI, ≥15 or
<30 events/h) and severe (AHI, ≥30 events/h).
Statistical analysis
The demographic, anthropometric and clinical
characteristics of the study participants were summarized using
either their mean and standard deviation (SD) and median (range)
for continuous variables, or absolute (N) and relative (%)
frequencies, for categorical ones. Differences in the distributions
of these characteristics across different categories of OSA
severity were assessed using one-way ANOVA for continuous variables
with normal distribution and using the Kruskal-Wallis test for
variables with a non-normal distribution, and for categorical
variables using the Chi-squared or Fisher's exact test.
Receiver operating characteristic (ROC) analysis was
performed for all four scores under investigation. The
corresponding results include graphs of the ROC curve and graphs of
sensitivity and specificity vs. varying values of the score's
cut-off. Results are also presented in tabular form where
sensitivity, specificity, percentage of correct classification,
positive predictive value (PPV) and NPV are given for the optimal
cut-off value.
The optimal cut-off value for each score was derived
using the method proposed by Liu (16), which is based on the maximization of
the product of the sensitivity and specificity. ROC curves are
graphically presented simultaneously for all scores and areas under
the curves are formally compared. Results from a global test are
provided along with tests for all scores against the NoSAS score
(with Sidak adjustments for multiple comparisons). All ROC analysis
results are provided for both definitions of disease as mentioned
above. P-values <0.05 were considered to indicate statistically
significant differences. All analyses were conducted utilizing
Stata version 15.1 (Stata Corp LLC).
Results
Study population
A total of 347 participants, 243 males and 104
females, were included in the present study, of whom 96 (27.7%)
were aged ≥65 years and 251 (72.3%) were aged <65 years. Of the
participants, 50 (14.4%) were not diagnosed with OSA, while 15
(4.3%) were diagnosed with mild OSA, 30 (8.6%) were diagnosed with
moderate OSA and 252 (72.6%) were diagnosed with severe OSA. The
characteristics of the study population based on OSA severity are
presented in Table I. Questionnaire
scores in relation to OSA severity are summarized in Table II.
 | Table IPopulation characteristics by OSA
severity. |
Table I
Population characteristics by OSA
severity.
| Variable | No OSA, n=50
(14.4%) | Mild, n=15
(4.3%) | Moderate, n=30
(8.6%) | Severe, n=252
(72.6%) | Overall, n=347
(100%) | P-value |
|---|
| Age, years; mean
(SD)a | 47.4 (14.3) | 53.5 (13.8) | 56.6 (11.0) | 57.6 (13.2) | 55.8 (13.7) | <0.001 |
| Height, cm; mean
(SD)a | 168.2 (9.9) | 173.6 (12.8) | 164.7 (7.0) | 170.8 (9.7) | 170.0 (9.8) | 0.104 |
| Weight, kg; mean
(SD)a | 88.0 (21.8) | 91.9 (10.8) | 88.5 (15.7) | 98.7 (19.2) | 96.0 (19.5) | <0.001 |
| BMI,
kg/m2; mean (SD)a | 31.3 (8.1) | 30.8 (4.6) | 32.7 (6.3) | 33.9 (6.0) | 33.3 (6.4) | <0.001 |
| Neck circumference,
cm; median (range)b | 37.0 (34.0-40.0) | 40.0 (38.0-41.0) | 37.5 (35.0-41.0) | 41.0 (39.0-43.0) | 40.0 (38.0-42.0) | <0.001 |
| Sex, n
(%)c | | | | | | <0.001 |
|
Male | 24 (48.0%) | 10 (66.7%) | 13 (43.3%) | 196 (77.8%) | 243 (70.0%) | |
|
Female | 26 (52.0%) | 5 (33.3%) | 17 (56.7%) | 56 (22.2%) | 104 (30.0%) | |
| Age, n
(%)c | | | | | | 0.003 |
|
<65 | 45 (90.0%) | 10 (66.7%) | 24 (80.0%) | 172 (68.3%) | 251 (72.3%) | |
|
≥65 | 5 (10.0%) | 5 (33.3%) | 6 (20.0%) | 80 (31.7%) | 96 (27.7%) | |
| BMI, n
(%)d | | | | | | <0.001 |
|
<25 | 12 (24.0%) | 0 (0.0%) | 1 (3.3%) | 13 (5.2%) | 26 (7.5%) | |
|
25-29 | 14 (28.0%) | 6 (40.0%) | 9 (30.0%) | 50 (19.8%) | 79 (22.8%) | |
|
≥30 | 24 (48.0%) | 9 (60.0%) | 20 (66.7%) | 189 (75.0%) | 242 (69.7%) | |
| Hypertension, n
(%)c | | | | | | 0.001 |
|
No | 33 (66.0%) | 12 (80.0%) | 15 (50.0%) | 113 (44.8%) | 173 (49.9%) | |
|
Yes | 17 (34.0%) | 3 (20.0%) | 15 (50.0%) | 139 (55.2%) | 174 (50.1%) | |
| Snoring, n
(%)d | | | | | | <0.001 |
|
No | 7 (14.0%) | 1 (6.7%) | 1 (3.3%) | 4 (1.6%) | 13 (3.7%) | |
|
Yes | 43 (86.0%) | 14 (93.3%) | 29 (96.7%) | 248 (98.4%) | 334 (96.3%) | |
| Feeling tired, n
(%)c | | | | | | 0.493 |
|
No | 11 (22.0%) | 3 (20.0%) | 3 (10.0%) | 40 (15.9%) | 57 (16.4%) | |
|
Yes | 39 (78.0%) | 11 (73.3%) | 27 (90.0%) | 211 (83.7%) | 288 (83.0%) | |
|
N/A | 0 (0.0%) | 1 (6.7%) | 0 (0.0%) | 1 (0.4%) | 2 (0.6%) | |
| Apnea, n
(%)c | | | | | | <0.001 |
|
No | 20 (40.0%) | 8 (53.3%) | 6 (20.0%) | 46 (18.3%) | 80 (23.1%) | |
|
Yes | 30 (60.0%) | 7 (46.7%) | 24 (80.0%) | 206 (81.7%) | 267 (76.9%) | |
 | Table IIScale scores and categories by OSA
severity. |
Table II
Scale scores and categories by OSA
severity.
