1. Introduction
Cirrhosis is a common long-term result of persistent
hepatic inflammation. Liver cirrhosis can be caused by various
toxic, metabolic, infectious, or autoimmune conditions such as
alcoholism, non-alcoholic fatty liver disease (NAFLD), autoimmune
hepatitis, viral hepatitis, primary biliary cholangitis (PBC), and
primary sclerosing cholangitis, as well as a variety of metabolic
disorders such as Wilson's disease, hemochromatosis and
alpha-1-antitrypsin deficiency (1).
The consequences in the function and anatomy of the liver include:
i) Hepatic insufficiency with reduced synthesis and impaired
metabolic functions; ii) the development of intrahepatic
portosystemic shunts between portal vessels and hepatic veins in a
sequential formation; and iii) portal hypertension (2,3).
Decompensated cirrhosis occurs when clinically relevant
complications and sequelae of portal hypertension (e.g., ascites,
variceal bleeding, hepatorenal syndrome) occur along with the
deterioration of liver function (e.g., decreased formation of
coagulation factors, insufficient degradation of ammonia resulting
in hepatic encephalopathy) (2,3).
Pulmonary complications may develop in patients with
or without liver decompensation (4).
Up to 70% of patients suffering from liver cirrhosis who are
evaluated for liver transplantation complain of dyspnea (5). In addition, as many as 45% of patients
with chronic liver disease who participated in screening studies
had abnormal arterial blood gas reports (6). These complications should be
distinguished from primary lung disorders, such as chronic
obstructive pulmonary disease (COPD), which can occur in patients
with liver diseases, but are not pathogenically linked to liver
cirrhosis (4).
Several causes of pulmonary dysfunction in liver
cirrhosis have been recognized, including intrinsic cardiopulmonary
disorders unrelated to liver disease and unique disorders related
to the presence of liver disease and/or portal hypertension
(7). Hepatic hydrothorax,
hepatopulmonary syndrome (HPS), spontaneous pulmonary empyema and
portopulmonary hypertension are the most common and clinically
important pulmonary consequences (4).
Conventional pulmonary function tests (PFTs) have
been used to measure the pulmonary function of patients with liver
cirrhosis (8). PFTs are a critical
diagnostic and monitoring modality for individuals with respiratory
disease. They provide vital information regarding the function of
the large and small airways, the lung parenchyma, as well as the
size and integrity of the pulmonary capillary bed. Although they do
not provide a diagnosis in and of themselves, diverse patterns of
anomalies are detected in various respiratory disorders, which aid
in diagnosis (9,10).
Pulmonary alterations may be present in
approximately one third of patients with decompensated liver
cirrhosis, leading to a decrease in arterial oxygen saturation and
sometimes to cyanosis (11).
Additionally, abnormalities in PFTs and impaired gas exchange may
develop in as many as 45-50% of patients with liver cirrhosis
(12). Certain pulmonary functions
may be impaired in chronic liver disease. In general, airway
obstruction, impairment in diffusion capacity for carbon monoxide
(DLCO) and a reduction in total lung capacity (TLC) indicating a
restrictive type of abnormality are manifested, leading to the
deterioration of gas exchange and hypoxemia (11,13).
Furthermore, apart from traditional PFTs, additional PFTs, such as
the calculation of the airway occlusion pressure 0.1 sec after the
onset of inspiratory flow (P0.1), which is a useful tool for the
evaluation of respiratory motor output (14), have been conducted in individuals
with liver cirrhosis and have been associated with disease severity
(15).
To the best of our knowledge, the present review is
the first to describe all the types of PFTs that have been
conducted in patients with liver cirrhosis and to discuss their
clinical significance.
2. Spirometry
Spirometry determines the maximum amount of air that
a patient can inhale and exhale while exerting maximum effort,
calculating volume or flow as a function of time. The most frequent
measurements include the forced vital capacity (FVC), which
quantifies the amount of air exhaled during a full and vigorous
expiration, the forced expiratory volume in one second (FEV1) and
peak expiratory flow rate (PEFR) (16). The mean forced expired flow when lung
volume declines from 75 to 25% of vital capacity [forced expiratory
flow between 25 and 75% of vital capacity (FEF25-75%)] is another
variable that may be assessed during the FVC maneuver and is linked
to small airway impairment (17).
Variations in the values of FEV1, FVC, PEFR and
FEF25-75% have been described in patients with liver cirrhosis
(13,15,18-40).
More specifically, some researchers (13,15,18,27,32) have
reported obstructive dysfunction, while others (19,21,23,25,26,31,33,38)
reported obstructive and/or restrictive ventilatory abnormalities,
and some researchers (11,20,22-24,28-30,32,34-37,39,40)
found isolated declines in the absolute values of FEV1, FVC, PEFR
and/or FEF25-75%.
Notably, in some studies, these dysfunctions were
shown to be associated with the severity of liver cirrhosis, as
assessed by various scores, such as the Child Pugh Score and the
Model for End-Stage Liver Disease (MELD) score, clinical
characteristics such as ascites and laboratory parameters such as
albumin (11,13,19,20,23,26-28,32,33,35,36,40).
Some studies (19,20,23,33,35) have
found a significant association between decreasing PEFR, FVC, FEV1
and FEF25-75% values, and increasing ascites. In addition, a
statistically significant positive correlation between FEV1 and
serum albumin, and between FVC and serum albumin has been described
in patients with liver cirrhosis (32). Moreover, FEV1 and FVC values have
been shown to be positively associated with the 6-min walking test
during the pre-transplant evaluation of patients with cirrhosis
(36).
In addition, a significant reduction in FEF25-75%
values has been observed in patients with esophageal varices, while
an obstructive dysfunction has been detected in patients with
alcoholic cirrhosis (19).
Of note, a restrictive spirometric alteration has
been statistically associated with a higher Child Pugh Score, a
higher MELD score, the presence of pleural effusions,
encephalopathy, ascites, hepatic hydrothorax, lower albumin levels,
the presence of hyperbilirubinemia and worse exercise capacity,
quality of life, and survival rates (19,33).
Moreover, a restrictive spirometric pattern has been associated
with tense ascites (26).
The Child-Pugh score has been found to be negatively
associated with FEV1 (11,28), FVC (32) and FEV1/FVC values (13), while the Glasgow Alcoholic Hepatitis
Scale (GAHS) has been negatively associated with FEV1/FVC values
(27).
Of note, in a study evaluating the presence of
non-specific impairment of lung functions (NILF), defined as the
observation of any two of three following criteria: i) FVC <80%
of predicted; ii) FEV1 <80% of predicted; iii) FEV1/FVC ≥70,
NILF was statistically associated with the female sex and with
increasing FibroScan scores (40).
3. Diffusing capacity for carbon
monoxide
DLCO testing is used to identify patients who have
exertional dyspnea, spirometric obstruction or restriction,
interstitial lung disease, pulmonary vascular disorders,
occupational pulmonary diseases and/or pulmonary side effects of
radiation or medications (41). A
low DLCO is value the most common lung function alteration
identified in chronic liver disease. Diffusion capacity is the
volume of any gas that diffuses across the alveolo-capillary
membrane in one unit of time (1 min) with a certain pressure
gradient (1 mmHg). DLCO/VA, on the other hand, is the diffusion
capacity of one liter of lung volume (23).
Decreased DLCO and DLCO/VA values have been reported
in patients with liver cirrhosis (6,21,23,24,25,34,38,39,42-44).
Similar to spirometric parameters, in some studies, alterations in
DLCO and DLCO/VA values have been found to be associated with the
severity of liver cirrhosis as assessed by various scores, clinical
features and laboratory data (23,24,27,38,43,44).
More specifically, both DLCO and DLCO/VA have been shown to
negatively correlate with the Child-Pugh score (23,24,27),
while DLCO/VA has also been shown to exhibit a negative correlation
with the MELD score (43). In
addition, DLCO has been found to exhibit a significant positive
correlation with serum albumin and cholinesterase levels (24), and a significant negative correlation
with esophageal varices and ascites (43). Moreover, DLCO and DLCO/VA values have
demonstrated an inverse linear correlation with the heart/liver
ratio (H/L) in thallium-201 per rectum scintigraphy, which
indirectly indicates a portosystemic shunt (44). Of note, DLCO has been described as a
significant predictor of ventilator time and both intensive care
unit (ICU) and hospital length of stay (LOS) following liver
transplantation (38).
4. Lung volumes
TLC and residual volume (RV), which indicates the
amount of air left in the respiratory tract at the end of a maximal
expiration, can be estimated using either gas dilution or
whole-body plethysmography (45).
The air volume that remains in the respiratory system following a
normal exhalation is referred to as functional residual capacity
(FRC). The FRC increases as lung volumes increase (46).
The RV, FRC and TLC values have been found to be
elevated, decreased, or normal in patients with liver cirrhosis.
