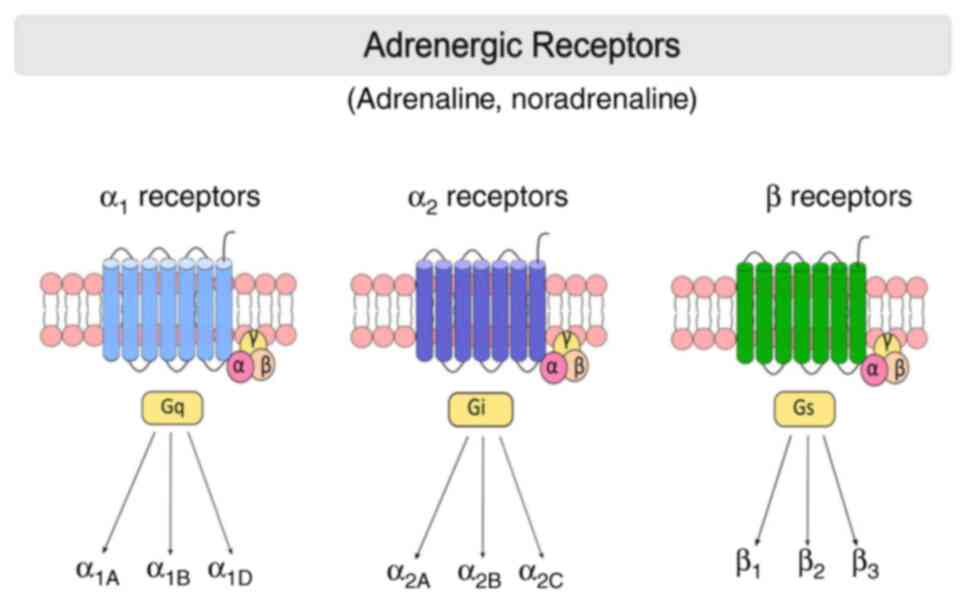
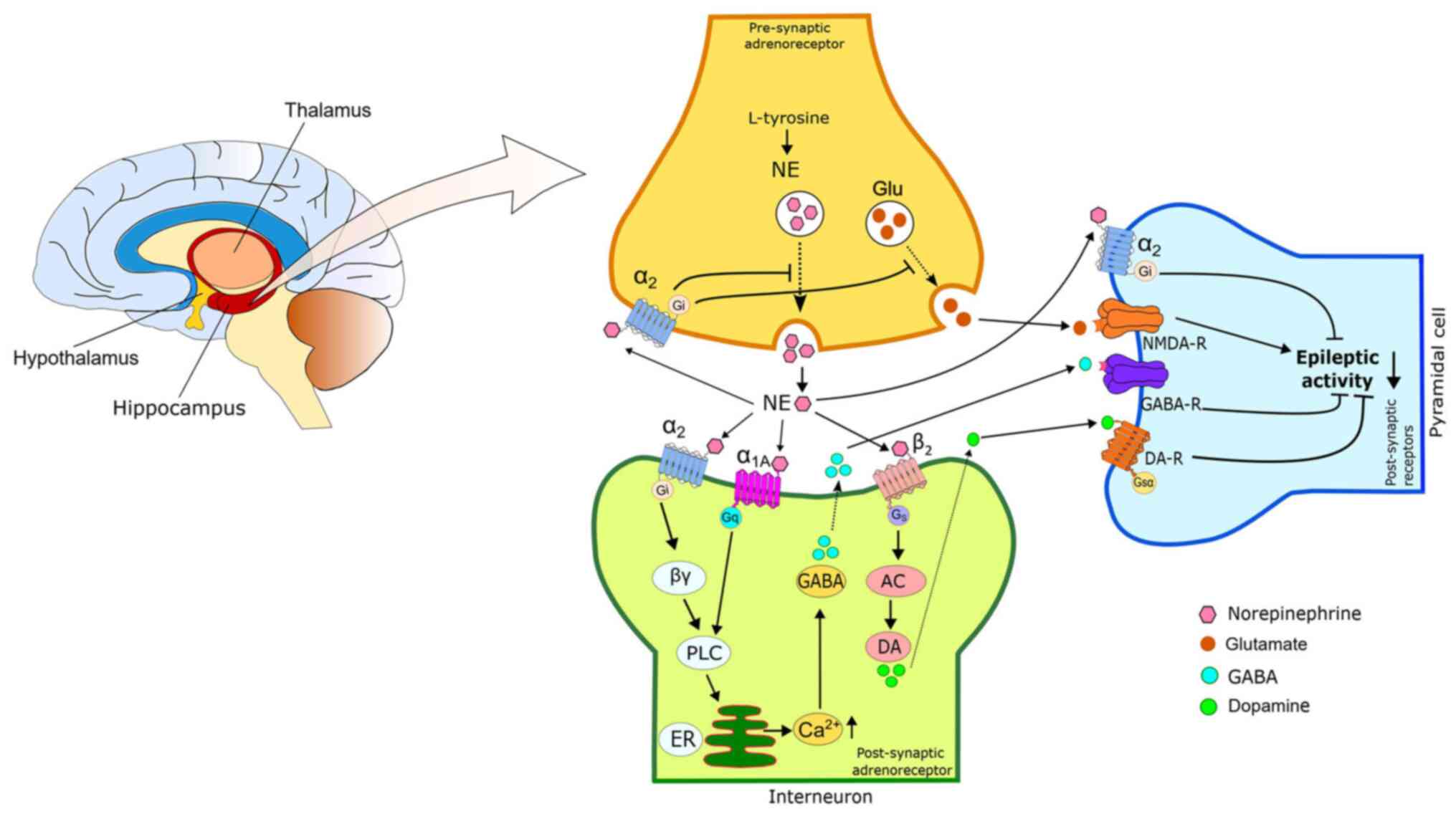
|
1
|
Fisher RS, van Emde Boas W, Blume W, Elger
C, Genton P, Lee P and Engel J Jr: Epileptic seizures and epilepsy:
Definitions proposed by the international league against epilepsy
(ILAE) and the international bureau for epilepsy (IBE). Epilepsia.
46:470–472. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
McHugh JC and Delanty N: Epidemiology and
classification of epilepsy: Gender comparisons. Int Rev Neurobiol.
83:11–26. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Weltha L, Reemmer J and Boison D: The role
of adenosine in epilepsy. Brain Res Bull. 151:46–54.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Berkovic SF and Scheffer IE: Febrile
seizures: Genetics and relationship to other epilepsy syndromes.
Curr Opin Neurol. 11:129–134. 1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bence AK, Worthen DR, Stables JP and
Crooks PA: An in vivo evaluation of the antiseizure activity and
acute neurotoxicity of agmatine. Pharmacol Biochem Behav.
74:771–775. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
DiNuzzo M, Mangia S, Maraviglia B and
Giove F: Physiological bases of the K+ and the glutamate/GABA
hypotheses of epilepsy. Epilepsy Res. 108:995–1012. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sahin B, Ozdemir E, Gumus E, Ergul M and
Taskiran AS: The 5-HT7 receptor antagonist SB-269970 alleviates
seizure activity and downregulates hippocampal c-Fos expression in
pentylenetetrazole-induced kindled rats. Neurol Res. 44:786–796.
2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Akyuz E, Doganyigit Z, Paudel YN, Koklu B,
