Introduction
Uterine carcinosarcoma (UCS) is a high-grade
endometrial cancer characterized by a carcinomatous component
(epithelial) and sarcomatous (stromal tissue) element (1). UCS is considered a rare tumor; however,
its incidence is increasing (1). UCS
is considered an aggressive tumor due to its components and it can
exhibit metastasis at the time of diagnosis in many cases (2).
The present study describes the case of a patient
with this type of tumor treated at the authors' hospital
(2020-2021) and also provides a brief review of the published
literature up to December, 2022, with the aim of offering an
overview of the pathology, presentation and management of UCS from
the point of view of General Surgery.
The limitation of the present study was there is not
much homogeneity in UCS treatment as this is a rare tumor and has
an aggressive pathology. In the search of published literature
using PudMed, no article on UCS with intestinal involvement was
found.
Case report
A 72-year-old female patient with no history of any
serious conditions, visited the emergency service at Principe de
Asturias Hospital due to a fever of 38˚C with 24 h of evolution,
rectal bleeding, vesical tenesmus with an evolution of 3 months and
occasional fecal incontinence that had become more severe over the
last few days. During her stay in the emergency room, the patient
was hemodynamically stable.
A physical examination revealed a soft and
depressible abdomen, with a painful area in the hypogastric region,
where a huge palpable mass was located. Laboratory test results
revealed the following: Leukocytes, 12.600 µl; neutrophils, 90%;
hemoglobin, 11 mg/dl; C reactive protein (CRP), 313 mg/l;