More specifically, the RV and TLC values have been observed to be
increased in patients with liver cirrhosis, indicating air trapping
(47), or to be normal (18). However, the majority of studies have
demonstrated that lung volumes are decreased in patients with liver
cirrhosis (20,24,27,35,38). As
regards the association between lung volumes and the severity of
liver cirrhosis and clinicolaboratory characteristics, TLC has been
shown to exhibit a significant positive correlation with serum
albumin levels (24) and a
significant negative correlation with the presence of ascites
(35). Furthermore, TLC has been
found to exhibit a significant negative correlation with the GAHS
scale (27), and both TLC and RV
have been found to be significant predictors of ventilator time and
both ICU and hospital LOS following liver transplantation (38).
5. Single breath gas washout
Small airway closure with increased lung volumes is
a feature of various respiratory disorders, including asthma and
chronic obstructive pulmonary disease, and identifying this
alteration may aid in the early detection of respiratory impairment
(48).
The reference method for investigating airway
closure is the single breath gas washout (SBW), commonly nitrogen.
The exhaled used gas concentration vs. exhaled volume trace after a
vital capacity inhalation of a used gas-free gas mixture exhibits
an initial rapid increase (phase II) to a slow-rising alveolar
plateau (phase III), and then an abrupt change in the slope that
signals the beginning of phase IV. The closing volume (CV)
represents the volume at the start of phase IV (49).
The CV has been reported to be increased in patients
with liver cirrhosis, suggesting that the narrowing or closure in
small airways may develop in these patients, while the association
of these alterations in CV with disease severity remains unclear
(18,47,50).
6. Airway occlusion pressure 0.1 sec after
the onset of inspiratory flow
Airway occlusion pressure 0.1 sec after the onset of
inspiratory flow (P0.1) is the negative airway pressure developed
during the first 100 msec of an obstructed inspiration. P0.1 is a
measure for the neuromuscular activation of the respiratory system,
which is a key predictor of breathing functions. It has been
demonstrated to be a good predictive indicator of efficient
mechanical ventilation weaning. The standard P0.1 measuring
procedures rely on occluding the inspiration for >100 msec
(51).
Increased values of P0.1 have been observed in
patients with liver cirrhosis (15).
Moreover, P0.1 has been shown to positively correlate with
FEV1/FVC. In addition, P0.1 has been found to positively correlate
with the MELD score, indicating the presence of abnormal increased
respiratory drive in these patients (15).
7. Measurement of maximal inspiratory
pressure and maximal expiratory pressure
Maximal inspiratory pressure (MIP) and maximal
expiratory pressure (MEP) are non-invasive, simple and practical
indicators of respiratory muscle strength at the mouth (52). MIP and MEP values have been described
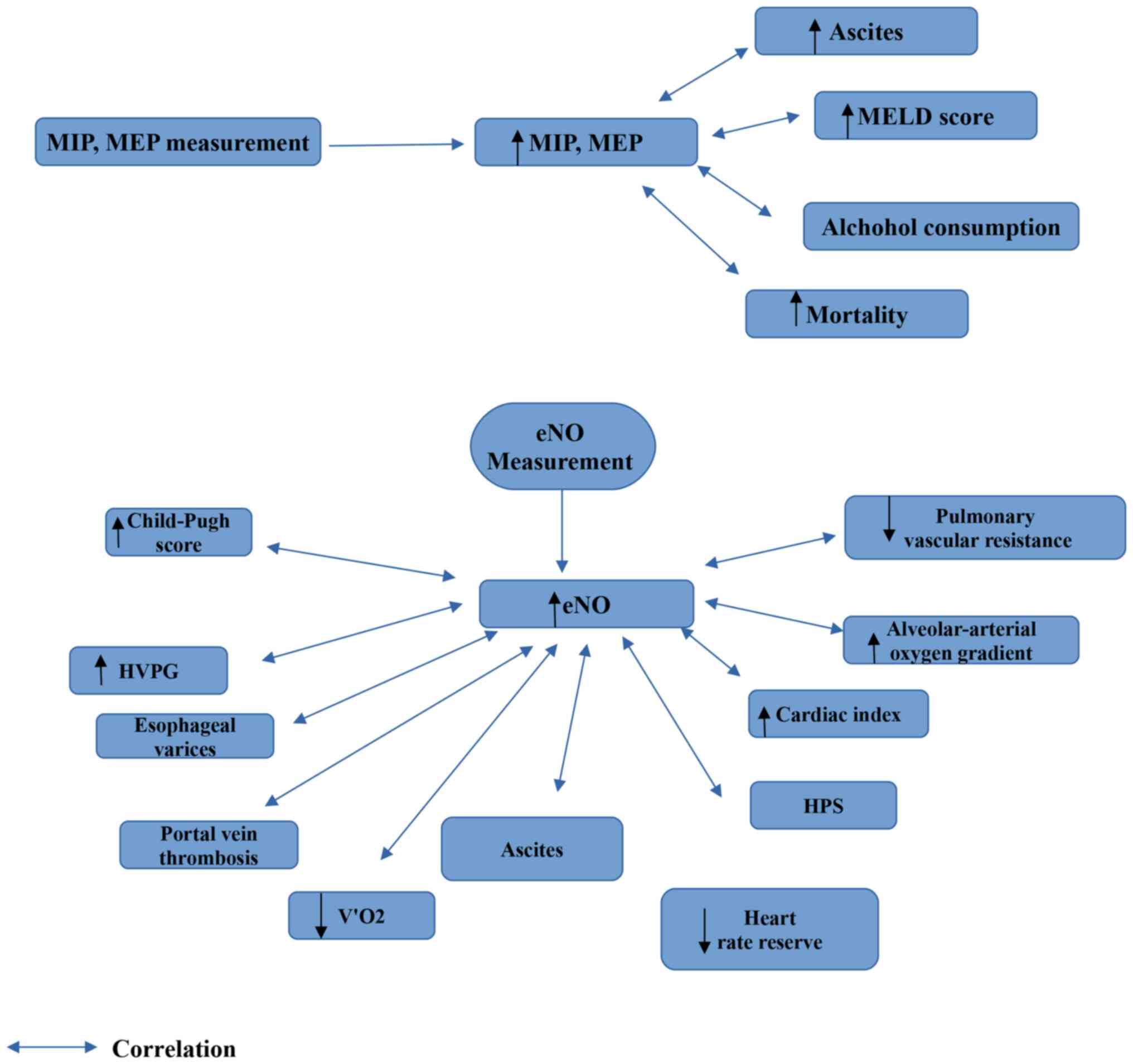
to be affected in patients with liver cirrhosis (35,53-55).
MIP and MEP values have been found to positively correlate with the
presence of ascites and with the MELD score (35). MIP and MEP values have also been
shown to correlate with the modified medical research council
dyspnea scale score in patients with liver cirrhosis (35). In addition, MIP and MEP values have
been reported to be lower in patients with liver cirrhosis due to
alcohol consumption compared to those with liver cirrhosis due to
hepatitis B virus and hepatitis C virus (54). Of note, MIP has been reported as a
predictive indicator of mortality in patients with liver cirrhosis
in a previous study (54).
8. Exhaled nitric oxide measurement
The role of nitric oxide (NO) in respiratory system
pathology has been widely researched. There are conflicting data
regarding the precise significance of NO in respiratory illnesses.
NO represents a pro-inflammatory factor exhibiting immunomodulatory
effects in pathological settings, predisposing to the onset of
airway hyperresponsiveness. In physiological circumstances, on the
contrary, NO weakly modulates smooth muscle relaxation and protects
against airway hyperresponsiveness. Exhaled NO (eNO) is produced by
airway epithelial cells. The measurement of NO has the greatest
clinical utility in allergic airway disease (56).
Endogenous pulmonary NO production estimated from
exhaled air is increased in individuals with cirrhosis and liver
failure (43,57-65).
A significant negative correlation has been observed between
pulmonary vascular resistance and eNO production, indicating that
increased NO production may also contribute to cirrhosis-induced
pulmonary vasodilatation (57). The
eNO concentration has been shown to significantly correlate with
the decrease in the alveolar-arterial oxygen gradient (58,59), and
the decrease in the eNO concentration following liver
transplantation has been found to correlate with the improvement in
oxygenation, reinforcing the hypothesis that NO is a key mediator
of impaired oxygenation in patients with cirrhosis (63). In addition, it has been reported that
there is a definite correlation between the Child-Pugh score
(60,61) and NO in exhaled air, and between peak
NO concentrations and alkaline phosphatase, aspartate
aminotransferase and alanine aminotransferase (ALT), serum albumin
and bilirubin (60). Moreover, an
increased NO output in exhaled air has been found to correlate with
cardiac index, suggesting an association with systemic circulatory
impairment in patients with liver cirrhosis (61,62).
In addition, eNO levels have been found to exhibit a
negative correlation with DLCO values in patients with liver
cirrhosis (43). Furthermore,
increased eNO can distinguish individuals with HPS when applying
specific cut-offs (63), and has
been positively correlated with ascites, portal vein thrombosis,
the mucosal red-color sign of varices, and a high hepatic venous
pressure gradient (64), and
negatively correlated with the average oxygen consumption over
45-60 min of work-time (V'O2) peak and a decrease in
heart rate reserve, indicating limiting aerobic capacity in
patients with liver cirrhosis (65).
