Introduction
The International League Against Epilepsy (ILAE)
defines epilepsy as a disorder of the brain characterized by an
enduring predisposition to generate epileptic seizures (1). The worldwide incidence of epilepsy
among children aged 11-17 years is 21-24 per 100,000 cases.
Epilepsies are multifactorial disorders with a poorly understood
etiology that are considered to be caused by an array of genes and
environmental factors (2). Abnormal
brain development during prenatal life is considered to play a
crucial role in certain neurological disorders with a clinical
onset later in life. Neuroimaging technology has further emphasized
this point (3). Preterm delivery and
a low birth weight have been found to be associated with an
increased risk of developing cerebral palsy (4), cognitive delay (5) and behavioral disorder (6).
A growing body of evidence has revealed associations
between preterm birth and an increased risk of developing epilepsy
in early life (7). The link between
the risk of developing epilepsy and post-term birth, however, has
not yet been elucidated. Ehrenstein et al (8) demonstrated an increased risk of
developing epilepsy with prolonged gestation. However, this may be
attributed to instrument-assisted and cesarean deliveries that can
cause complications at birth (8).
A nationwide cohort in Finland revealed a decreased
risk of developing epilepsy with an increased gestational age, with
the cumulative incidence of epilepsy in the cohort being 0.54%
(9). From both a clinical and public
health perspective, it is important to identify such risk factors.
This identification prompts the question of whether these factors
could be modifiable through alterations in obstetric practices.
Birth weight, a factor often influenced by
gestational age, has been found to be lower in patients with
epilepsy compared to healthy controls (10). The effect of sex on the development
of childhood epilepsy has been controversial. While some studies
have found that males have a higher tendency to develop this
condition (11), others have found
no significant difference (12).
The correlation between maternal age and a number of
neuropsychiatric diseases has been studied. An extreme maternal age
range appears to be a risk factor (13,14). A
young maternal age, and specifically teenage motherhood, may
contribute to socioeconomic disadvantages and in numerous diseases,
including epilepsy, there appears to be a social gradient
suggesting that children born to parents of a lower socioeconomic
position face an elevated risk of developing the disease (15). In addition, an advanced maternal age
has been linked to increasing offspring disease risk due to higher
rates of genomic alterations and a higher risk of perinatal and
obstetric complications (16). The
present study aimed to investigate these variables in relation to
epilepsy. To the best of our knowledge, variables such as birth
weight, maternal age and sex have been negligibly explored in
previous studies (8,9).
Patients and methods
Patient information
A case-control study was conducted on 154 patients
with epilepsy and 299 controls. The study was approved by the
Ethics Committee of Iashvili Children's Central Hospital (a
university hospital of Tbilisi State Medical University), Tbilisi,
Georgia (approval no. N 22-0929-1311a). Signed informed consent was
collected from the parents/legal guardians of each study
participant. The inclusion criteria were an age range of 1-18 years
and the diagnosis of epilepsy. All patients were recruited from two
hospitals (Iashvili Children's Central Hospital and Medison Clinic)
in Tbilisi, Georgia. The controls were children in the same age
range, who were hospitalized without having been diagnosed with
epilepsy. The exclusion criteria included children who had severe
chronic or multisystemic health conditions, severe developmental
delays, or any other neurological conditions. Information such as
gestational age, birth weight, maternal age and sex were collected
from patients' medical records. Patient seizures were classified
based on ILAE 2017 as generalized, focal and unspecified onset
seizures (17).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 9.3.1 for macOS (Dotmatics). Data were analyzed
using the Chi-Squared and Fisher's exact tests, and odds ratios
(ORs) and 95% confidence intervals (CIs) were calculated.. All
statistical tests were planned to be two-sided. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
To examine the association between gestational age
and the risk of developing epilepsy, the participants were
categorized into the pre-term (<37 weeks of gestation), term
(37-41 weeks) and post-term groups (>41 weeks). There was a
statistically significant difference between the gestational ages
of the cases and controls (Chi-square test value, 11.84; P=0.0027).