| Variable | No OSA, n=50
(14.4%) | Mild, n=15
(4.3%) | Moderate, n=30
(8.6%) | Severe, n=252
(72.6%) | Overall, n=347
(100%) | P-value |
|---|
| NoSAS score, median
(range)a | 7.5 (7.0-11.0) | 11.0
(7.0-13.0) | 11.0
(7.0-11.0) | 13.0
(11.0-17.0) | 13.0
(9.0-15.0) | <0.001 |
| STOP BANG score,
median (range)a | 4.0 (3.0-5.0) | 4.0 (3.0-5.0) | 4.5 (4.0-6.0) | 6.0 (5.0-6.0) | 5.0 (4.0-6.0) | <0.001 |
| ESS score, median
(range)a | 7.0 (4.0-11.0) | 6.0 (3.0-9.0) | 8.0 (6.0-12.0) | 7.0 (4.0-11.0) | 7.0 (4.0-11.0) | 0.382 |
| BQ score, mean
(SD)b | 2.1 (0.8) | 2.0 (0.8) | 2.5 (0.6) | 2.5 (0.6) | 2.4 (0.7) | <0.001 |
| AHI (episodes/h),
median (range)a | 1.1 (0.0-2.7) | 13.2
(7.9-14.6) | 24.3
(19.7-27.2) | 64.3
(49.5-79.8) | 54.6
(27.2-75.2) | <0.001 |
| NoSAS category, n
(%)c | | | | | | <0.001 |
|
Low risk for
OSA | 25 (37.9%) | 5 (7.6%) | 7 (10.6%) | 29 (43.9%) | 66 (100.0%) | |
|
High risk
for OSA | 25 (8.9%) | 10 (3.6%) | 23 (8.2%) | 223 (79.4%) | 281 (100.0%) | |
| STOP BANG category,
n (%)d | | | | | | <0.001 |
|
Low risk for
OSA | 11 (68.8%) | 2 (12.5%) | 1 (6.3%) | 2 (12.5%) | 16 (100.0%) | |
|
High risk
for OSA | 39 (11.8%) | 13 (3.9%) | 29 (8.8%) | 250 (75.5%) | 331 (100.0%) | |
| ESS category, n
(%) | | | | | | 0.145 |
|
Normal
sleepiness | 36 (14.9%) | 15 (6.2%) | 21 (8.7%) | 169 (70.1%) | 241 (100.0%) | |
|
Mild/moderate
excessive sleepiness | 7 (10.1%) | 0 (0.0%) | 8 (11.6%) | 54 (78.3%) | 69 (100.0%) | |
|
Severe
excessive sleepiness | 7 (18.9%) | 0 (0.0%) | 1 (2.7%) | 29 (78.4%) | 37 (100.0%) | |
| BQ category, n
(%) | | | | | | 0.001 |
|
Low risk for
OSA | 10 (34.5%) | 3 (10.3%) | 2 (6.9%) | 14 (48.3%) | 29 (100.0%) | |
|
High risk
for OSA | 40 (12.6%) | 12 (3.8%) | 28 (8.8%) | 238 (74.8%) | 318 (100.0%) | |
Results of ROC analysis. NoSAS
questionnaire
By performing ROC analysis, the discriminative
ability of the NoSAS score for moderate and severe OSA was found to
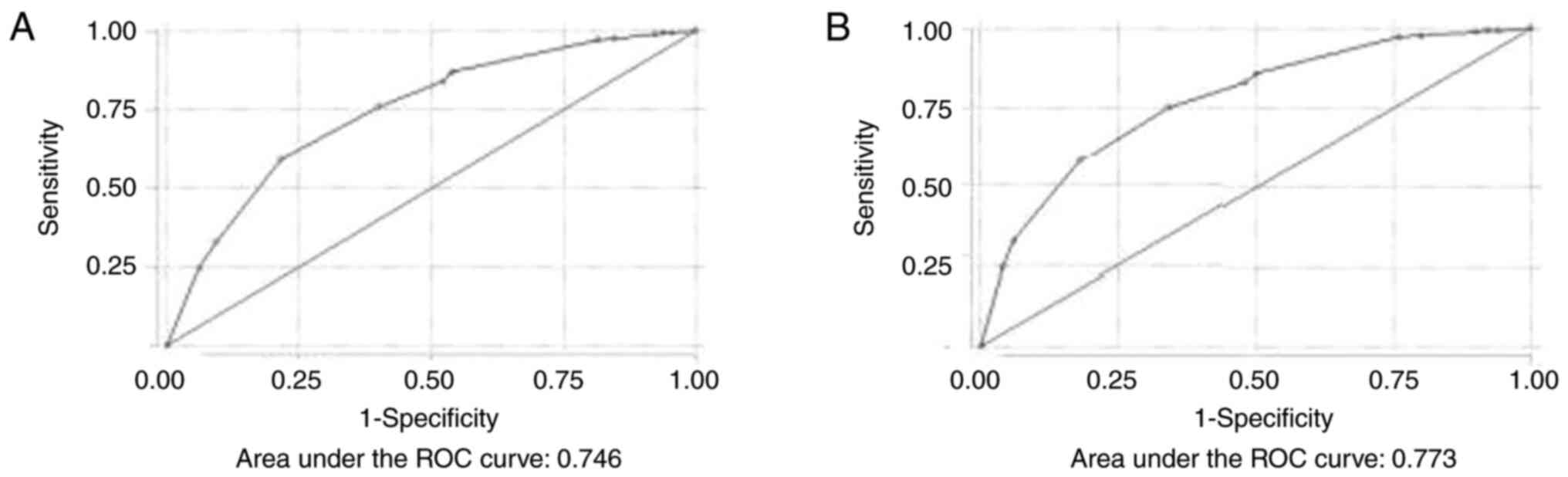
be excellent [area under the curve (AUC), 0.746] (Fig. 1A).