Of interest, according to a study a low peak exercise oxygen
consumption (VO2) and reduced eNO may facilitate
identifying patients who are at risk to develop perioperative
sepsis when undergo liver transplantation (66).
The findings which can be derived from the
performance of PFTs in patients with liver cirrhosis are
illustrated in Fig. 1, Fig. 2 and Fig.
3. In addition, diagrams of PFTs are illustrated in Fig. S1, Fig.
S2, Fig. S3, Fig. S4, Fig.
S5 and Fig. S6.
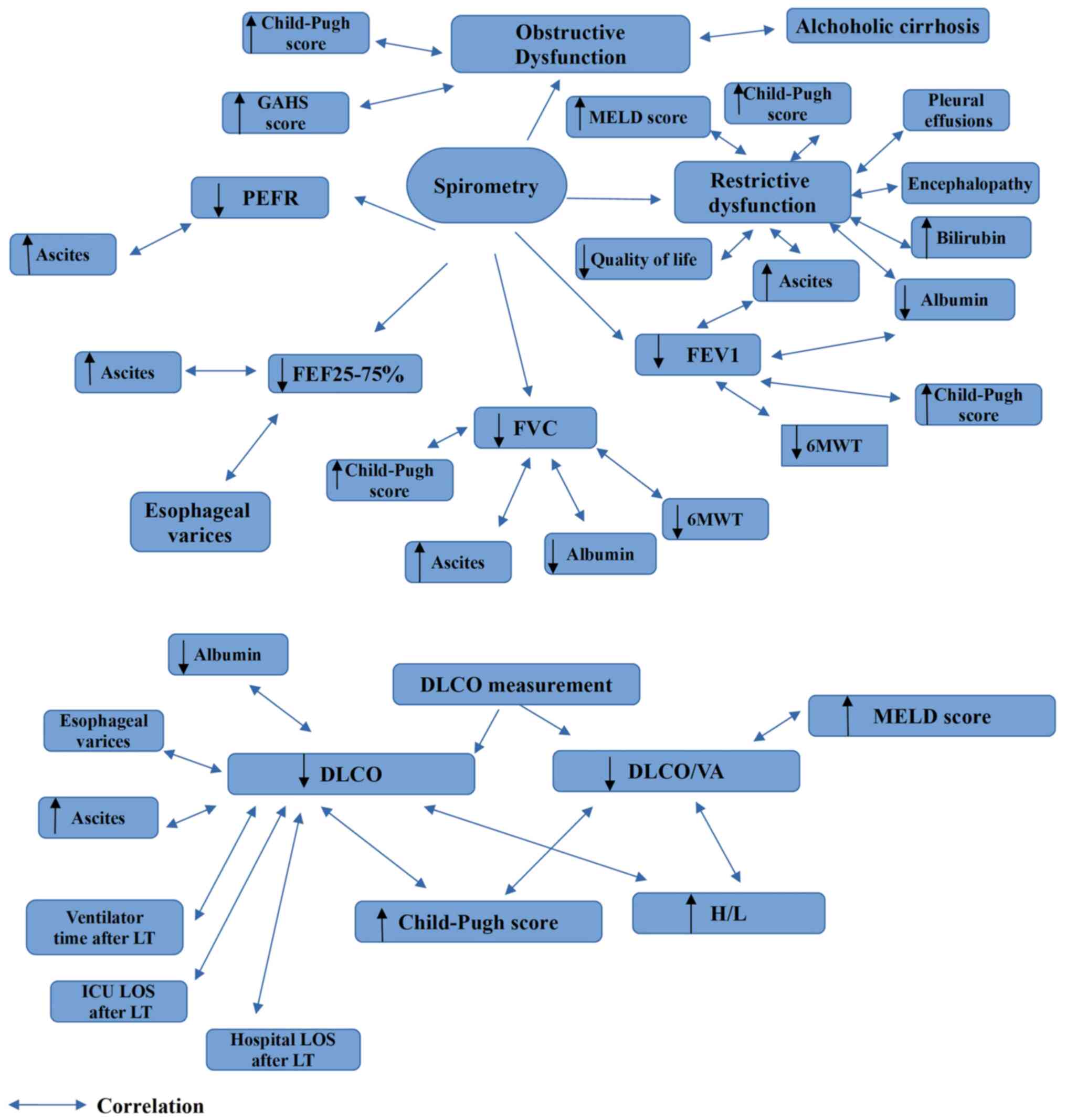 | Figure 1Findings which are obtained from
spirometry and DLCO measurements in liver cirrhosis. GAHS, Glasgow
alchoholic hepatitis score; DLCO, diffusion capacity for carbon
monoxide; H/L, heart liver ratio; FEV1, forced expiratory volume in
1 sec; FVC, forced vital capacity; FEF25-75%, mean forced expired
flow as lung volume decreases from 75 to 25% of vital capacity;
ICU, intensive care unit; LT, liver transplantation; LOS, length of
stay; MELD, Model for End-Stage Liver Disease; PEFR, peak
expiratory flow rate; 6MWT, six minute walking test; VA, alveolar
volume. |
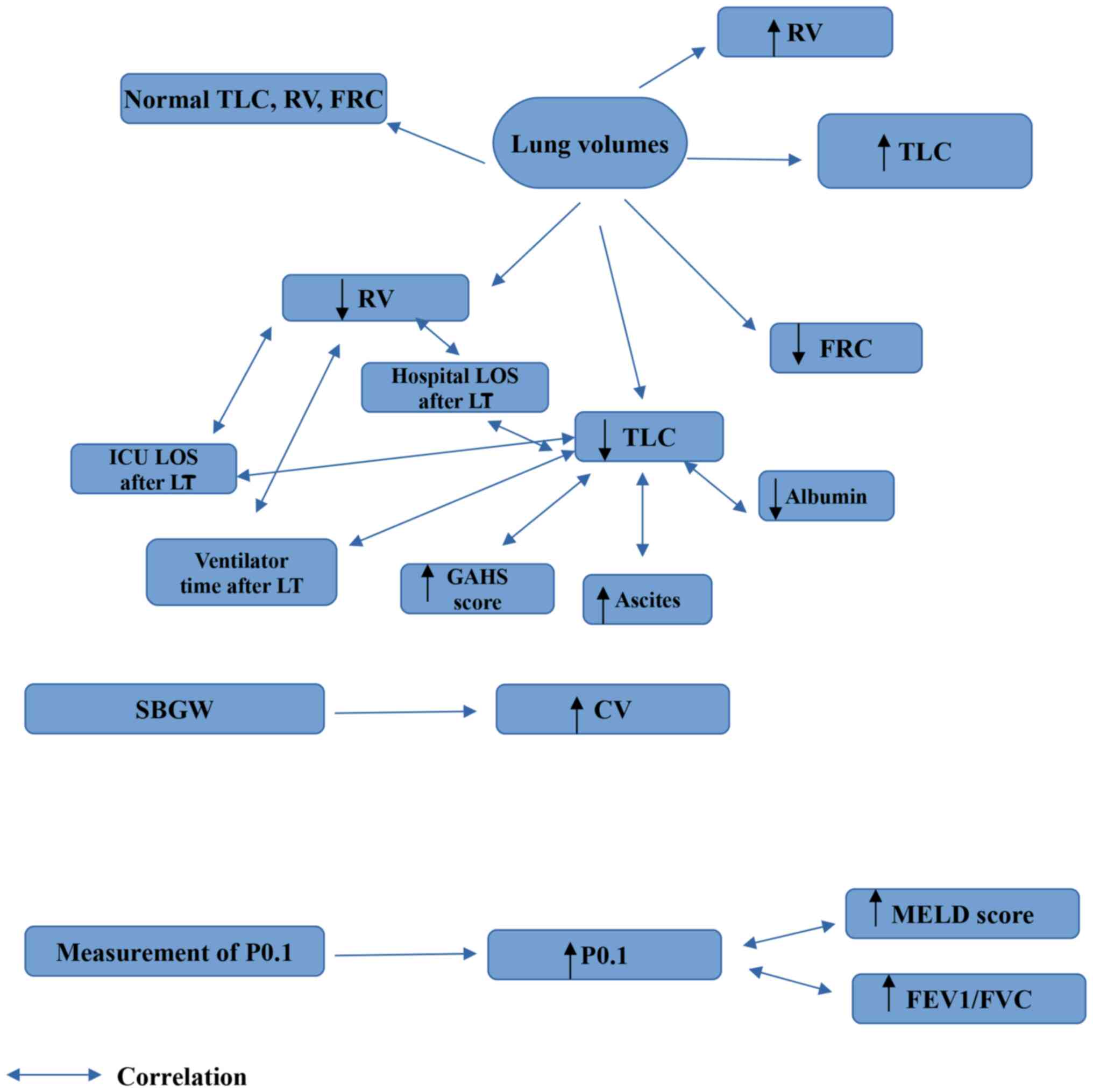 | Figure 2Findings which are obtained from the
measurement of lung volumes, SBGW and measurement of P0.1 in liver
cirrhosis. CV, closing volume; FEV1, forced expiratory volume in 1
sec; FVC, forced vital capacity; FRC, functional residual capacity;
GAHS, Glasgow alchoholic hepatitis score; P0.1, airway occlusion
pressure 0.1 sec after the onset of inspiratory flow; RV, residual
volume; TLC, total lung capacity; ICU, intensive care unit; LT,
liver transplantation; LOS, length of stay; MELD, Model for
End-Stage Liver Disease; SBGW, single breath gas washout. |
9. Pulmonary function testing in children
with liver cirrhosis
To the best of our knowledge, only a few studies to
date have documented the performance of PFT in children with liver
cirrhosis (67-69).