Compared with children whose gestational age at birth was 37-41
weeks, children with a gestational age <37 weeks had an almost
2-fold greater risk of having epilepsy (OR, 2.3; 95% CI, 1.4-3.7).
Post-term birth was not associated with an increased risk of
developing epilepsy compared to the term children (Table I).
 | Table IRisk of developing epilepsy according
to gestational age, sex, maternal age and birth weight. |
Table I
Risk of developing epilepsy according
to gestational age, sex, maternal age and birth weight.
| Parameter | Cases, n (%) | Controls, n (%) | OR (95% CI) | OR P-value | P-valuea |
|---|
| Gestational age | | | | | |
|
Pre-term | 38 (24.6%) | 38 (12.7%) | 2.3 (1.4-3.7) | 0.0011b | |
|
Term | 110 (71.4%) | 254 (84.9%) | 1.00 Ref | | |
|
Post-term | 6 (3.9%) | 7 (2.3%) | 1.9 (0.6-5.4) | 0.2293 | 0.0027b (χ2=11.84) |
| Sex | | | | | |
|
Male | 74 (48.0%) | 178 (59.5%) | 0.6 (0.4-0.9) | 0.0202b | |
|
Female | 80 (51.9%) | 121 (40.4%) | 1.00 Ref | | 0.0218b |
| Maternal age,
years | | | | | |
|
>35 | 20 (41.6%) | 114 (40.8%) | 1.0 (0.5-1.8) | 0.9164 | |
|
<35 | 28 (58.3%) | 165 (59.1) | 1.00 Ref | | >0.9999 |
| Birth weight, kg | | | | | |
|
<2.5 | 23 (15.2%) | 24 (8.1%) | 2.0 (1.1-3.6) | 0.0243b | |
|
>2.5 | 128 (84.7%) | 269 (91.8%) | 1.00 Ref | | 0.0332b |
The male sex was found to be associated with a lower
risk of developing epilepsy compared to the female sex (OR, 0.6;
95% CI, 0.4-0.9). However, a maternal age >35 years was not
found to be a significant risk factor (OR, 1.0; 95% CI, 0.5-1.8)
(Table I).
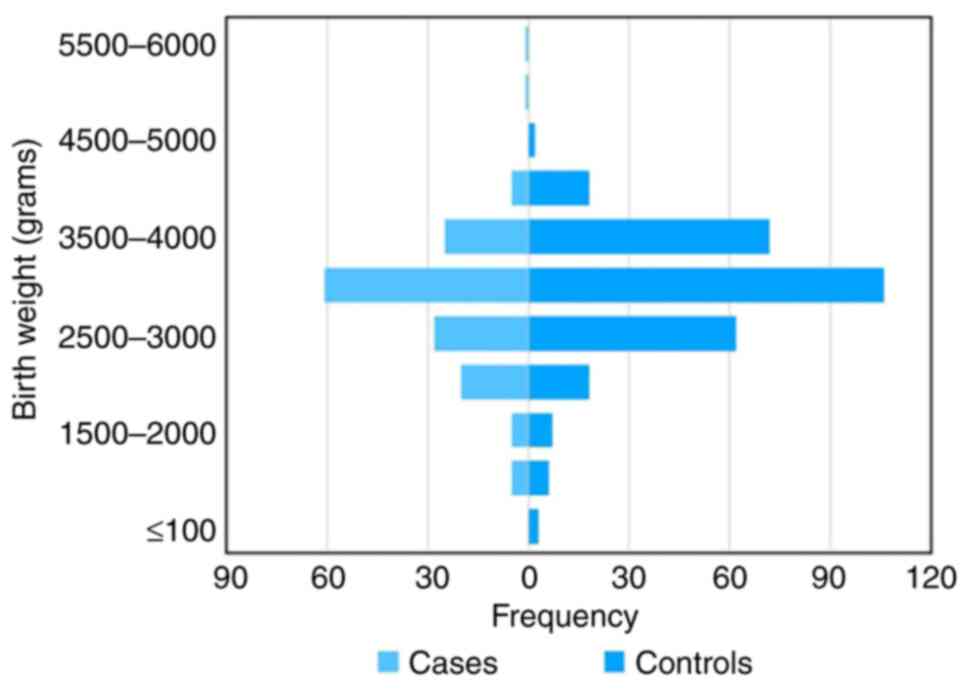
The distribution of birthweight in the study
population is presented in Fig. 1.