Using the NoSAS score, for scores >7 to predict
moderate and severe OSA, the sensitivity and specificity were 86.88
and 46.15%, respectively. The optimal cut-off value was >11,
where the sensitivity and specificity were 59.2 and 78.4%,
respectively, the PPV was 92.27% and the NPV was 30.72%. The
percentage of correct classification was 62.82%.
The discriminative ability of the NoSAS score for
all severity categories of OSA was also excellent (AUC, 0.773)
(Fig. 1B). Using the NoSAS score,
for scores >7 to predict OSA (all severity categories), the
sensitivity and specificity were 85.8 and 50%, respectively. The
PPV for scores >7 to predict OSA (all severity categories) was
91.1% and the NPV was 30.9%. The optimal cut-off value was >9,
where the sensitivity and specificity were 75 and 66% respectively,
the PPV was 92.92% and the NPV was 30.84%. The percentage of
correct classification was 73.78%. Table III displays the sensitivity and
specificity of different cut-off values of the NoSAS score for
detecting moderate and severe OSA and OSA of all severity
categories.
 | Table IIINoSAS, STOP-BANG, Berlin
questionnaire and ESS score vs. moderate and severe OSA and OSA of
all severity categories: Sensitivity and specificity for various
cut-off values. |
Table III
NoSAS, STOP-BANG, Berlin
questionnaire and ESS score vs. moderate and severe OSA and OSA of
all severity categories: Sensitivity and specificity for various
cut-off values.
| Moderate and severe
OSA |
|---|
| NoSAS score | Sensitivity
(%) | Specificity
(%) |
|---|
| 2 | 99.29 | 4.62 |
| 3 | 99.29 | 6.15 |
| 4 | 98.94 | 7.69 |
| 5 | 97.52 | 15.38 |
| 6 | 97.16 | 18.46 |
| 7 | 86.88 | 46.15 |
| 8 | 84.04 | 47.69 |
| 9 | 75.89 | 60.00 |
| 11 | 59.22 | 78.46 |
| 13 | 32.98 | 90.77 |
| 15 | 24.82 | 93.85 |
| 17 | 0.00 | 100.00 |
| OSA of all severity
categories |
| NoSAS score | Sensitivity
(%) | Specificity
(%) |
| 2 | 99.33 | 6.00 |
| 3 | 99.33 | 8.00 |
| 4 | 98.99 | 10.00 |
| 5 | 97.64 | 20.00 |
| 6 | 97.31 | 24.00 |
| 7 | 85.86 | 50.00 |
| 8 | 83.16 | 52.00 |
| 9 | 75.08 | 66.00 |
| 11 | 57.91 | 82.00 |
| 13 | 32.32 | 94.00 |
| 15 | 24.24 | 96.00 |
| 17 | 0.00 | 100.00 |
| Moderate and severe
OSA |
| STOP-BANG
score | Sensitivity
(%) | Specificity
(%) |