procalcitonin, 8.15 ng/ml; lactate, 3.1 mg/dl; hematuria,
leukocyturia and bacteriuria for Escherichia coli.
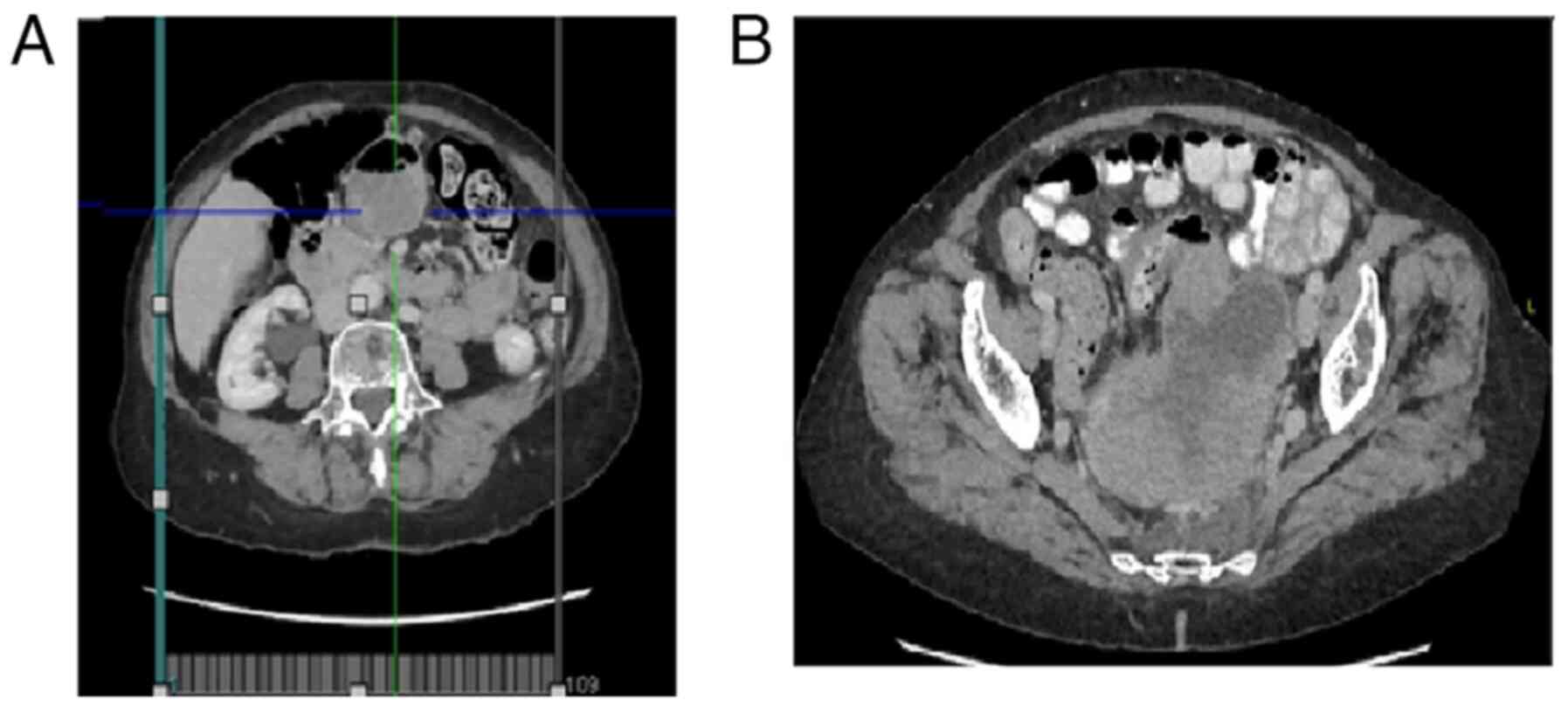
Computed tomography (CT) of the abdomen and pelvis
with intravenous contrast demonstrated a large solid polylobulated
mass with peripheral enhancement with the intravenous contrast and
wide areas of necrosis. The mass was contacting, compressing and
moving the rectus and sigmoid colon to the posterolateral side with
no intestinal obstruction signals. The tumor measured
12.5x8.23x8.87 cm (anteroposterior x transverse x craniocaudal
diameters). There was a small edematous zone in the presacral fatty
tissue, two small calcifications in the right mass zona and a
significant growth in the vascular are around the described injury.
Left paraaortic nodes with abnormal size, and a small adenopathy
near the iliac bifurcation and around the fatty tissue of the mass
were observed. The bladder and uterus were displaced anteriorly,
and the ovaries were not visible (Fig.
1).
An ultrasound of the pelvis revealed a solid mass of
10 x6 cm in size in the pouch of Douglas with intense Doppler
imaging. Due to the worsening of the patient's general condition
and the laboratory test results during her hospitalization 4 days
later, the authors' requested to perform an evaluation of the
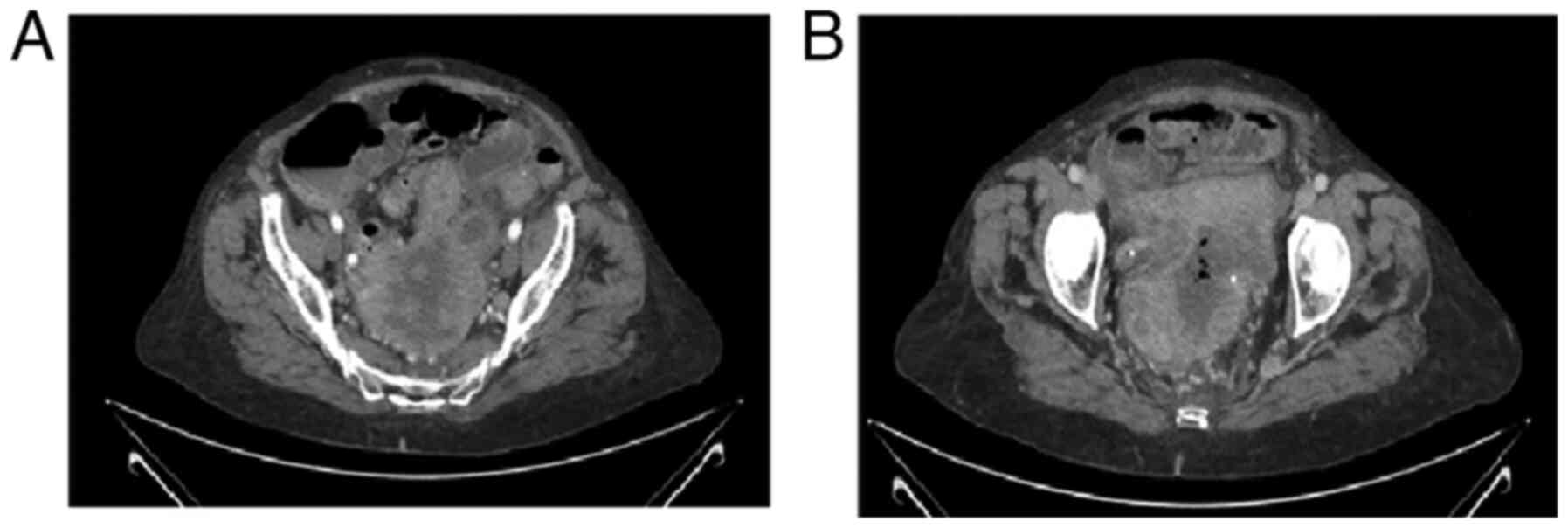
patient. A new CT scan was performed to compare the new findings
with the previous ones. This also revealed a solid pelvic mass with
wider areas of necrosis and pneumoperitoneum. Traces of free fluid
were found in the pelvis and between the intestines. In a 3.5 cm
segment of the jejunum, inflammation and and/or focal ischemia were
observed (Fig. 2).