Compared with the children whose birth weight was >2,500 g,
those whose birth weight was <2,500 g had a 2-fold greater risk
of developing epilepsy (OR, 2; 95% CI, 1.1-3.6). The multiple risk
factors that were studied in association with epilepsy are
presented in Table I.
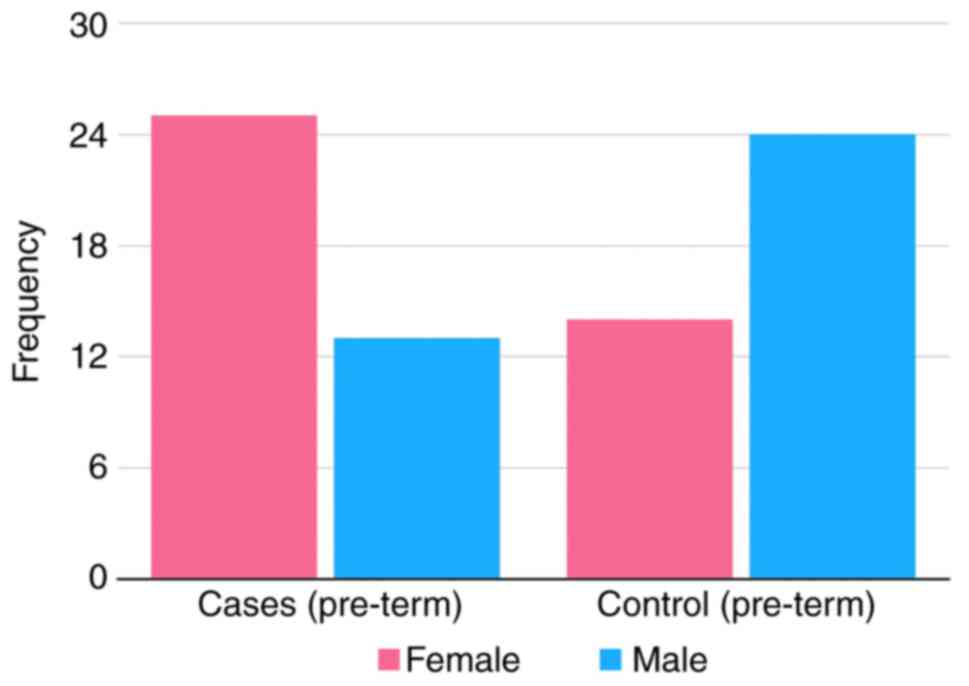
The proportion of male and female patients in the
pre-term epilepsy cases compared to the proportion of male and
female patients in the pre-term controls was also investigated
(Fig. 2). There was a statistically
significant association (P=0.0211) between the female sex and the
risk of developing epilepsy only in pre-term children (OR, 3.2; 95%
CI, 1.2-8.8). The risk of a pre-term female child being diagnosed
with epilepsy was ~3.2-fold that of a pre-term male child being
diagnosed with epilepsy (Table
II).
 | Table IIRisk of epilepsy according to sex in
pre-term cases and controls. |
Table II
Risk of epilepsy according to sex in
pre-term cases and controls.
| Sex | Pre-term cases, n
(%) | Pre-term controls, n
(%) | OR (95% CI) | P-value |
|---|
| Female | 25 (65.7%) | 14 (36.8%) | 3.2 (1.2-8.8) | 0.0211a |
| Male | 13 (34.2%) | 24 (63.1%) | 1.00 Ref | |
In addition, the association between the type of
seizures and the study variables, including gestational age, sex,
maternal age and birth weight was examined (Table III). It was found that there was a
statistically significant difference between the type of seizure
and gestational age; however, lager studies with higher sample
numbers are required for a definitive conclusion.