As regards spirometry, some researchers have reported obstructive
dysfunction (67), others have
reported obstructive and/or restrictive ventilatory abnormalities
(68), and some studies have found
isolated declines in the absolute values of FEV1 and FVC (67,69).
FEV1 and FVC values have been observed to be lower in children with
HPS compared to those without HPS; however, this difference has not
reached statistical significance (69). In addition, alterations in
spirometric values have not been related to the duration,
histological severity, or grading of fibrosis in children with
liver cirrhosis. Decreased DLCO values have been reported in
children with liver cirrhosis (67).
However, no correlation has been found between a decrease in DLCO
values and the duration, histological severity, or grading of
fibrosis in children with liver cirrhosis (67).
10. Pathogenetic mechanisms for PFT changes
in patients with liver cirrhosis
Massive hepatomegaly, ascites, atelectasis and
pleural effusions all reduce lung compliance in patients with liver
cirrhosis (25). Restrictive
dysfunction has been linked to the increasing severity of liver
disease and consequences, such as encephalopathy, ascites and
hepatic hydrothorax. The mechanism underlying the link between
encephalopathy and limitation remains unknown; however, it may be
due to the difficulty performing spirometric maneuvers or weakness
in the context of end-stage liver disease. Restriction has also
been related to lower levels of aminotransferases and albumin
(33). The reasons for this are
unknown; however, reduced ALT levels in elderly individuals have
been linked to frailty and sarcopenia (70). As a result, ALT may be a biomarker of
frailty in liver cirrhosis. Restrictive dysfunction is usually
associated with ascites and/or pleural effusions, although some
patients with restriction abnormalities have neither ascites nor
pleural effusions (33). In
addition, respiratory muscle weakness is another component that may
have contributed to a restrictive dysfunction in these patients
(33).
Notably, obesity, systemic inflammation and insulin
resistance, all of which have a marked pathophysiological
association with diseases responsible for liver cirrhosis, such as
NAFLD, can all affect lung function. It has been shown that
worsening hepatic steatosis is accompanied by a more rapid loss of
lung function. By contrast, an improvement in hepatic steatosis is
accompanied by a steady deterioration in pulmonary function,
suggesting the presence of a temporal link between changes in fatty
liver status and lung function deterioration (71). Obesity, inflammation and insulin
resistance are all metabolic risk factors that can affect lung
function by activating pulmonary fibrosis or bronchial inflammation
and inhibiting airway smooth muscle (72). In a cross-sectional investigation,
the homeostasis model assessment of insulin resistance, which
represents an indicator of insulin resistance, was found to have an
inverse connection with FEV1 and FVC (73). In a previous observational study,
individuals with COPD who used antidiabetic medicines that lowered
insulin resistance (i.e., insulin sensitizers) had a lower risk of
exacerbation of airway inflammation (74).
To date, there have been a few comparable
hypotheses, such as insulin resistance causing dysregulation of
airway smooth muscle receptors; however, the process by which
insulin resistance and lung function degradation are linked is not
yet fully understood (75). In
addition, a number of inflammatory mechanisms in adipose tissue,
skeletal muscle and the liver contribute to insulin resistance
development (76). Moreover, serum
high-sensitivity C-reactive protein levels have been evaluated as a
systemic inflammatory marker linked to decreased lung function
(77).
Small airway dysfunction, as indicated by an
increased FEF25-75% and an increase in CV, may be due to intrinsic
alterations in the small airways, such as muscle edema or muscle
spasm, or to changes in the transmural pressure of the airways
caused by peribronchial and interstitial edema (18). According to Ruff et al
(50), the CV in the majority of
patients with cirrhosis was higher than predicted, and gas trapping
was found in the dependent zone of the lungs. They hypothesized
that these abnormalities were caused by interstitial pulmonary
edema (50). This is supported by
additional evidence provided by laboratory experiments on animals.
The small airways in early pulmonary edema models are easily
compressed by peribronchial and perivascular cuffing in edema, and
there is a significant increase in CV and trapped gas volume in the
dependent zone of the lungs (78,79).
Interstitial pulmonary edema in liver cirrhosis can be caused by
systemic and local factors, such as i) decreased colloid osmotic
pressure due to hypoalbuminemia; ii) impairment in lymphatic
drainage from the lungs; and iii) an increase in fluid movement
from capillaries to interstitial spaces due to increased
hydrostatic pressure or altered permeability of the pulmonary
capillary membrane due to elevated levels of vasoactive substances
(80) and endotoxins (18). Endotoxins can cause hyperdynamic
states of circulation in patients with cirrhosis; thus, endotoxins
may play a role in the development of interstitial pulmonary edema,
leading to small airway dysfunction (18). Intrapulmonary vascular dilatations,
widespread interstitial lung illness, pulmonary vaso-occlusive
disease, and/or ventilation-perfusion imbalance may all account for
gas transfer impairment, as indicated by abnormal DLCO values. An
increased capillary plasma volume associated with alveolar
capillary dilatation in some patients with severe hepatic disease
would be predicted to increase the diffusion distance for carbon
monoxide (as well as oxygen) from the alveoli to the red blood
cells in the capillary bloodstream, resulting in an increase in the
membrane component of diffusion resistance and subsequent hypoxemia
(6). Alternative explanations for
the decrease in DLCO include early diffuse interstitial lung
disease that affects gas exchange, blood flowing through
non-ventilated alveoli, anatomic communications between pulmonary
arteries and veins that bypass the capillary-alveolar interfaces,
and other pulmonary vascular diseases (6).
Although pulmonary hypertension has been linked to
cirrhosis and portal hypertension, it is rare, with only a few
cases recorded (80,81). Individuals with liver cirrhosis, on
the other hand, frequently have low or normal pulmonary vascular
resistance (38). Cirrhosis has been
shown to be associated with diffuse pulmonary emboli (82) or pulmonary vascular disease with
concentric wall thickening of the arteries and veins (83). Even though pulmonary thromboembolism
from the portal circulation has been proposed as a cause of
pulmonary hypertension in liver disease, Matsubara et al
(83) failed to demonstrate a
statistically significant occurrence of thrombi in the portal and
pulmonary vascular beds of patients with hepatic failure. When the
restrictive defect is caused by parenchymal pulmonary disease,
there is usually a corresponding reduction in DLCO that is
disproportionate to the reduction in lung capacity, due to the
diffuse parenchymal process involving the microcirculation
(6). Interstitial lung disease has
been linked to primary biliary cirrhosis (82). There is a considerable link between
PBC and Sjogren's syndrome (84),
with the latter occurring in half of patients with PBC. Pulmonary
function abnormalities linked with Sjogren's syndrome have been
thoroughly documented (85), and
they include both obstructive and restrictive ventilatory defects.
Some researchers have reported that Sjogren's syndrome contributes
to the lung abnormalities reported in patients with PBC (86). Chronic active hepatitis has also been
linked to interstitial lung disease (87).
The increased alveolar-arterial oxygen gradient seen
in liver cirrhosis may be the result of ventilation-perfusion
mismatch, right-to-left shunting, or perfusion-diffusion imbalance.
Ventilation-perfusion mismatch can occur dur to the following: i)
The narrowing and early closure of airways to dependent lung zones
due to interstitial edema, pleural effusion, or ascites and/or ii)
an imbalance between vasoconstrictor and vasodilator substances
that are abnormally metabolized by an impaired liver, with the
resultant impairment in hypoxic vasoconstriction leading to
relative overperfusion of poorly ventilated (6). Lung ‘spiders’, which are arterial
changes in the lungs related to liver cirrhosis, may also
contribute to the diffusion abnormalities without restriction,
causing right-to-left shunting and/or diffusion-perfusion imbalance
(88). Another potential source of
the observed oxygenation defect is right-to-left shunting of blood
through arteriovenous fistulae, which has been well-described in
patients with advanced liver failure and may have accounted for the
moderately severe hypoxemia observed in a large proportion of
patients with concurrent diffusion abnormalities (88).
The most prevalent acid-base disorder in cirrhotic
individuals is the decreased partial pressure of carbon dioxide and
respiratory alkalosis (89,90). The precise cause of the aberrant
hyperventilation in these patients remains unknown. However,
hyperammonemia, ascites, HPS, increased chemosensitivity to
CO2 and hypoxia, and poor progesterone and estradiol
metabolism may all contribute to hyperventilation in individuals
with decompensated cirrhosis (90,91).