| 1 | 100.00 | 6.15 |
| 2 | 98.94 | 20.00 |
| 3 | 92.91 | 43.08 |
| 4 | 73.40 | 67.69 |
| 5 | 49.29 | 90.77 |
| 6 | 19.50 | 93.85 |
| 7 | 4.96 | 98.46 |
| 8 | 0.00 | 100.00 |
| OSA of all severity
categories |
| STOP-BANG
score | Sensitivity
(%) | Specificity
(%) |
| 1 | 100.00 | 8.00 |
| 2 | 98.32 | 22.00 |
| 3 | 91.25 | 44.00 |
| 4 | 71.38 | 68.00 |
| 5 | 47.47 | 92.00 |
| 6 | 18.86 | 94.00 |
| 7 | 4.71 | 98.00 |
| 8 | 0.00 | 100.00 |
| Moderate and severe
OSA |
| ESS score | Sensitivity
(%) | Specificity
(%) |
| 0 | 96.45 | 1.54 |
| 1 | 92.91 | 9.23 |
| 2 | 88.30 | 12.31 |
| 3 | 79.79 | 21.54 |
| 4 | 71.28 | 30.77 |
| 5 | 65.60 | 33.85 |
| 6 | 57.80 | 49.23 |
| 7 | 51.06 | 56.92 |
| 8 | 44.33 | 60.00 |
| 9 | 38.30 | 66.15 |
| 10 | 31.91 | 78.46 |
| 11 | 24.11 | 81.54 |
| 12 | 18.44 | 84.62 |
| 13 | 12.77 | 87.69 |
| 14 | 11.35 | 89.23 |
| 15 | 10.28 | 89.23 |
| 16 | 8.16 | 90.77 |
| 17 | 6.38 | 95.38 |
| 18 | 3.19 | 96.92 |
| 19 | 2.48 | 98.46 |
| 20 | 1.77 | 98.46 |
| 21 | 0.71 | 100.00 |
| 22 | 0.00 | 100.00 |
| OSA of all severity
categories |
| ESS score | Sensitivity
(%) | Specificity
(%) |
| 0 | 96.63 | 2.00 |
| 1 | 92.59 | 8.00 |
| 2 | 87.88 | 10.00 |
| 3 | 79.46 | 20.00 |
| 4 | 71.38 | 32.00 |
| 5 | 65.32 | 32.00 |
| 6 | 56.90 | 46.00 |
| 7 | 50.51 | 56.00 |
| 8 | 43.77 | 58.00 |
| OSA of all severity
categories |
| ESS score | Sensitivity
(%) | Specificity
(%) |
| 9 | 37.37 | 62.00 |
| 10 | 30.30 | 72.00 |
| 11 | 22.90 | 76.00 |
| 12 | 17.51 | 80.00 |
| 13 | 12.12 | 84.00 |
| 14 | 10.77 | 86.00 |
| 15 | 9.76 | 86.00 |
| 16 | 7.74 | 88.00 |
| 17 | 6.06 | 94.00 |
| 18 | 3.03 | 96.00 |
| 19 | 2.36 | 98.00 |
| 20 | 1.68 | 98.00 |
| 21 | 0.67 | 100.00 |
| 22 | 0.00 | 100.00 |
| Moderate and severe
OSA |
| BQ score | Sensitivity
(%) | Specificity
(%) |
| 0 | 100.00 | 4.62 |
| 1 | 94.33 | 20.00 |
| 2 | 55.67 | 66.15 |
| 3 | 0.00 | 100.00 |
| OSA of all severity
categories |
| BQ score | Sensitivity
(%) | Specificity
(%) |
| 0 | 99.66 | 4.00 |
| 1 | 93.60 | 20.00 |
| 2 | 54.21 | 64.00 |
| 3 | 0.00 | 100.00 |
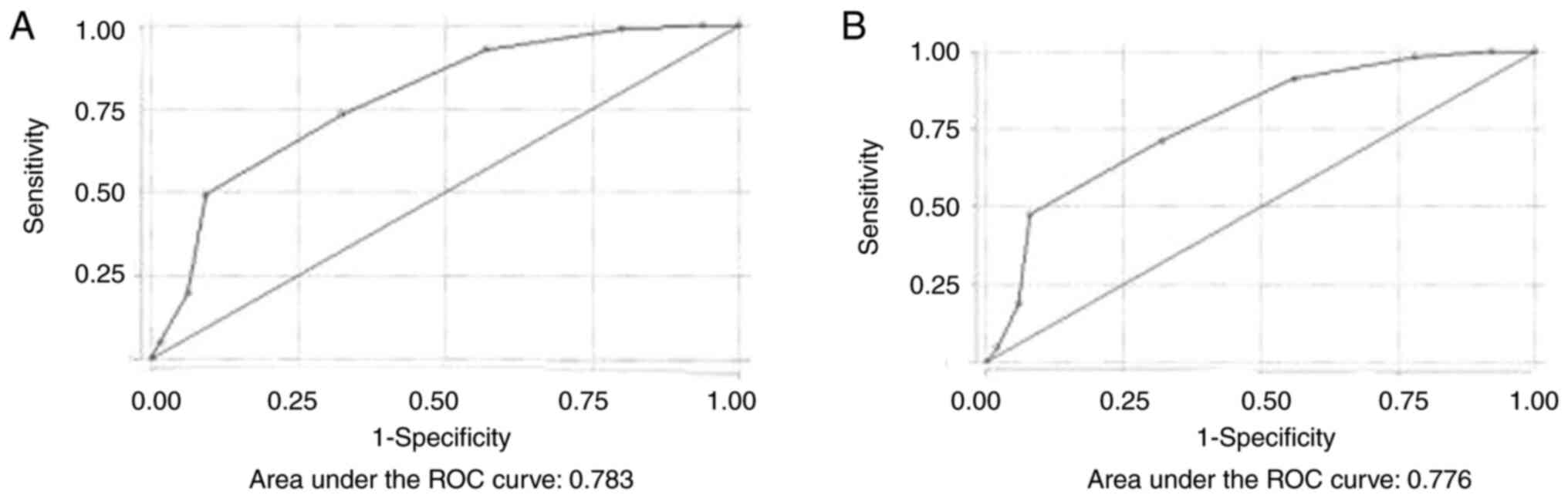
STOP-BANG questionnaire. By performing ROC
analysis, the discriminative ability of the STOP-BANG questionnaire
for moderate and severe OSA was excellent (AUC, 0.783) (Fig. 2A).