 | Table IIIAssociation between the type of
seizure and gestational age, sex, maternal age and birth
weight. |
Table III
Association between the type of
seizure and gestational age, sex, maternal age and birth
weight.
| Parameter | Generalized onset
seizure, n (%) | Focal onset seizure,
n (%) | Unspecified onset
seizure, n (%) | P
valuea |
|---|
| Gestational
age | | | |
<0.0001b |
|
Pre-term | 25 (80.6%) | 22 (27.5%) | 10 (27.0%) | |
|
Term | 6 (19.3%) | 58 (72.5%) | 27 (72.9%) | |
| Sex | | | | 0.1 |
|
Male | 15 (46.8%) | 46 (54.7%) | 13 (34.2%) | |
|
Female | 17 (53.1%) | 38 (45.2%) | 25 (65.8%) | |
| Maternal age,
years | | | | 0.5 |
|
>35 | 4 (33.3%) | 5 (55.5%) | 12 (44.4%) | |
|
<35 | 8 (66.6%) | 4 (44.4%) | 15 (55.5%) | |
| Birth weight,
kg | | | | 0.6 |
|
<2.5 | 5 (15.6%) | 14 (16.6%) | 4 (10.5%) | |
|
>2.5 | 27 (84.3%) | 70 (83.3%) | 34 (89.4%) | |
Discussion
The present study identified an increased risk of
epilepsy in children who were born prematurely, before 37 weeks of
gestation, compared to children born full term. Arpino et al
(18) conducted a study on neonatal
seizures in the first week of life and found that neonatal seizures
were strongly associated with a low gestational age and a low birth
weight, two related factors that can predispose neonates to
seizures. They found that the key etiology of neonatal seizures was
hypoxic-ischemic encephalopathy (30%), which can be mediated by a
low birth weight and gestational age (18). In the present study, the risk of
developing epilepsy in pre-term births was >2-fold higher (OR,
2.3) than that in term births. Brain volume markedly increases
during the last trimester, with a 4-fold increase in gray matter
and a 5-fold increase in white matter (19). Premature birth can increase the risk
of developing epilepsy, as the last trimester is a critical time
for the brain development of the fetus (20). In addition, premature birth is often
accompanied by other risk factors during pregnancy, such as
infection and preeclampsia (21,22).
Prenatal stress can affect the fetus via multiple mechanisms in the
long term. In addition to increasing preterm birth and low birth
weight, prenatal stress can affect the central nervous system of
the fetus by stress hormones released from the body of the pregnant
mother. Neurotransmitters in the brain can be disturbed by being
exposed to stress hormones, such as glucocorticoids and
corticotrophin-releasing hormones. There is supporting literature
on the general association between stress in the early stages of
life and seizures (23). Considering
the adverse effects of preterm gestational age and prenatal stress
on the chances of developing epilepsy, the multidisciplinary
management of pregnant women needs to be implemented in order to
reduce preterm birth and stress. Ehrenstein et al
demonstrated that prolonged gestation may be a risk factor for
epilepsy (8); however, in the
present study, this could not be confirmed with the data
obtained.
Another variable in the present study was birth
weight, specifically a birth weight <2,500 g. After analyzing
the data, it was observed that patients born with a birth weight
<2,500 g were 2-fold more likely to develop epilepsy. This may
be due to the fact that the premature brain is more susceptible to
seizures (7). Febrile seizures are
also more common in preterm children and those with a low
birthweight (24).
In the present study, there was a significant
associated risk between developing epilepsy and the female sex.