Respiratory muscle weakness and a raised diaphragm due to ascites
are two major causes of hyperventilation in individuals with
cirrhosis (92). Previous research
has linked inspiratory muscle fatigue to higher P0.1 in healthy
individuals (93). P0.1 levels have
also been found to be elevated in patients suffering from various
disorders that induce impaired respiratory muscle strength and
dyspnea (94). The impact of
inspiratory muscle strength training (IMST) on inspiratory motor
drive (P0.1) in healthy volunteers has been investigated, and it
has been demonstrated that IMST significantly enhances MIP, which
has also been associated with a decrease in P0.1(95). In patients with liver cirrhosis,
hyperventilation causes respiratory muscle weakening, which results
in increased respiratory motor output and P0.1(15).
Hyperventilation induces the overuse of the
respiratory muscles, which may exhibit impairment (35). As regards the lower values of
respiratory muscle strength indices in patients with ascites, one
possible reason is that these patients have more severe liver
disease and a varied degree of mechanical compromise due to ascites
(35). Moreover, patients with liver
cirrhosis have less muscle mass due to a variety of causes, the
most notable of which is protein-calorie deficiency. Another aspect
that contributes to muscle mass loss is a decrease in anabolism and
an increase in protein catabolism. These nutritional and catabolic
consequences on skeletal muscles occur throughout the body,
resulting in reduced muscle function in patients with cirrhosis
(53).
In individuals with cirrhosis, an increase in portal
pressure causes the dilatation of visceral arterial blood vessels
throughout the body, as well as a hyperkinetic circulatory
condition in combination with portosystemic collateral circulation.
Endotoxins and additional gut-produced metabolites can directly
stimulate blood vessels or cytokines to derive NO synthase (NOS)
and lead to an increased in vivo synthesis and release of NO
due to decreased liver metabolism, toxin accumulation, increased
permeability of the intestinal wall, damaged intestinal motility,
and alteration and translocation of the intestinal flora (61). NO is a signaling molecule with a
marked involvement in inflammation and tissue damage, and it can
widen visceral blood vessels with the elevation of visceral blood
flow and aggravate portal hypertension. Increased levels of
inflammatory cytokines and endotoxins in the bloodstream of
patients with cirrhosis can activate pulmonary vascular endothelial
cells to generate NO, which is exhaled outside the body via the
respiratory tract (64). An
increased NOS expression and elevated NO concentrations in
peripheral blood are related to a decrease in the inactivating
effects of the liver on endotoxins, and an increase in endotoxin
concentrations in the blood circulation in cirrhotic individuals.
However, serum NO levels are mostly assessed by detecting its
metabolites, nitrate and nitrite, and the results may be incorrect
(96). Under the catalysis of NOS,
NO is generated in vivo from L-arginine and oxygen.
Increased NO levels in the pulmonary small airways and alveolar
areas of cirrhotic individuals can result in elevation of eNO
concentration (58). Excessive eNO
production is mostly related to an increase in eNO production by
pulmonary vascular endothelial cells, airway epithelial cells and
peripheral inflammatory cells (61).
11. Alterations in PFTs following specific
interventions
Large-volume paracentesis (LVP) is a well-accepted
therapeutic option for cirrhotic individuals with tense ascites.
Following LVP, the majority of patients experience a symptomatic
improvement in breathing (97). The
effects of LVP on PFTs in patients with liver cirrhosis have been
extensively investigated (20,34,37,97-103).
The majority of available studies have reported that LVP results in
an increase in lung volumes (34,97-101)
and an increase in the values of the spirometric parameters, FEV1,
FVC, FEV/FVC, FEF25-75% and PEFR (20,34,37,97-103).
As regards DLCO, some studies have demonstrated an increase in its
values following LVP (34,100), whereas others have mentioned no
change following LVP (97,98,101).
Of note, one study on LVP in individuals with tense cirrhotic
ascites demonstrated a lack of effect on MIP values, suggesting
that the cause is not solely mechanical (102). In addition, the administration of
diuretics, such as spironolactone and furosemide has been shown to
have positive effects both on the values of spirometric parameters
(37,100) and the values of DLCO and lung
volumes (100).
Respiratory rehabilitation is an important
intervention that has been reported to result in an increase in MIP
and MEP values and in the values of FEF25-75% in patients with
liver cirrhosis (104).
Furthermore, liver transplantation leads to an improvement in
arterial oxygenation, but no change in the values of DLCO (105,106).
However, liver transplantation results in the normalization of
increased eNo values (63).
12. Hepatopulmonary syndrome
HPS, which is present in 10-17% of individuals with
cirrhosis, is characterized by dilated intrapulmonary vessels,
particularly in the basal regions of the lungs. Hypoxemia develops
and oxygen therapy may be necessary. Liver transplantation is the
sole curative method as it may prevent HPS by closing the shunts.
Alveolar-arterial oxygen gradient calculation and contrast
echocardiography are two methods used for the diagnosis of HPS. The
severity of HPS can be a standalone indication for liver
transplantation and is unrelated to the severity of liver disease.
Since patients with partial pressure of oxygen levels <50 mmHg
and no reversibility to 100% oxygen may be at risk of developing
irreversible respiratory failure in the post-transplant period, and
having a significant risk of perioperative mortality, it is crucial
to accurately identify the severity of HPS (107).
It has been reported that an impaired DLCO is a
very common finding when performing PFTs in these patients. DLCO
levels have been found to be lower in patients with HPS compared to
other patients with cirrhosis, candidates for liver transplantation
(108). An impaired DLCO has been
described to be independently associated with HPS and has been
found to be able to predict the diagnosis of HPS with a
considerable discriminative ability (area under the receiver
operating characteristic curve, 0.890) (109,110).
13. Special considerations
The use of pulmonary PFTs in combination with DLCO
to evaluate signs of primary lung disease or the HPS is debatable,
and there are significant variations in practice. While some
transplantation institutions only screen individuals with symptoms,
a history of smoking, or a history of established lung illness,
others perform testing to all transplant candidates. More
specifically, according to the European Association for the Study
of the Liver clinical practice guidelines for liver transplantation
candidates, there is a recommendation for performing PFTs to
evaluate the respiratory function in all liver transplant
candidates (107).
As regards the role of PFTs in the prognosis of
patients with liver cirrhosis, as it was mentioned above, DLCO, TLC
and RV have been described as significant predictors of ventilator
time and both ICU and hospital LOS (P<0.05), but not of patients
of graft survival undergoing liver transplantation (38). Moreover, according to another study,
although abnormal PFTs are found in a great proportion of patients
undergoing liver transplants, they are not associated with
complications, graft failure, or mortality following liver
transplantation (111).
14. Conclusions
Pulmonary complications in patients with liver
cirrhosis are accompanied by alterations in PFTs. More
specifically, patients with liver cirrhosis present with lower
values of the spirometric parameters, FEV1, FVC, FEV1/FVC, PEFR and
FEF25-75%, of DLCO, lung volumes, MIP and MEP. In addition, they
present with increased values of CV, P0.1 and eNO. These
alterations have been shown to be associated with disease severity,
clinical features and laboratory parameters in adult patients with
liver cirrhosis. Patients with ascites may require therapeutic
paracentesis to improve their pulmonary function. Such findings
should be considered when assessing patients with liver disease,
particularly those who may require surgical intervention. As an
impaired lung function and in particular, restrictive dysfunction,
can affect post-transplant outcomes such as ventilator time, the
hospital LOS and post-operative pulmonary complications, it is
critical for the transplant care team to be aware of its prevalence
and significance.
Supplementary Material
Diagram of spirometry. (A) Flow
(L/s)-time (sec) Curve. FVC, forced vital capacity; MEF25%, flow at
25% of FVC; MEF 50%, flow at 50% of FVC; MEF75%, flow at 75% of
FVC; PEFR, peak expiratory flow rate. (B) Volume-time curve. FEV1,
forced expiratory volume in 1 sec; FVC, forced vital capacity; ATS,
American Thoracic Society.
Diagram of DLCO. CO, carbon monoxide;
CH4, methane; DLCO, diffusion capacity for carbon monoxide; VC,
vital capacity.
Diagram of lung volumes. Functional
residual capacity (FRC) is the sum of expiratory reserve volume
(ERV) and residual volume (RV). Total lung capacity (TLC) is the
sum of FRC and inspiratory capacity (IC).
Diagram for MIP and MEP [Pmouth
(cmH2O)-time (sec) curve]. MIP, maximal inspiratory
pressure; MEP, maximal expiratory pressure.
Diagram for single breath gas washout
test. Phase I is the very beginning of exhalation where only oxygen
is being exhaled and consists primarily of test system and airway
deadspace. Phase II is where the gas concentration rises rapidly
and consists of mixture of airway and alveolar gas. Phase III is
where the gas concentration plateaus and its slope depends on the
uniform distribution of gas in the lung. Phase IV is where the gas
concentration rises abruptly from the plateau and is considered to
be part of the closing volume.