Using the STOP-BANG questionnaire, for scores >2
to predict moderate and severe OSA, the sensitivity and specificity
were 98.94 and 20%, respectively. The optimal cut-off value was
>4, where the sensitivity and specificity were 73.4 and 67.6%,
respectively, the PPV was 90.79% and the NPV was 36.97%. The
percentage of correct classification was 72.33%. The discriminative
ability of the STOP-BANG questionnaire for all severity categories
of OSA was also excellent (AUC, 0.776) (Fig. 2B).
Using the STOP-BANG questionnaire for scores >2
to predict OSA (all severity categories), the sensitivity and
specificity were 98.32 and 22%, respectively. The optimal cut-off
value was >4, where the sensitivity and specificity were 71.3
and 68%, respectively, the PPV was 92.98% and the NPV was 28.57%.
The percentage of correct classification was 70.89%. Table III displays the sensitivity and
specificity of different cut-off values of the STOP-BANG score for
detecting moderate and severe OSA and OSA of all severity
categories.
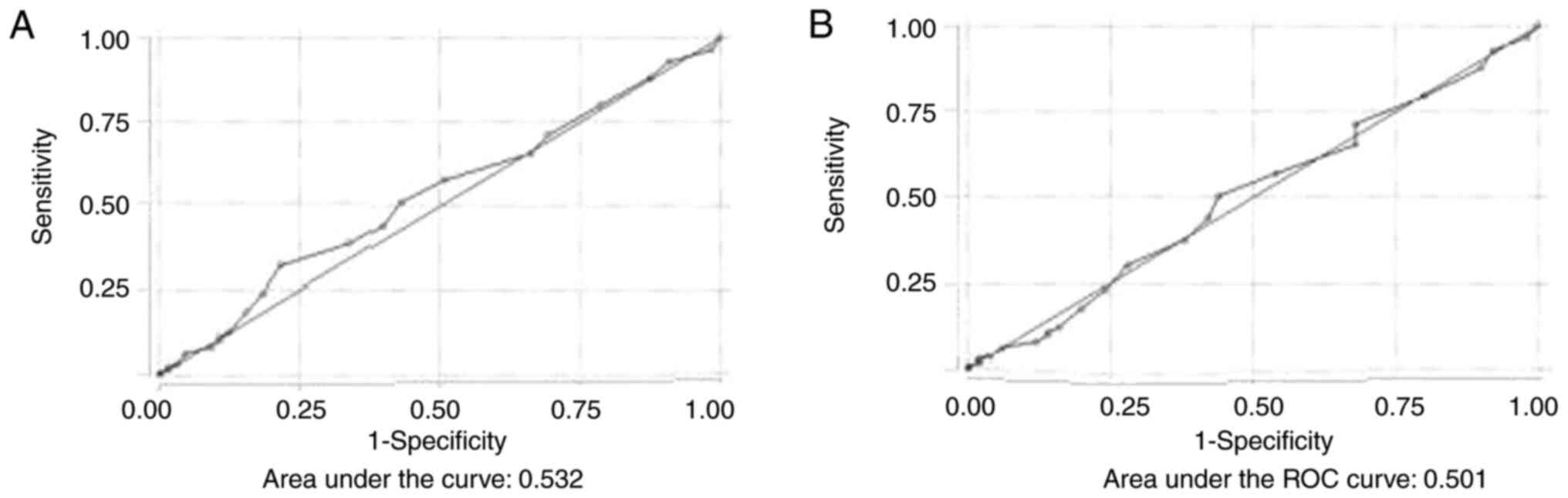
ESS. By performing ROC analysis, the
discriminative ability of the ESS for moderate and severe OSA was
poor (AUC, 0.532) (Fig. 3A). Using
the ESS, for scores >10 to predict moderate and severe OSA, the
sensitivity and specificity were 31.9 and 78.4%, respectively. The
optimal cut-off value was >7, where the sensitivity and
specificity were 51 and 56.9%, respectively, the PPV was 83.72% and
the NPV was 21.14%. The percentage of correct classification was
52.16%. The discriminative ability of the ESS for all severity
categories of OSA was also poor (AUC, 0.501) (Fig. 3B).
Using the ESS, for score >10 to predict OSA (all
severity categories), sensitivity and specificity were 30.3 and
72%, respectively. The optimal cut-off value was >7, where the
sensitivity and specificity were 50.5 and 56%, respectively, the
PPV was 87.21% and the NPV was 16%. The percentage of correct
classification was 51.3%. Table
III displays the sensitivity and specificity of different
cut-off values of ESS for detecting moderate and severe OSA and OSA
of all severity categories.