According to the results obtained, the risk of a preterm female
child being diagnosed with epilepsy was ~3.3-fold greater than that
of a preterm male child being diagnosed with epilepsy. Following
the analysis of all the subgroups together, females were found to
be more likely to develop epilepsy. Epidemiological research has
suggested that boys are more likely than girls to develop febrile
seizures, and are 1.5-2-fold more likely to develop multifocal
epileptic syndromes (25). However,
idiopathic generalized epilepsy is more frequent among females. The
reason behind these differences is not fully understood; however,
it may be related to sex hormones, as they directly influence the
development of the mammalian brain and modulate brain activity
(11,12). Since sex remains controversial in
association with the development of epilepsy, further, more
extensive and diverse research in a similar vein is warranted to
elucidate this matter. An advanced maternal and paternal age has
been recognized to affect the risk of developing a number of
congenital diseases, including neuropsychological disorders
(26). For example, a significantly
increased risk of developing psychosis has been found in the
offspring of mothers at an advanced age (27). However, no significant difference was
found in the risk of developing epilepsy between the offspring of
mothers >35 years and <35 years of age in the population in
the present study.
The present study has certain limitations which
should be mentioned. The study was limited by a lack of detailed
clinical data on the types of epilepsy, limitation of size and
follow-up. There are more factors to be considered as variables in
future research, such as the form of delivery (natural vs. cesarean
sections) or complicated birth (with or without the assistance of
instruments).
In conclusion, the findings of the present study
demonstrate that gestational age at birth and birth weight are
associated with a subsequent risk of developing epilepsy.
Environmental factors during fetal development or a shorter
gestation period could potentially contribute to the developing of
epilepsy, particularly in young children.
Acknowledgements
The authors would like to express their gratitude to
Dr Nona Janikashvili and Dr Nino Shiukashvili (both from Tbilisi
State Medical University) for their administrative support as
module coordinators.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NO conceptualized the study. NO, SA, LI, AB and TG
were involved in the methodology, collection and analysis of the
data, and wrote the draft of the manuscript. All authors
contributed to manuscript revisions and have read and approved the
final version of the manuscript. NO and SA confirm the authenticity
of all of the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Iashvili Children's Central Hospital (a university
hospital of Tbilisi State Medical University), Georgia under
(approval no. N 22-0929-1311a) with the following members: Chair
Levan Kharashvili, Marina Dzidziguri, Tamar Balanchivadze, Mariam
Tsilosani, Sophio Nikoladze. Signed informed consent was obtained
from the parents/legal guardians of each study participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fisher RS, Acevedo C, Arzimanoglou A,
Bogacz A, Cross JH, Elger CE, Engel J Jr, Forsgren L, French JA,
Glynn M, et al: ILAE official report: A practical clinical
definition of epilepsy. Epilepsia. 55:475–482. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marini C, Scheffer IE, Crossland KM,
Grinton BE, Phillips FL, McMahon JM, Turner SJ, Dean JT, Kivity S,
Mazarib A, et al: Genetic architecture of idiopathic generalized
epilepsy: Clinical genetic analysis of 55 multiplex families.
Epilepsia. 45:467–478. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nelson KB and Ellenberg JH: Antecedents of
cerebral palsy. Multivariate analysis of risk. N Engl J Med.
315:81–86. 1986.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ellenberg JH and Nelson KB: Birth weight
and gestational age in children with cerebral palsy or seizure
disorders. Am J Dis Child. 133:1044–1048. 1979.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Woodward LJ, Anderson PJ, Austin NC,
Howard K and Inder TE: Neonatal MRI to predict neurodevelopmental
outcomes in preterm infants. N Engl J Med. 355:685–694.
2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Linnet KM, Wisborg K, Agerbo E, Secher NJ,
Thomsen PH and Henriksen TB: Gestational age, birth weight, and the
risk of hyperkinetic disorder. Arch Dis Child. 91:655–660.
2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sun Y, Vestergaard M, Pedersen CB,
Christensen J, Basso O and Olsen J: Gestational age, birth weight,
intrauterine growth, and the risk of epilepsy. Am J Epidemiol.
167:262–270. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ehrenstein V, Pedersen L, Holsteen V,
Larsen H, Rothman KJ and Sørensen HT: Postterm delivery and risk
for epilepsy in childhood. Pediatrics. 119:e554–e561.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hirvonen M, Ojala R, Korhonen P, Haataja
P, Eriksson K, Gissler M, Luukkaala T and Tammela O: The incidence
and risk factors of epilepsy in children born preterm: A nationwide
register study. Epilepsy Res. 138:32–38. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jackson DC, Lin JJ, Chambers KL,
Kessler-Jones A, Jones JE, Hsu DA, Stafstrom CE, Seidenberg M and
Hermann BP: Birth weight and cognition in children with epilepsy.