Immediate surgery was performed with medium
laparotomy with intraoperative findings of purulent free fluid,
small intestinal plastron that encompassed the superior rectus,
left ovary and fallopian tube. A complete mesorectal dissection was
made with the mass mobilization of the left ovary and tube. An
oncological ultralow anterior resection and colostomy in left iliac
fossa were performed with and appropriate splenic angle descent.
There were no intraoperative and postoperative incidents.
The results of the pathological analysis revealed
the following: Carcinosarcoma (mixed malignant Mullerian tumor) of
probable ovarian origin that involved all the intestinal wall with
large areas of necrosis. The resection margins were not assessable.
Ki67 positivity was 90%, and immunohistochemical analysis
(performed by the anatomic pathology service) yielded positive
findings for CK AE1AE3, CK7, p63, Pax8, CD10 and vimentin (data not
shown).
The clinical case was evaluated by the
multidisciplinary oncological committee for oncological surgery
enlargement, although after surgery, the patient was revaluated to
determine whether she was a candidate for chemotherapy.
Intraoperatively, there was a bleeding mass in the small intestine
of 5 cm in size in the left iliac fossa. There are several tumoral
implants in the omentum, intestine and presacral region. A simple
hysterectomy with the excision of the right ovary was made, and
also the resection of hepatic angle mesentery, transverse colon
mesentery, the complete resection of the greater omentum, terminal
colostomy of the antimesenteric border, and the resection of the
injury in the proximal jejunum. Multiple lesions were observed in
the terminal ileum; thus, a resection of 50 cm was made and 20 cm
of the distal jejunum with all adenopathies visualized.
Latero-lateral manual biplane anastomosis was performed between the
terminal ileum and distal jejunum. In addition, there was an
tumoral implant in the iliac artery bifurcation and left iliac
vein, and thus there was a high risk of vascular damage. An R2
resection was made with a peritoneal carcinomatosis index of
18/39.
All the dissected sections were sent to the anatomic
pathology service for analysis, which revealed carcinosarcomatous
infiltration and 16 nodes of the ileum and 2 of the jejunum were
reactive. The patient did not experience any incidences
post-operatively, and was thus discharged from the hospital 9 days
later.
After 1 month, the patient visited the emergency
service with abdominal pain, a fever of 38.4˚C, with no functioning
colostomy and peritoneal irritation. An examination revealed the
following: Leukocytes, 13.100 µl; neutrophils, 90%; hemoglobin, 9.7
g/dl; CRP, 98 mg/l. A CT scan revealed cancer progression with new
hepatic lesions in the VIII segment 2.6 cm in diameter, several
tumoral implants in the sacral region next to the bladder, in the
right iliac fossa, retroperitoneum (anterior to left psoas muscle)
and between the small intestine. Following a consensus with the
patient and her family, palliative care was commenced and in the
following days, the patient succumbed to her condition.
Discussion
UCS is considered a rare tumor, comprising ~5% of
endometrial cancer cases; however, its incidence is increasing
(1). The annual incidence of UCS is
0.5-3.3 cases per 100,000 females (2). European statistics have revealed an
incidence of 5.1-6.9 cases per 1.000.000 individuals per year,
between the years 1989-2008(1). UCS
mainly affects individuals of 70-79 years of age, although in
recent decades, younger patients have also been identified
(1).
Women of African origin have the highest risk of
developing this type of cancer, even though in recent years, the
incidence among Hispanic women has increased compared with that in
other races (1). UCS is considered
to derive from a dedifferentiated sarcoma component (1). Recent research has demonstrated that
carcinomatous and sarcomatous elements are derived from a common
precursor with mutations that are typical of carcinomas (3).
A significant discovery was that
epithelial-mesenchymal transition (EMT) plays a pivotal role in the
pathogenesis of sarcomatous dedifferentiation and that heterologous
sarcoma is associated with a higher EMT signature compared with
homologous sarcoma (1). Mutations
and genes in this type of tumor have not been extensively studied.