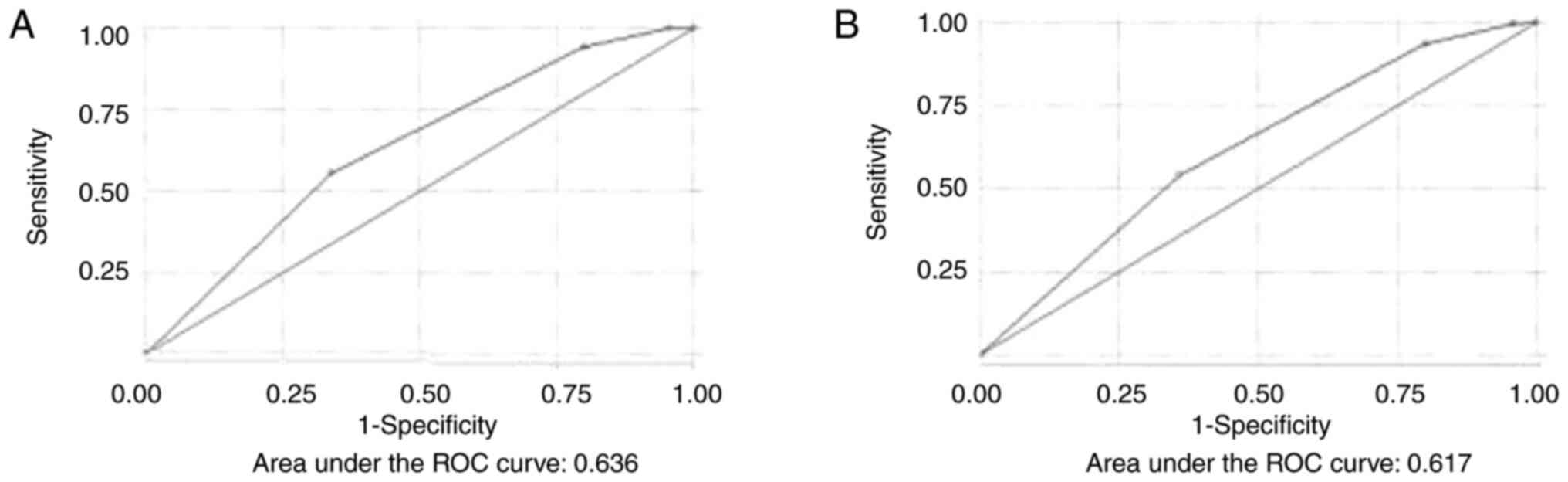
Berlin questionnaire. By performing ROC
analysis, the discriminative ability of the Berlin questionnaire
for moderate and severe OSA was acceptable (AUC, 0.636) (Fig. 4A). Using the Berlin questionnaire
score, for a score >1 to predict moderate and severe OSA, the
sensitivity and specificity were 94.33 and 20%, respectively. The
optimal cut-off value was >2, where the sensitivity and
specificity were 55.6 and 66.1%, respectively, the PPV was 87.71%
and the NPV was 25.60%. The percentage of correct classifications
was 57.64%. The discriminative ability of the Berlin questionnaire
for all severity categories of OSA was also acceptable (AUC, 0.617)
(Fig. 4B).
Using the Berlin questionnaire score, for a score
>1 to predict OSA (all severity categories), the sensitivity and
specificity were 93.6 and 20%, respectively. The optimal cut-off
value was >2, where the sensitivity and specificity were 54.2
and 64%, respectively, the PPV was 89.94% and the NPV was 19.05%.
The percentage of correct classification was 55.62%. Table III displays the sensitivity and
specificity of different cut-off values of Berlin questionnaire
score for detecting moderate and severe OSA and OSA of all severity
categories.
Comparison of the diagnostic
performance of the NoSAS, ESS, STOP-BANG and Berlin
questionnaires
The NOSAS and STOP-BANG scores clearly performed
better in detecting moderate and severe OSA, and in detecting OSA
of all severity categories, compared to the ESS and the Berlin
score with a very slight superiority of STOP-BANG.
A statistically significant difference was observed
in the AUC of all scores for the detection of moderate and severe
OSA and for the detection of OSA of all severity categories
(P<0.001). There was no statistically significant difference
between the AUC of NoSAS and the AUC of STOP-BANG for the detection
of moderate and severe OSA, and for the detection of OSA of all
severity categories (P=0.488 and P=0.999, respectively) (Table IV).
 | Table IVArea under the ROC curve for all
scores and equality tests. |
Table IV
Area under the ROC curve for all
scores and equality tests.
| Moderate and severe
OSA |
|---|
| Score | AUC (95% CI) | P-value (vs.
NoSAS) | Global test
P-value |
|---|
| NoSAS | 0.746
(0.680-0.813) | Reference | <0.001 |
| STOP-BANG | 0.783
(0.720-0.847) | 0.488 | |
| ESS | 0.532
(0.456-0.609) | <0.001 | |
| BQ | 0.636
(0.565-0.707) | 0.011 | |
| OSA of all severity
categories |
| Score | AUC (95% CI) | P-value (vs.