Diagram of P0.1. VT, volume tidal;
P0.1, airway occlusion pressure 0.1 sec after the onset of
inspiratory flow.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
VEG and EC conceptualized the study. VEG, SA and EC
examined the data from the literature for inclusion in the review,
and wrote and prepared the draft of the manuscript. EC and VEG
provided critical revisions. All authors contributed to manuscript
revision and have read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schuppan D and Afdhal NH: Liver cirrhosis.
Lancet. 371:838–851. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Iwakiri Y, Shah V and Rockey DC: Vascular
pathobiology in chronic liver disease and cirrhosis-current status
and future directions. J Hepatol. 61:912–924. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Iwakiri Y: Pathophysiology of portal
hypertension. Clin Liver Dis. 18:281–291. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Benz F, Mohr R, Tacke F and Roderburg C:
Pulmonary complications in patients with liver cirrhosis. J Transl
Int Med. 8:150–158. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sood G, Fallon MB, Niwas S, Tutton T, Van
Leeuwen DJ, Bloomer JR and McGuire BM: Utility of a dyspnea-fatigue
index for screening liver transplant candidates for hepatopulmonary
syndrome [abstract]. Hepatolog. 28 (Suppl)(S742)1998.
|
|
6
|
Hourani JM, Bellamy PE, Tashkin DP, Batra
P and Simmons MS: Pulmonary dysfunction in advanced liver disease:
Frequent occurrence of an abnormal diffusing capacity. Am J Med.
90:693–700. 1991.PubMed/NCBI
|
|
7
|
Fallon MB and Abrams GA: Pulmonary
dysfunction in chronic liver disease. Hepatology. 32:859–865.
2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Møller S, Krag A, Henriksen JH and
Bendtsen F: Pathophysiological aspects of pulmonary complications
of cirrhosis. Scand J Gastroenterol. 42:419–427. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Miller MR, Crapo R, Hankinson J, Brusasco
V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CP,
Gustafsson P, et al: General considerations for lung function
testing. Eur Respir J. 26:153–161. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Georgakopoulou VE, Tarantinos K, Papalexis
P, Spandidos DA, Damaskos C, Gkoufa A, Chlapoutakis S, Sklapani P,
Trakas N and Mermigkis D: Role of pulmonary function testing in
inflammatory bowel diseases (review). Med Int (Lond).
2(25)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Roque L, Sankarankutty AK, Silva OC Jr and
Mente ED: Evaluation of lung function in liver transplant
candidates. Transplant Proc. 50:762–765. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yao EH, Kong BC, Hsue GL, Zhou AC and Wang
H: Pulmonary function changes in cirrhosis of the liver. Am J
Gastroenterol. 82:352–354. 1987.PubMed/NCBI
|
|
13
|
Awad NF, Elbalsha AAM, Amer MZA and
Ibrahim MHE: Study of the relationship between severity of liver
cirrhosis and pulmonary function tests. Egypt J Hosp Med.
76:4570–4576. 2019.
|
|
14
|
Whitelaw WA, Derenne JP and Milic-Emili J:
Occlusion pressure as a measure of respiratory center output in
conscious man. Respir Physiol. 23:181–199. 1975.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gholamipoor D, Nassiri-Toosi M, Azadi M
and Asadi Gharabaghi M: The relationship between airway occlusion
pressure and severity of liver cirrhosis in candidates for liver
transplantation. Middle East J Dig Dis. 12:111–115. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Graham BL, Steenbruggen I, Miller MR,
Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA,
McCarthy K, McCormack MC, et al: Standardization of spirometry 2019
update. An official American thoracic society and european
respiratory society technical statement. Am J Respir Crit Care Med.
200:e70–e88. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Riley CM, Wenzel SE, Castro M, Erzurum SC,
Chung KF, Fitzpatrick AM, Gaston B, Israel E, Moore WC, Bleecker
ER, et al: Clinical implications of having reduced mid forced
expiratory flow rates (FEF25-75), independently of FEV1, in adult
patients with asthma. PLoS One. 10(e0145476)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hara N, Yoshida T, Furukawa T and Inokuchi
K: Abnormalities in maximum flow volume curve and closing volume in
patients with hepatic cirrhosis. Jpn J Surg. 10:265–269.
1980.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Caruso G, Catalano D, Corsaro A, Salerno
M, Sciuto L, Sciuto V and Mazzone O: Respiratory function and liver
cirrhosis. Riv Eur Sci Med Farmacol. 12:83–89. 1990.PubMed/NCBI
|
|
20
|
Nagral A, Kolhatkar VP, Bhatia SJ, Taskar
VS and Abraham P: Pulmonary function tests in cirrhotic and
non-cirrhotic portal hypertension. Indian J Gastroenterol.
12:36–40. 1993.PubMed/NCBI
|
|
21
|
Al-Moamary MS, Gorka T, Al-Traif IH,
Al-Jahdali HH, Al-Shimemeri AA, Al-Kanway B and Abdulkareeem AA and
Abdulkareeem AA: Pulmonary changes in liver transplant candidates
with hepatitis C cirrhosis. Saudi Med J. 22:1069–1072.
2001.PubMed/NCBI
|
|
22
|
Tüzün A, Uzun K, Yüksekol I and Taş D:
Evaluation of pulmonary function tests in end stage liver diseases.
Solunum. 3:117–120. 2001.
|
|
23
|
Yigit IP, Hacievliyagil SS, Seckin Y, Oner
RI and Karincaoglu M: The relationship between severity of liver
cirrhosis and pulmonary function tests. Dig Dis Sci. 53:1951–1956.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Drenth JPH, Jansen JBMJ and Dekhuijzen
PNR: Reduced diffusion in liver cirrhosis is related to impairment
of protein liver synthesis. Scand J Gastroenterol. 37:1338–1340.
2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bozbas SS, Yilmaz EB, Dogrul I, Ergur FO,
Savas N, Eyuboglu F and Haberal M: Preoperative pulmonary
evaluation of liver transplant candidates: Results from 341 adult
patients. Ann Transplant. 16:88–96. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ghayumi SM, Mehrabi S, Zamirian M, Haseli
J and Bagheri Lankarani K: Pulmonary complications in cirrhotic
candidates for liver transplantation. Hepat Mon. 10:105–109.
2010.PubMed/NCBI
|
|
27
|
Siemieniako A, Pogorzelska J, Łapiński TW
and Flisiak R: Respiratory functional impairment in patients with
liver cirrhosis. Pol Merkur Lekarski. 31:274–277. 2011.PubMed/NCBI(In Polish).
|
|
28
|
Corlateanu O, Tcaciuc E and Corlateanu A:
Evaluation of pulmonary function and functional capacity in
patients with liver cirrhosis. Eur Respir J. 40 (Suppl
56)(P588)2012.
|
|
29
|
Thenmozhi R, Ratna Manjushree J, Heber A
and Vishwanatha RB: Pulmonary functions and respiratory efficiency
in patients with cirrhosis and portal hypertension. Int J Sci
Study. 4:114–117. 2016.
|
|
30
|
Osni Leão Perin P, de Fátma Ferreira
Santana Boin I, Oliveira da Silva AM, Chueiri Neto F and Martins
LC: Lung ultrasound and pulmonary function test in cirrhotic
patients. Transplant Proc. 49:824–828. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shahzad M, Iqbal J, Imran Aslam M, Tahir
M, Javed M and Ashfaq Zia M: Examine the association between
patients of liver cirrhosis and pulmonary dysfunctions. PJMHS.
13:573–575. 2019.
|
|
32
|
Alkhayat K, Moustafa G, Zaghloul A and
Elazeem AA: Pulmonary dysfunction in patients with liver cirrhosis.
Arch Med. 9(4)2017.
|
|
33
|
DuBrock HM, Krowka MJ, Krok K, Forde K,
Mottram C, Scanlon P, Al-Naamani N, Patel M, McCormick A, Fallon MB
and Kawut SM: Prevalence and impact of restrictive lung disease in
liver transplant candidates. Liver Transpl. 26:989–999.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Makhlouf NA, Mahran ZG, Sadek SH, Magdy DM
and Makhlouf HA: Six-minute walk test before and after large-volume
paracentesis in cirrhotic patients with refractory ascites: A pilot
study. Arab J Gastroenterol. 20:81–85. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kaltsakas G, Antoniou E, Palamidas AF,
Gennimata SA, Paraskeva P, Smyrnis A, Koutsoukou A, Milic-Emili J
and Koulouris NG: Dyspnea and respiratory muscle strength in
end-stage liver disease. World J Hepatol. 5:56–63. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mizuno Y, Ito S, Hattori K, Nagaya M,
Inoue T, Nishida Y, Onishi Y, Kamei H, Kurata N, Hasegawa Y and
Ogura Y: Changes in muscle strength and six-minute walk distance
before and after living donor liver transplantation. Transplant
Proc. 48:3348–3355. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nitrini AMS, Rolim EG and Stirbulov R:
Influence of ascites on pulmonary function in patients with portal
hypertension. J Bras Pneumol. 30:1–9. 2004.
|
|
38
|
Kia L, Cuttica MJ, Yang A, Donnan EN,
Whitsett M, Singhvi A, Lemmer A and Levitsky J: The utility of
pulmonary function testing in predicting outcomes following liver
transplantation. Liver Transpl. 22:805–811. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Demirel S, Funda Coskun N, Giray Nak S and
Ozyener F: Assessment of pulmonary functions with spirometry method
in hepatic impairment patients. Ann Med Res. 28:1–7. 2021.
|
|
40
|
Zuberi FF, Zuberi BF, Rasheed T and Nawaz
Z: Non-specific impairment of lung function on spirometery in
patients with chronic hepatitis-C. Pak J Med Sci. 35:360–364.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Enright Md P: Office-based DLCO tests help
pulmonologists to make important clinical decisions. Respir
Investig. 54:305–311. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Degano B, Mittaine M, Guénard H, Rami J,
Garcia G, Kamar N, Bureau C, Péron JM, Rostaing L and Rivière D:
Nitric oxide and carbon monoxide lung transfer in patients with
advanced liver cirrhosis. J Appl Physiol (1985). 107:139–143.