The most common of these are probably PT53, PTEN and FBXW7(1). The disease can also exhibit chromosomal
instability and a complex karyotype (2).
Traditionally, UCS was considered a sarcoma, being
the most frequent uterus sarcoma. It is also known as a malignant
mixed mesodermal tumor or a mixed Mullerian malignant tumor
(2). The carcinomatous epithelial
element can be of low or high grade, while the sarcomatous part can
be heterologous/no gynecological tissue (rhabdomyosarcoma,
chondrosarcoma and osteosarcoma) or, homologous/gynecological
tissue (leiomyosarcoma, fibrosarcoma and endometrial stromal
sarcoma) (1,2).
The most frequent element is carcinomatous,
highlighting high grade endometrioid and serous carcinoma (2). This type of element is the tissue that
tends to metastasize and appeal (2).
Carcinoma with no dominant homologous sarcoma is the most prevalent
type of UCS (1). The main risk
factors for UCS are presented in Table
I.
 | Table IRisk factors of uterine
carcinosarcoma. |
Table I
Risk factors of uterine
carcinosarcoma.
| Risk factors for UCS
(2) |
|---|
| • Elevated levels of
estrogens |
| • Nulliparous |
| • Obesity |
| • African race |
| • Tamoxifen use |
| • Pelvic
radiation |
Clinically, UCS is similar other uterine
adenocarcinomas, with symptoms such as vaginal bleeding (most
common symptom), abdominal pain and a large uterus (2,4). It may
present as a pelvic mass shown on an imaging test or as a prolapsed
cervix (2). Due to this malignancy,
the extrinsic compression of the bladder or intestines may occur,
leading to urinary retention, bowel obstruction, constipation and
tenesmus (5). Approximately 10% of
patients exhibit metastasis at the time of diagnosis (4) and 30-40% are positive for adenopathies
(6). Metastasis can occur in the
lungs (49%), peritoneum (44%), bones (17%), liver (15%) and central
nervous system (7).
The diagnoses of UCS is based on laboratory and
imaging tests. Although there is no specific laboratory test to
indicate this condition, ~10% of patients can present anemia due to
vaginal bleeding (3). Ca125 levels
can be elevated due to serous epithelial tissue and deep myometrial
invasion (2).
The first step for imaging tests is a pelvic
ultrasound, which may reveal hyperechoic lesions which are
difficult to differentiate from adenocarcinoma (4). As patients frequently exhibit an
extra-uterine pathology, CT scan is recommended for classification
and/or magnetic resonance (MR) imaging prior to surgery (2). However, a CT scan is more effective
than MR imaging for adenopathies (5). Positron emission tomography is more
sensitive (68 vs. 50%), but less specific (88 vs. 93%) than MR
imaging to detect adenopathies (7).
The definitive diagnosis is anatomopathological (4). Due to the high risk of micrometastasis,
a lymphadenectomy with posterior analysis is the gold standard
(7).
The stages of carcinosarcomas are similar to those
of the endometrial carcinoma system (5). The stages of stages of UCS are listed
in Table II and in TNM staging
(AJCC UICC 2017) is presented in Table
III (8). Although treatment is
multimodal, the prognosis of patients is poor, with a median
survival rate of <2 years (1).
More than half of the cases are diagnosed at an early stage, while
the other half at an advanced stage (stage I, 43.9%; stage II,
8.7%; stage III, 22.9%; and stage IV, 24.4%) (1). The survival for rates of patients with
different stages of the disease are generally as follows: Stage I,
78 months; stage II, 30 months; stage III, 19 months; and stage IV,
8 months (1). Factors associated
with patient prognosis are presented in Table IV.
 | Table IIStages of uterine carcinosarcoma
(8). |
Table II
Stages of uterine carcinosarcoma
(8).
| Carcinosarcoma
stage |
|---|
| Stage | Description | Subgroup |
|---|
| I | Body uterine
tumor | • IA: <50%
myometrial invasion |
| | | • IB: >50%
myometrial invasion |
| II | Cervical stroma is
invaded by tumor but no across uterus | |
| III | Local and/or regional
dissemination | • IIIA: Serous and/or
annexes |
| | | • IIIB: Vagina and/or
parametrium |
| | | • IIIC1: Pelvic
adenopathies |
| | | • IIIC2: Paraaortic
adenopathies |
| IV | Bladder, intestine
invasion or metastasis | IVA: Bladder and/or
intestine |
| | | IVB: Metastasis or
inguinal adenopathies |
 | Table IIITNM staging AJCC UICC 2017(8). |
Table III
TNM staging AJCC UICC 2017(8).