Epilepsia. 55:901–908. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
McHugh JC and Delanty N: Chapter 2
epidemiology and classification of epilepsy: Gender comparisons.
Int Rev Neurobiol. 83:11–26. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Christensen J, Kjeldsen MJ, Andersen H,
Friis ML and Sidenius P: Gender differences in epilepsy. Epilepsia.
46:956–960. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
McGrath JJ, Petersen L, Agerbo E, Mors O,
Mortensen PB and Pedersen CB: A comprehensive assessment of
parental age and psychiatric disorders. JAMA Psychiatry.
71:301–309. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sandin S, Hultman CM, Kolevzon A, Gross R,
MacCabe JH and Reichenberg A: Advancing maternal age is associated
with increasing risk for autism: A review and meta-analysis. J Am
Acad Child Adolesc Psychiatry. 51:477–486.e1. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Heaney DC, MacDonald BK, Everitt A,
Stevenson S, Leonardi GS, Wilkinson P and Sander JW: Socioeconomic
variation in incidence of epilepsy: Prospective community based
study in south east England. BMJ. 325:1013–1016. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Idring S, Magnusson C, Lundberg M, Ek M,
Rai D, Svensson AC, Dalman C, Karlsson H and Lee BK: Parental age
and the risk of autism spectrum disorders: Findings from a Swedish
population-based cohort. Int J Epidemiol. 43:107–115.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fisher RS, Cross JH, French JA, Higurashi
N, Hirsch E, Jansen FE, Lagae L, Moshé SL, Peltola J, Roulet Perez
E, et al: Operational classification of seizure types by the
international league against epilepsy: Position paper of the ILAE
commission for classification and terminology. Epilepsia.
58:522–530. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Arpino C, Domizio S, Carrieri MP,
Brescianini DS, Sabatino MG and Curatolo P: Prenatal and perinatal
determinants of neonatal seizures occurring in the first week of
life. J Child Neurol. 16:651–656. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hüppi PS, Warfield S, Kikinis R, Barnes
PD, Zientara GP, Jolesz FA, Tsuji MK and Volpe JJ: Quantitative
magnetic resonance imaging of brain development in premature and
mature newborns. Ann Neurol. 43:224–235. 1998.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Carrasco M and Stafstrom CE: How early can
a seizure happen? Pathophysiological considerations of extremely
premature infant brain development. Dev Neurosci. 40:417–436.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Goldenberg RL, Hauth JC and Andrews WW:
Intrauterine infection and preterm delivery. N Eng J Med.
342:1500–1507. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Basso O, Rasmussen S, Weinberg CR, Wilcox
AJ, Irgens LM and Skjaerven R: Trends in fetal and infant survival
following preeclampsia. JAMA. 296:1357–1362. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Saboory E, Mohammadi S, Dindarian S and
Mohammadi H: Prenatal stress and elevated seizure susceptibility:
Molecular inheritable changes. Epilepsy Behav. 96:122–131.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Vestergaard M, Basso O, Henriksen TB,
Østergaard JR and Olsen J: Risk factors for febrile convulsions.
Epidemiology. 13:282–287. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Velís̆ková J, Claudio OI, Galanopoulou AS,
Lado FA, Ravizza T, Velís̆ek L and Moshé SL: Seizures in the
developing brain. Epilepsia. 45:6–12. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tarín JJ, Brines J and Cano A: Long-term
effects of delayed parenthood. Hum Reprod. 13:2371–2376.
1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
El-Saadi O, Pedersen CB, McNeil TF, Saha
S, Welham J, O'Callaghan E, Cantor-Graae E, Chant D, Mortensen PB
and McGrath J: Paternal and maternal age as risk factors for
psychosis: Findings from Denmark, Sweden and Australia. Schizophr
Res. 67:227–236. 2004.PubMed/NCBI View Article : Google Scholar
|