2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jung JY, Jun DW and Lee JH: Lung diffusion
capacity in early cirrhosis: Is lung diffusion capacity a predictor
of esophageal varices and ascites? Dig Dis Sci. 56:1229–1234.
2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Park MS, Lee MH, Park YS, Kim SH, Kwak MJ
and Kang JS: Abnormal gas diffusing capacity and portosystemic
shunt in patients with chronic liver disease. Gastroenterology Res.
5:182–189. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
45
|
O'Donnell CR, Bankier AA, Stiebellehner L,
Reilly JJ, Brown R and Loring SH: Comparison of plethysmographic
and helium dilution lung volumes: Which is best for COPD? Chest.
137:1108–1115. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hopkins E and Sharma S: Physiology,
functional residual capacity. [Updated 2022 Jan 4]. In: StatPearls
[Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-.
Available from: https://www.ncbi.nlm.nih.gov/books/NBK500007/.
|
|
47
|
Lover MB: Cirrhosis and regional lung
function. Anesthesiology. 36(527)1972.
|
|
48
|
Milic-Emili J, Torchio R and D'Angelo E:
Closing volume: A reappraisal (1967-2007). Eur J Appl Physiol.
99:567–583. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Veneroni C, Van Muylem A, Malinovschi A,
Michils A and Dellaca' RL: Closing volume detection by
single-breath gas washout and forced oscillation technique. J Appl
Physiol (1985). 130:903–913. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ruff F, Hughes JM, Stanley N, McCarthy D,
Greene R, Aronoff A, Clayton L and Milic-Emili J: Regional lung
function in patients with hepatic cirrhosis. J Clin Invest.
50:2403–2413. 1971.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kuhlen R, Mohnhaupt R, Slama K, Hausmann
S, Pappert D, Rossaint R and Falke K: Validation and clinical
application of a continuous P0.1 measurement using standard
respiratory equipment. Technol Health Care. 4:415–424.
1996.PubMed/NCBI
|
|
52
|
Evans JA and Whitelaw WA: The assessment
of maximal respiratory mouth pressures in adults. Respir Care.
54:1348–1359. 2009.PubMed/NCBI
|
|
53
|
Corrêa FCCR, Mira PAC, Pace FHL, Laterza
MC, Trevizan PF and Martinez DG: Reduced peripheral and inspiratory
muscle endurance in patients with liver cirrhosis: A
cross-sectional study. Arq Gastroenterol. 58:308–315.
2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Faustini Pereira JL, Galant LH, Rossi D,
Telles da Rosa LH, Garcia E, de Mello Brandão AB and Marroni CA:
Functional capacity, respiratory muscle strength, and oxygen
consumption predict mortality in patients with cirrhosis. Can J
Gastroenterol Hepatol. 2016(6940374)2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Galant LH, Forgiarini Junior LA, Dias AS
and Marroni CA: Functional status, respiratory muscle strength, and
quality of life in patients with cirrhosis. Rev Bras Fisioter.
16:30–34. 2012.PubMed/NCBI(In English, Portuguese).
|
|
56
|
Taylor DR, Pijnenburg MW, Smith AD and De
Jongste JC: Exhaled nitric oxide measurements: Clinical application
and interpretation. Thorax. 61:817–827. 2006.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sogni P, Garnier P, Gadano A, Moreau R,
Dall'Ava-Santucci J, Dinh-Xuan AT and Lebrec D: Endogenous
pulmonary nitric oxide production measured from exhaled air is
increased in patients with severe cirrhosis. J Hepatol. 23:471–473.
1995.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Rolla G, Brussino L, Colagrande P,
Scappaticci E, Morello M, Bergerone S, Ottobrelli A, Cerutti E,
Polizzi S and Bucca C: Exhaled nitric oxide and impaired
oxygenation in cirrhotic patients before and after liver
transplantation. Ann Intern Med. 129:375–378. 1998.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Söderman C, Leone A, Furst V and Persson
MG: Endogenous nitric oxide in exhaled air from patients with liver
cirrhosis. Scand J Gastroenterol. 32:591–597. 1997.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Delclaux C, Mahut B, Zerah-Lancner F,
Delacourt C, Laoud S, Cherqui D, Duvoux C, Mallat A and Harf A:
Increased nitric oxide output from alveolar origin during liver
cirrhosis versus bronchial source during asthma. Am J Respir Crit
Care Med. 165:332–337. 2002.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Matsumoto A, Ogura K, Hirata Y, Kakoki M,
Watanabe F, Takenaka K, Shiratori Y, Momomura S and Omata M:
Increased nitric oxide in the exhaled air of patients with
decompensated liver cirrhosis. Ann Intern Med. 123:110–113.
1995.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Degano B, Mittaine M, Hervé P, Rami J,
Kamar N, Suc B, Rivière D and Rostaing L: Nitric oxide production
by the alveolar compartment of the lungs in cirrhotic patients. Eur
Respir J. 34:138–144. 2009.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Lam Shin Cheung J, Naimi M, Sykes J and
Gupta S: A role for alveolar exhaled nitric oxide measurement in
the diagnosis of hepatopulmonary syndrome. J Clin Gastroenterol.
54:278–283. 2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Huang X, Thansamay S, Yang K, Luo T and
Chen S: Measurement of exhaled nitric oxide in cirrhotic patients
with esophageal and gastric varices. Biomed Res Int.
2019(9673162)2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Hulo S, Edme JL, Inamo J, Van Bulck R,
Dharancy S and Neviere R: Elevated alveolar nitric oxide is linked
to poor aerobic capacity and chronotropic incompetence in liver
transplant candidates. J Breath Res. 12(046008)2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Neviere R, Trinh-Duc P, Hulo S, Edme JL,
Dehon A, Boleslawski E, Dharancy S and Lebuffe G: Predictive value
of exhaled nitric oxide and aerobic capacity for sepsis
complications after liver transplantation. Transpl Int.
29:1307–1316. 2016.PubMed/NCBI View Article : Google Scholar
|
|
67
|
El-Shabrawi MHF, El-Karaksy HM, Okasha SH,
El-Sayed HM, Kotb MA, Hassan AM and Ibrahim AM: Pulmonary function
testing in children with chronic liver disease. Alex J Pediatr.
16:405–409. 2002.
|
|
68
|
Alves L, Sant'Anna CC, March Mde F,
Ferreira S, Marsillac M, Tura M and Oñate H: Preoperative pulmonary
assessment of children for liver transplantation. Pediatr
Transplant. 12:536–540. 2008.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Dehghani SM, Aleyasin S, Honar N,
Eshraghian A, Kashef S, Haghighat M and Malek-Hosseini SA:
Pulmonary evaluation in pediatric liver transplant candidates.
Indian J Pediatr. 78:171–175. 2011.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Vespasiani-Gentilucci U, De Vincentis A,
Ferrucci L, Bandinelli S, Antonelli Incalzi R and Picardi A: Low
alanine aminotransferase levels in the elderly population: Frailty,
disability, sarcopenia, and reduced survival. J Gerontol A Biol Sci
Med Sci. 73:925–930. 2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Lee HW, Chung GE, Koo BK, Sim H, Choi M,
Lee DH, Choi SH, Kwak SH, Kim DK and Kim W: Innovative Target
Exploration of NAFLD (ITEN) consortium. Impact of evolutionary
changes in nonalcoholic fatty liver disease on lung function
decline. Gut Liver. 17:139–149. 2023.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Forno E, Han YY, Muzumdar RH and Celedón
JC: Insulin resistance, metabolic syndrome, and lung function in US
adolescents with and without asthma. J Allergy Clin Immunol.
136:304–311.e8. 2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Kim SH, Kim HS, Min HK and Lee SW:
Association between insulin resistance and lung function trajectory
over 4 years in South Korea: Community-based prospective cohort.