| Primary tumor, T
category | FIGO stage | T criteria |
|---|
| Tx | | Primary tumor cannot
be assessed |
| T0 | | No evidence of
primary tumor |
| T1 | I | Tumor confined to the
corpus uteri, including endocervical glandular involvement |
| T1a | IA | Tumor limited to
endometrium or invading les than half the myometrium |
| T1b | IB | Tumor invading one
half or more of myometrium |
| T2 | II | Tumor invading
stromal connective tissue of cervix but not extending beyond the
uterus (not included endocervical tissue) |
| T3 | III | Tumor involving
serosa, adnexa, vagina or parametrium |
| T3a | IIIA | Tumor involving
serosa and/or adnexa (direct or metastasis) |
| T3b | IIIB | Vaginal involvement
(direct or metastasis) or parametrial involvement |
| T4 | IVA | Tumor invading the
bladder mucosa and/or bowel mucosa (bullous edema is not sufficient
to classify a tumor as T4) |
| Regional lymph
nodes, | FIGO stage | |
| N category | | N criteria |
| Nx | | Regional lymph
nodes cannot be assessed |
| N0 | | No regional lymph
nodes |
| N0(i+) | | Isolated tumor
cells in regional lymph nodes not >0.2 mm |
| N1 | IIIC1 | Regional lymph
nodes to pelvic lymph nodes |
| N1m | IIIC1 | Regional lymph
nodes (>0.2 mm but not >2.0 mm) to pelvic lymph nodes |
| N1a | IIIC1 | Regional lymph
nodes (>2.0 mm in diameter) to pelvic lymph nodes |
| N2 | IIIC2 | Regional lymph
nodes to para-aortic lymph nodes with or without positive pelvic
involvement |
| N2Mi | IIIC2 | Regional lymph
nodes (>0.2 mm but not >2.0 mm in diameter) to para-aortic
lymph nodes with or without positive pelvic involvement |
| N2a | I; IIC2 | Regional lymph
nodes (>2.0 mm in diameter) to para-aortic lymph nodes with or
without positive pelvic involvement |
| Distant
metastasis, | FIGO stage | |
| M category | | M criteria |
| M0 | | No distant
metastasis |
| M1 | IVB | Distant
metastasis |
| | | - Included:
inguinal lymph nodes, intraperitoneal disease, lungs, liver,
bones |
| | | - Excluded: pelvic
and para-aortic lymph nodes, vagina, uterus serosa and adnexa |
 | Table IVPrognosis of patients with
carcinosarcoma. |
Table IV
Prognosis of patients with
carcinosarcoma.
| Relevant factors in
survival (1) | Aggressiveness
factors (1) | Independent
survival factors (2) |
|---|
| • Histology | • Size >5 cm (it
is related to venous thrombosis) | • <40 years
old |
| • Sarcomatous
dominancea | • Myometrial
invasion | • Caucasian
race |
| • Tumor size | • Lymphovascular
invasion | • Radiotherapy
post-operatively |
| • Depth
invasion | • Adenopathies (25%
pelvic, 15% paraaortic) | • Lymphadenectomy
x |
| • Lymphovascular
invasion | | • Early stages |
| • Malignant
peritoneal cytology | | |
| • Positive
adenopathies | | |
| • Metastasis | | |
The concept of sarcomatous dominance is associated
with a >50% decrease in survival (1). The worse survival subtype is carcinoma
with heterologous sarcoma (1).
Lymphatic invasion in recent years has increased the incidence of
distant metastasis (1). The
carcinoma element can metastasize to distant zones, while the
sarcomatous element to regional zones (1). Approximately 60% of cases have
disseminated illness, and 50% of tumors are treated using surgery
and adjuvant therapy (2). The
majority of recurrences occur outside the pelvis. Research has
confirmed a poorer overall survival when compared to high grade
endometrial carcinomas (9).
In addition, elevated levels of Ca125 after surgery
are associated with a poor prognosis (2). In the case described herein, due to the
rapid progression of the patient's tumor, the levels of Ca125 were
not measured; however, these may be useful to evaluate prognosis
and perform diagnosis, as these levels can be elevated due to
serous epithelial tissue and the deep myometrial invasion. This is
a limitation of the present study.