NoSAS) | Global test
P-value |
| NoSAS | 0.774
(0.705-0.843) | Reference | <0.001 |
| STOP-BANG | 0.777
(0.705-0.848) | 0.999 | |
| ESS | 0.502
(0.414-0.590) | <0.001 | |
| BQ | 0.617
(0.536-0.698) | <0.001 | |
Discussion
The NoSAS score was first created and validated
using participants from a population in Lausanne, Switzerland
(HypnoLaus cohort) and was separately validated in a population
undergoing PSG due to indicating symptoms (EPISONO cohort)
(11). In that previous study, in
the HypnoLaus cohort, the NoSAS score ≥8 to detect moderate and
severe OSA had an AUC of 74%, a PPV of 47%, and an NPV of 90%. In
the EPISONO cohort, the NoSAS score for a value ≥8 had an AUC of
0.810, PPV of 33%, and NPV of 98% for the detection of moderate and
severe OSA. When comparing the ability to correctly classify the
participants, the NoSAS score clearly outperformed the other scores
in both cohorts. In the HypnoLaus cohort, the AUC was 0.740 for the
NOSAS score, 0.670 for the STOP-BANG score, and 0.630 for the
Berlin score to detect moderate and severe OSA. In the EPISONO
cohort, the AUC was 0.810 for the NoSAS score, 0.680 for the
STOP-BANG score, and 0.650 for the Berlin score for the detection
of moderate and severe OSA (11).
In a study from a Turkish Sleep Unit for the
detection of OSA of all severity categories, the sensitivity,
specificity, PPV and NPV of the NoSAS score ≥8 were 81, 51.2, 88.2
and 37.5%, respectively (17). For
the detection of moderate and severe OSA, for a value ≥8 the
sensitivity, specificity, PPV and NPV of the NoSAS score were 84.5,
38.2, 66 and 63.4%, respectively. The STOP-BANG questionnaire had
the highest sensitivity for all OSA severity categories, but also
had the lowest specificity. The Berlin questionnaire exhibited
similar results to the STOP-BANG questionnaire (17).
In another study on a multi-ethnic Asian cohort, the
sensitivity, specificity, and NPV and PPV of the NoSAS score ≥8 for
predicting severe OSA were 69.2, 73.1, 95.2 and 23.7%, respectively
(18). The STOP-BANG and Berlin
questionnaires performed similarly to the NoSAS score, with the
AUCs of all three questionnaires having a range of 0.682-0.748.
Compared with the STOP-BANG (94.8%) and Berlin (96.3%)
questionnaires, the NoSAS score (95.2%) had an equally high NPV in
ruling out severe OSA (18).
In a large study from China, the AUC for the NoSAS
score for predicting moderate and severe OSA was 0.707(7). In contrast to the present study, the
NoSAS score performed significantly better than the STOP-BANG
questionnaire (AUC 0.704) and the ESS (AUC 0.642), and was similar
to the Berlin questionnaire (AUC, 0.697) for detecting
moderate-to-severe OSA (7). In
addition, in contrast to the present study, in another study on 479
participants from China, the NoSAS score for the detection of OSA
of all severity categories had an AUC of 0.734 and the Berlin
questionnaire had an AUC of 0.732. Both exhibited better predictive
values than the ESS and the STOP-BANG questionnaire (19).
In a study from Portugal with 294 participants,
using the NoSAS score to predict OSA of all severity categories,
moderate/severe OSA and severe OSA, a score of 12 had the optimal
performance, an AUC 0.770, a sensitivity of 57.5% and a specificity
of 83%. In the same study, using the STOP-BANG score to predict OSA
of all severity categories, moderate/severe OSA and severe OSA, a
score of 5 had the best performance with an AUC of 0.813,
sensitivity of 77.3% and specificity of 66.1% (20). Furthermore, in contrast to the
present study, in a study from Switzerland, the NoSAS score had the
highest AUC (0.780) compared to STOP-BANG (0.710) and Berlin
(0.620) for detecting moderate/severe OSA (21).
Of particular interest is a meta-analysis of 10
studies, involving a total of 14,510 patients, which demonstrated
that the NoSAS score for the detection of OSA of all severity
categories was satisfactorily with an AUC of 0.770, similar to the
present study. The same meta-analysis demonstrated that the NoSAS
score ≥8 had a sensitivity of 79.8% and a specificity of 58.2% for
the detection of OSA of all severity categories, while in our
study, the corresponding sensitivity is 85.86% and the specificity
50% (22).
The present study had certainly some limitations.
The study was conducted retrospectively, and NoSAS scores were
calculated based on different answers previously given by the
patients, a fact that may have an effect on the studied performance
of the scores. In addition, the present study sample was derived
from a single sleep clinic in Greece, which limits the
generalization of conclusions for the entire Greek population.