BMC Pulm Med. 21(110)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Wang MT, Lai JH, Huang YL, Kuo FC, Wang
YH, Tsai CL and Tu MY: Use of antidiabetic medications and risk of
chronic obstructive pulmonary disease exacerbation requiring
hospitalization: A disease risk score-matched nested case-control
study. Respir Res. 21(319)2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Baffi CW, Wood L, Winnica D, Strollo PJ
Jr, Gladwin MT, Que LG and Holguin F: Metabolic syndrome and the
lung. Chest. 149:1525–1534. 2016.
|
|
76
|
Wu H and Ballantyne CM: Metabolic
Inflammation and insulin resistance in obesity. Circ Res.
126:1549–1564. 2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Kalhan R, Tran BT, Colangelo LA, Rosenberg
SR, Liu K, Thyagarajan B, Jacobs DR Jr and Smith LJ: Systemic
inflammation in young adults is associated with abnormal lung
function in middle age. PLoS One. 5(e11431)2010.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Hughes JM and Rosenzweig DY: Factors
affecting trapped gas volume in perfused dog lungs. J Appl Physiol.
29:332–339. 1970.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Lemen R, Jones JG, Graf PD and Cowan G:
‘Closing volume’ changes in alloxan-induced pulmonary edema in
anesthetized dogs. J Appl Physiol. 39:235–241. 1975.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Rüttner JR, Bärtschi JP, Niedermann R and
Schneider J: Plexogenic pulmonary arteriopathy and liver cirrhosis.
Thorax. 35:133–136. 1980.PubMed/NCBI View Article : Google Scholar
|
|
81
|
McDonnell PJ, Toye PA and Hutchins GM:
Primary pulmonary hypertension and cirrhosis: Are they related? Am
Rev Respir Dis. 127:437–441. 1983.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Golding PL, Smith M and Williams R:
Multisystem involvement in chronic liver disease. Studies on the
incidence and pathogenesis. Am J Med. 55:772–782. 1973.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Matsubara O, Nakamura T, Uehara T and
Kasuga T: Histometrical investigation of the pulmonary artery in
severe hepatic disease. J Pathol. 143:31–37. 1984.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Rodriguez-Roisin R, Pares A, Bruguera M,
Coll J, Picado C, Agusti-Vidal A, Burgos F and Rodes J: Pulmonary
involvement in primary biliary cirrhosis. Thorax. 36:208–212.
1981.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Segal I, Fink G, Machtey I, Gura V and
Spitzer SA: Pulmonary function abnormalities in Sjögren's syndrome
and the sicca complex. Thorax. 36:286–289. 1981.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Krowka MJ and Cortese DA: Pulmonary
aspects of chronic liver disease and liver transplantation. Mayo
Clin Proc. 60:407–418. 1985.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Ji FP, Li ZX, Deng H, Xue HA, Liu Y and Li
M: Diagnosis and management of interstitial pneumonitis associated
with interferon therapy for chronic hepatitis C. World J
Gastroenterol. 16:4394–4399. 2010.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Robin ED, Horn B, Goris ML, Theodore J,
Kessel AV, Mazoub J and Tilkian A: Detection, quantitation and
pathophysiology of lung ‘spiders’. Trans Assoc Am Physicians.
88:202–216. 1975.PubMed/NCBI
|
|
89
|
Henriksen JH, Bendtsen F and Møller S:
Acid-base disturbance in patients with cirrhosis: Relation to
hemodynamic dysfunction. Eur J Gastroenterol Hepatol. 27:920–927.
2015.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Jiménez JV, Carrillo-Pérez DL,
Rosado-Canto R, García-Juárez I, Torre A, Kershenobich D and
Carrillo-Maravilla E: Electrolyte and acid-base disturbances in
end-stage liver disease: A physiopathological approach. Dig Dis
Sci. 62:1855–1871. 2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Passino C, Giannoni A, Mannucci F,
Prontera C, Filipponi F, Carrai P, Emdin M and Catapano G: Abnormal
hyperventilation in patients with hepatic cirrhosis: Role of
enhanced chemosensitivity to carbon dioxide. Int J Cardiol.
154:22–26. 2012.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Scheiner B, Lindner G, Reiberger T,
Schneeweiss B, Trauner M, Zauner C and Funk GC: Acid-base disorders
in liver disease. J Hepatol. 67:1062–1073. 2017.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Clague JE, Carter J, Pearson MG and
Calverley PM: Effect of sustained inspiratory loading on
respiratory sensation and CO2 responsiveness in normal humans. Clin
Sci (Lond). 91:513–518. 1996.PubMed/NCBI View Article : Google Scholar
|
|
94
|
El-Gamal H, Khayat A, Shikora S and
Unterborn JN: Relationship of dyspnea to respiratory drive and
pulmonary function tests in obese patients before and after weight
loss. Chest. 128:3870–3874. 2005.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Huang CH, Martin AD and Davenport PW:
Effect of inspiratory muscle strength training on inspiratory motor
drive and RREP early peak components. J Appl Physiol (1985).
94:462–468. 2003.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Moreau R and Lebrec D: Endogenous factors
involved in the control of arterial tone in cirrhosis. J Hepatol.
22:370–376. 1995.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Angueira CE and Kadakia SC: Effects of
large-volume paracentesis on pulmonary function in patients with
tense cirrhotic ascites. Hepatology. 20:825–828. 1994.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Berkowitz KA, Butensky MS and Smith RL:
Pulmonary function changes after large volume paracentesis. Am J
Gastroenterol. 88:905–907. 1993.PubMed/NCBI
|
|
99
|
Chao Y, Wang SS, Lee SD, Shiao GM, Chang
HI and Chang SC: Effect of large-volume paracentesis on pulmonary
function in patients with cirrhosis and tense ascites. J Hepatol.
20:101–105. 1994.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Chang SC, Chang HI, Chen FJ, Shiao GM,
Wang SS and Lee SD: Therapeutic effects of diuretics and
paracentesis on lung function in patients with non-alcoholic
cirrhosis and tense ascites. J Hepatol. 26:833–838. 1997.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Geoum MS, Kim YT, Choi SG, Lee CH, Kweon
YO, Kim SK, Choi YH and Chung JM: The effect of paracentesis on
pulmonary function in patients with cirrhosis. Korean J Hepatol.
3:50–57. 1997.
|
|
102
|
Duranti R, Laffi G, Misuri G, Riccardi D,
Gorini M, Foschi M, Iandelli I, Mazzanti R, Mancini M, Scano G and
Gentilini P: Respiratory mechanics in patients with tense cirrhotic
ascites. Eur Respir J. 10:1622–1630. 1997.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Gupta D, Lalrothuama Agrawal PN, Aggarwal
AN, Dhiman RK, Behera D and Chawla Y: Pulmonary function changes
after large volume paracentesis. Trop Gastroenterol. 21:68–70.
2000.PubMed/NCBI
|
|
104
|
Limongi V, dos Santos DC, da Silva AM,
Ataide EC, Mei MF, Udo EY, Boin IF and Stucchi RS: Effects of a
respiratory physiotherapeutic program in liver transplantation
candidates. Transplant Proc. 46:1775–1777. 2014.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Ewert R, Mutze S, Schachschal G, Lochs H
and Plauth M: High prevalence of pulmonary diffusion abnormalities
without interstitial changes in long-term survivors of liver
transplantation. Transpl Int. 12:222–228. 1999.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Battaglia SE, Pretto JJ, Irving LB, Jones
RM and Angus PW: Resolution of gas exchange abnormalities and
intrapulmonary shunting following liver transplantation.
Hepatology. 25:1228–1232. 1997.PubMed/NCBI View Article : Google Scholar
|
|
107
|
European Association for the Study of the
Liver. Electronic address: simpleeasloffice@easloffice.eu.
EASL clinical practice guidelines: Liver transplantation. J
Hepatol. 64:433–485. 2016.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Lima BLG, França AVC, Pazin-Filho A,
Araújo WM, Martinez JAB, Maciel BC, Simões MV, Terra-Filho J and
Martinelli ALC: Frequency, clinical characteristics, and
respiratory parameters of hepatopulmonary syndrome. Mayo Clin Proc.
79:42–48. 2004.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Khiangte B, Kothakota SR, Sasidharan M,
Kareem H, Joshi S, Kumar VV, Kanala JR, Kumar CP and Nair AK:
Prevalence and determinants of hepatopulmonary syndrome in
decompensated chronic liver disease. Indian J Gastroenterol.
39:362–369. 2020.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Martínez GP, Barberà JA, Visa J, Rimola A,
Paré JC, Roca J, Navasa M, Rodés J and Rodriguez-Roisin R:
Hepatopulmonary syndrome in candidates for liver transplantation. J
Hepatol. 34:651–657. 2001.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Buggs J, LaGoy M, Ermekbaeva A, Rogers E,
Nyce S, Patiño D, Kumar A and Kemmer N: Cost utilization and the
use of pulmonary function tests in preoperative liver transplant
patients. Am Surg. 86:996–1000. 2020.PubMed/NCBI View Article : Google Scholar
|