Recent research suggests that Aurora kinase
expression is a poor prognostic marker due to positive
adenopathies, vascular invasion and omental dissemination (6). The presence of lymphovascular invasion
or >50% myometrial invasion is associated with a higher risk of
positive adenopathies (10). The
gold standard of treatment for UCS is hysterectomy, double
adnexectomy with lymphadenectomy, even in patients with stage I
disease, due to the high prevalence of micrometastasis (1,2).
In the early stages (stages I-II) of the disease,
surgery with chemotherapy and pelvic radiotherapy or vaginal
brachytherapy are used for local control. In the earlier stages,
chemotherapy can improve tumor recurrence and progression-free
survival, but not overall survival (4). Patients may have benefits from adjuvant
multimodality therapy to reduce the chance of tumor recurrence with
the potential to improve overall survival (11). However, it should be noted that, in
stage I disease, radiotherapy does not improve survival (1). In patients with advanced stages of the
disease, chemotherapy and cytoreduction is considered, with the
possible inclusion of palliative radiotherapy (2).
Adjuvant chemoradiation is an independent good
prognostic factor (12). The
National Comprehensive Cancer Network guidelines (NCCN) recommends
the use of carboplatin/paclitaxel (1). The use of radiotherapy with
chemotherapy in the case of sarcomatous dominance is usually
considered (1). Palliative
radiotherapy can be used in non-resectable tumors or in cases in
which surgery is not possible for clinical reasons, to treat
endometrial bleeding or pelvic pain, always individualizing each
patient (13).
In the case described herein, surgery was first
performed due to severe illness (the results of the clinical
analysis revealed sepsis, with radiological images indicating
pneumoperitoneum; a physical examination did not reveal any notable
comorbidities). This surgery was performed with the informed
consent of the patient and her family. /the second surgery was
performed following an evaluation by the multidisciplinary
oncological team with an oncologist, gynecologist, general surgeon,
radiologist, anatomic pathologist and a radiotherapy team, and
following a discussion with the patient and her family. The second
surgery was performed due to the consensus of the oncological
committee in a patient with previously good quality of life, with
intentions of resection the mass and completion of the treatment,
always knowing the prognosis of this type of tumor. The results of
the second surgery were not evident until after the surgery, as at
the time of the first surgery, there was no distant metastases and
neither in the previous CT scan. However, the second one revealed
several metastatic implants. In the present study, the patient was
operated on twice in a period of <2 months (urgent surgery was
first performed and the second surgery was an attempt to complete
the treatment); however, the tumor progressed rapidly.
It is well known in the literature that tumor
progression is possible. For the patient described herein, after
considering all the risks and benefits, the optimal treatment for
the patient was offered; however, effective treatment was not
possible for this case and the patient succumbed to the disease,
most probably due to tumor progression. This type of tumor accounts
for 16.4% of all deaths from uterine malignancies (14). This type of tumor progresses rapidly,
even with treatment, as demonstrated in the case described herein.
Consequently, this led to the death of the patient. However, in the
patient in the present study, it was difficult to assess the main
cause of death.
Sentinel node efficacy for this tumor is not yet
well known (1), and hormonotherapy
is not common treatment (6). There
are currently studies available on treatment with tyrosine kinase
inhibitors, Her2 and immune checkpoint inhibitors (5). Immunotherapies may hold promising
outcomes for patients with tumors positive for mismatch repair
(MMR) deficiency (MMR-D) and programmed cell death ligand-1 (PD-L1)
(6). The most common mutations were
observed are in TP53 (86%), PIK3CA (34%),
FBXW7 (23%), PTEN (18%), KRAS (16%) and
PPP2R1A (10%) (15).
Microsatellite instability (MSI)-high is detected in 15-30% of
endometrial cancer cases, and some studies have demonstrated that
immune checkpoint inhibitors are effective for MSI-high solid
tumors (16,17). High-frequency microsatellite
instability is absent and is observed in ~5% of carcinosarcomas.
The loss of heterozygosity for chromosome 11 is present in 17% of
uterine sarcomas and 19% if carcinosarcomas (16).
Immunostaining for p53 defects is used in these
types of tumors and is frequently between 67-85%, and is almost
always consistent in the carcinomatous and sarcomatous component
(18,19). Previous studies have revealed that
deficiencies in MMR expression in UCS are rare, ~4% (15,19).