In conclusion, the present study demonstrates that
the NoSAS score is a simple, effective, and easy method for the
detection of OSAS in clinical practice in the Greek population. The
NoSAS score performs similarly to well-established questionnaires,
such as the Berlin questionnaire, the ESS and the STOP BANG
questionnaire, for the detection of OSA of all severity categories
and of moderate-to-severe-severe OSA in particular.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PS and AA conceptualized the study. VEG, XT, NP, AA
and EN made a substantial contribution to data interpretation and
analysis, and wrote and prepared the draft of the manuscript. PS
and AA analyzed the data and provided critical revisions. VEG and
AA confirm the authenticity of all the raw data. All authors
contributed to manuscript revision, and have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in line with the
Declaration of Helsinki and gained approval by the regional
Institutional Review Board (protocol no. 5974/05.04.2021). Written
informed was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Archontogeorgis K, Nena E, Papanas N and
Steiropoulos P: Biomarkers to improve diagnosis and monitoring of
obstructive sleep apnea syndrome: Current status and future
perspectives. Pulm Med. 2014(930535)2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Benjafield AV, Ayas NT, Eastwood PR,
Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin
JL, et al: Estimation of the global prevalence and burden of
obstructive sleep apnoea: A literature-based analysis. Lancet
Respir Med. 7:687–698. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pafili K, Steiropoulos P and Papanas N:
The relationship between obstructive sleep apnoea and coronary
heart disease. Curr Opin Cardiol. 30:439–446. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Perantoni E, Filos D, Archontogeorgis K,
Steiropoulos P and Chouvarda IC: Pre-diabetic patients with severe
obstructive sleep apnea: Novel parameters of hypoxia during sleep
correlate with insulin resistance. Annu Int Conf IEEE Eng Med Biol
Soc. 2019:5002–5005. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bouloukaki I, Grote L, McNicholas WT,
Hedner J, Verbraecken J, Parati G, Lombardi C, Basoglu OK, Pataka
A, Marrone O, et al: Mild obstructive sleep apnea increases
hypertension risk, challenging traditional severity classification.
J Clin Sleep Med. 16:889–898. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Garvey JF, Pengo MF, Drakatos P and Kent
BD: Epidemiological aspects of obstructive sleep apnea. J Thorac
Dis. 7:920–929. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hong C, Chen R, Qing S, Kuang A, Yang H,
Su X, Zhao D, Wu K and Zhang N: Validation of the NoSAS score for
the screening of sleep-disordered breathing: A hospital-based
retrospective study in China. J Clin Sleep Med. 14:191–197.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Johns MW: A new method for measuring
daytime sleepiness: The Epworth sleepiness scale. Sleep.
14:540–545. 1991.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chung F, Yegneswaran B, Liao P, Chung SA,
Vairavanathan S, Islam S, Khajehdehi A and Shapiro CM: STOP
questionnaire: A tool to screen patients for obstructive sleep
apnea. Anesthesiology. 108:812–821. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Netzer NC, Stoohs RA, Netzer CM, Clark K
and Strohl KP: Using the Berlin questionnaire to identify patients
at risk for the sleep apnea syndrome. Ann Intern Med. 131:485–491.
1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Marti-Soler H, Hirotsu C, Marques-Vidal P,
Vollenweider P, Waeber G, Preisig M, Tafti M, Tufik SB, Bittencourt
L, Tufik S, et al: The NoSAS score for screening of
sleep-disordered breathing: A derivation and validation study.
Lancet Respir Med. 4:742–748. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tsara V, Serasli E, Amfilochiou A,
Constantinidis T and Christaki P: Greek version of the Epworth
sleepiness scale. Sleep Breath. 8:91–95. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pataka A, Chavouzis N, Passa KF, Bagalas
V, Pitsiou G, Stanopoulos I, Kalamaras G, Paspala A, Sourla E,
Vaitsi E and Argyropoulou P: Validation of the stop bang
questionnaire in a sleep clinic in Greece for the prediction of
mobstructive sleep apnea. Eur Respir J. 42(P4052)2013.
|
|
14
|
Bouloukaki I, Komninos ID, Mermigkis C,
Micheli K, Komninou M, Moniaki V, Mauroudi E, Siafakas NM and
Schiza SE: Translation and validation of Berlin questionnaire in
primary health care in Greece. BMC Pulm Med. 13(6)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Berry RB, Budhiraja R, Gottlieb DJ, Gozal
D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF,
et al: Rules for scoring respiratory events in sleep: Update of the
2007 AASM manual for the scoring of sleep and associated events.
Deliberations of the sleep apnea definitions task force of the
American academy of sleep medicine. J Clin Sleep Med. 8:597–619.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu X: Classification accuracy and cut
point selection. Stat Med. 31:2676–2686. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Oktay Arslan B, Uçar ZZ, Batum Ö and Orman
MN: Validation of the NoSAS score for screening sleep-disordered
breathing: A sleep clinic- based study in Turkey. Turk J Med Sci.
51:319–327. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tan A, Hong Y, Tan LWL, van Dam RM, Cheung
YY and Lee CH: Validation of NoSAS score for screening of
sleep-disordered breathing in a multiethnic Asian population. Sleep
Breath. 21:1033–1038. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Peng M, Chen R, Cheng J, Li J, Liu W and
Hong C: Application value of the NoSAS score for screening
sleep-disordered breathing. J Thorac Dis. 10:4774–4781.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Coutinho Costa J, Rebelo-Marques A,
Machado JN, Gama JMR, Santos C, Teixeira F and Moita J: Validation
of NoSAS (Neck, Obesity, Snoring, Age, Sex) score as a screening
tool for obstructive sleep apnea: Analysis in a sleep clinic.
Pulmonology. 25:263–270. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Herschmann S, Berger M, Haba-Rubio J and
Heinzer R: Comparison of NoSAS score with Berlin and STOP-BANG
scores for sleep apnea detection in a clinical sample. Sleep Med.
79:113–116. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen H, Zheng Z, Chen R, Zeng Y, Li N, Zhu
J, Zhong Y, Liu H, Lu J, Zhang N and Hong C: A meta-analysis of the
diagnostic value of NoSAS in patients with sleep apnea syndrome.
Sleep Breath. 26:519–531. 2022.PubMed/NCBI View Article : Google Scholar
|