PD-L1 expression in these tumors may predict the
response to checkpoint inhibitor therapies. The majority of cases
of UCS exhibit at least a focal PD-L1 expression. Carcinosarcomas
with an endometrioid morphology are significantly more likely to
have high levels of PD-L1. MMR-deficient carcinosarcomas are also
more likely to have high levels of PD-L1, although this has not
reached statistical significance (P=0.2) (20).
The type of tumor has several mutations that are
possible therapeutic targets; however, there are limited treatment
options for this type of aggressive tumor with poor outcomes. Thus,
further clinical trials are required to validate new treatments.
The different treatments available for this type of tumor are
presented in Tables V and VI.
 | Table VCurrent possible treatments available
for uterine carcinosarcoma. |
Table V
Current possible treatments available
for uterine carcinosarcoma.
| Surgery | • Gold standard
treatment is hysterectomy, double adnexectomy with
lymphadenectomy |
| Chemotherapy | • It is an
independent good prognostic factor |
| | • In earlier stages
improve tumor recurrence and progression free survival, but not
global survival |
| | • Recommended use
of carboplatin/paclitaxel |
| Radiotherapy | • Using
radiotherapy with chemotherapy to improve the effectiveness as long
as sarcomatous dominance is present |
| | • The addition of
radiotherapy for patients with stage I disease does not improve
survival |
| | • Palliative
radiotherapy can be used in non-resectable tumors, to treat
endometrial bleeding or pelvic pain, always individualizing each
patient. |
| Immunotherapy | Immunotherapies
appear to be promising for patients with tumors positive for MMR-D
and PD-L1, although further studies are required. |
| Other
treatments | • The efficacy of
hormone therapy for this tumor is not yet well known |
| | • Sentinel node
efficacy is not well known |
 | Table VITreatment according to clinical
stage. |
Table VI
Treatment according to clinical
stage.
| Gold standard:
Hysterectomy, double adnexectomy with lymphadenectomy. In all
stages, it is important to individualize patient treatments. |
|---|
| Early stages
(I-II) | Surgery +
chemotherapy ± pelvic radiotherapy or vaginal brachytherapy for
local control |
| Advanced stages
(III-IV) | Important to
evaluate all patient factors (comorbidities, mobility, patient's
opinions, resectability of tumors and operative factors, possible
complications, etc.) |
| | • Possible good
prognosis: Chemotherapy + surgery (cytoreduction) +
radiotherapy |
| | • Possible poor
prognosis: Palliative treatment |
Several clinical trials have examined the
therapeutic efficacy in recurrent/metastatic UCS. The median
response rates were shown to be 37.5% and 5.9 months for
progression-free survival; however, after later therapies, the
outcomes were worse (5.5% and 1.8 months, respectively) (1).
A limitation of the present study was that the Ca125
levels were not measured, as well as the rapid tumor progression
and the worsening of the patient's clinical condition during the
completion of treatment (chemotherapy, radiotherapy, etc.). No
immunophenotypic analysis was performed for this tumor.
In conclusion, UCS is a rare tumor, comprised of two
main elements, carcinomatous and sarcomatous. This type of tumor is
usually more frequent in older-aged women of African origin. The
most common symptom is vaginal bleeding, as with other uterine
sarcomas. The first imaging test that needs to be performed is an
ultrasound, followed by a CT scan in order to diagnose metastasis.
UCS is a tumor with a poor prognosis due to its malignant component
and the possibility for recurrence and metastasis. The gold
standard of treatment is surgery, followed by adjuvant therapy
based on the carcinosarcoma stages: Patients with the early stages
of the disease may receive use chemotherapy and radiotherapy, and
in those at the advanced stages, cytoreductive surgery may be
considered with palliative radiotherapy to control symptoms, but
always individualizing patients. There are several therapies in
development that may be promising in future years.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (PLH, FMM, MBA, RAH, SSS, ESY, YAM, FMJ,
RJM, LJA, DCG, MMDA and AGC) contributed to the diagnosis and
treatment of the patient, and in the design of the study. PLH was a
major contributor in the writing of the manuscript. PLH and FMM
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study followed international and
national regulations and was in agreement with the Declaration of
Helsinki, and ethical principles. The patient signed an informed
consent form before the surgery was performed.
Patient consent for publication
The patient provided written informed consent for
the publication of any data and/or accompanying images, before the
surgery was performed. Patients have a right to anonymity and
privacy, and authors have a legal and ethical responsibility to
respect this right.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matsuzaki S, Klar M, Matsuzaki S, Roman
LD, Sood AK and Matsuo K: Uterine carcinosarcoma: Contemporary
clinical summary, molecular updates, and future research
opportunity. Gynecol Oncol. 160:586–601. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cantrell LA, Blank SV and Duska LR:
Uterine carcinosarcoma: A review of the literature. Gynecol Oncol.
137:581–588. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Soror NN, Woredekal D, Hemrock L, Gibson G
and Bennett R: Uterine carcinosarcoma in a young female: Case
report and literature review. Cureus. 13(e12642)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Denschlag D and Ulrich UA: Uterine
carcinosarcomas-diagnosis and management. Oncol Res Treat.
41:675–679. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kord A, Rabiee B, Elbaz Younes I and Xie
KL: Uterine carcinosarcoma: A case report and literature review.
Case Rep Obstet Gynecol. 2020(8816348)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pezzicoli G, Moscaritolo F, Silvestris E,
Silvestris F, Cormio G, Porta C and D'Oronzo S: Uterine
carcinosarcoma: An overview. Crit Rev Oncol Hematol.
163(103369)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bužinskienė D, Mikėnas S, Drąsutienė G and
Mongirdas M: Uterine sarcoma: A clinical case and a literature
review. Acta Med Litu. 25:206–218. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
American Joint Committee on Cancer: Corpus
uteri-carcinoma and carcinosarcoma. In: AJCC cancer staging manual.
8th edition. New York, NY: Springer, pp661-669, 2017.
|
|
9
|
Terblanche L and Botha MH: Uterine
carcinosarcoma: A 10-year single institution experience. PLoS One.
17(e0271526)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sagebiel TL, Bhosale PR, Patnana M, Faria
SC and Devine CE: Uterine carcinosarcomas. Semin Ultrasound CT MR.
40:295–301. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Elshaikh MA, Modh A, Jhingran A, Biagioli
MC, Coleman RL, Gaffney DK, Harkenrider MM, Heskett K, Jolly S,
Kidd E, et al: Executive summary of the American Radium
Society® appropriate use criteria for management of
uterine carcinosarcoma. Gynecol Oncol. 158:460–466. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chiang CY, Huang HJ, Chang WY, Yang LY, Wu
RC, Wang CC, Tung HJ, Chao A and Lai CH: Adjuvant therapy and
prognosis in uterine carcinosarcoma. J Formos Med Assoc.
120:1977–1987. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Toboni MD, Crane EK, Brown J, Shushkevich
A, Chiang S, Slomovitz BM, Levine DA, Dowdy SC, Klopp A, Powell MA
and Thaker PH: Uterine carcinosarcomas: From pathology to practice.
Gynecol Oncol. 162:235–241. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Almeida PC, Amaral Ferreira L and Donato
P: Uterine carcinosarcoma with extensive extrauterine component.
BMJ Case Rep. 14(e247643)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Crane E, Naumann W, Tait D, Higgins R,
Herzog T and Brown J: Molecular variations in uterine
carcinosarcomas identify therapeutic opportunities. Int J Gynecol
Cancer. 30:480–484. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Amant F, Dorfling CM, Dreyer L, Vergote I,
Lindeque BG and Van Rensburg EJ: Microsatellite instability in
uterine sarcomas. Int J Gynecol Cancer. 11:218–223. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Taylor NP, Zighelboim I, Huettner PC,
Powell MA, Gibb RK, Rader JS, Mutch DG, Edmonston TB and Goodfellow
PJ: DNA mismatch repair and TP53 defects are early events in
uterine carcinosarcoma tumorigenesis. Mod Pathol. 19:1333–1338.
2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kunc M, Gabrych A, Rękawiecki B,
Gorczyński A, Haybaeck J, Biernat W and Czapiewski P:
Immunohistochemical evaluation of mismatch repair proteins and p53
expression in extrauterine carcinosarcoma/sarcomatoid carcinoma.
Contemp Oncol (Pozn). 24:1–4. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jenkins TM, Hanley KZ, Schwartz LE,
Cantrell LA, Stoler MH and Mills AM: Mismatch repair deficiency in
uterine carcinosarcoma: A multi-institution retrospective review.
Am J Surg Pathol. 44:782–792. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jenkins TM, Cantrell LA, Stoler MH and
Mills AM: PD-L1 and mismatch repair status in uterine
carcinosarcomas. Int J Gynecol Pathol. 40:563–574. 2021.PubMed/NCBI View Article : Google Scholar
|