Kaymak E, Villa C, Arulsamy A, Shaikh MF and Devinsky O:
Immunoreactivity of muscarinic acetylcholine M2 and serotonin
5-HT2B receptors, norepinephrine transporter and Kir channels in a
model of epilepsy. Life (Basel). 11(276)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen C, Zhu T, Gong L, Hu Z, Wei H, Fan J,
Lin D, Wang X, Xu J, Dong X, et al: Trpm2 deficiency in microglia
attenuates neuroinflammation during epileptogenesis by upregulating
autophagy via the AMPK/mTOR pathway. Neurobiol Dis.
186(106273)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kuang X, Chen S and Ye Q: The role of
histone deacetylases in NLRP3 inflammasomes-mediated epilepsy. Curr
Mol Med 2023: doi: 10.2174/1566524023666230731095431, 2023.
|
|
11
|
Rana A and Musto AE: The role of
inflammation in the development of epilepsy. J Neuroinflammation.
15(144)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gunes H, Ozdemir E and Arslan G: Coenzyme
Q10 increases absence seizures in WAG/Rij rats: The role of the
nitric oxide pathway. Epilepsy Res. 154:69–73. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Taskıran AS, Ozdemir E, Gumus E and Ergul
M: The effects of salmon calcitonin on epileptic seizures,
epileptogenesis, and postseizure hippocampal neuronal damage in
pentylenetetrazole-induced epilepsy model in rats. Epilepsy Behav.
13(107501)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Strac DS, Pivac N, Smolders IJ, Fogel WA,
Deurwaerdere PD and Giovanni GD: Monoaminergic mechanisms in
epilepsy may offer innovative therapeutic opportunity for
monoaminergic multi-target drugs. Front Neurosci.
10(492)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Giorgi FS, Pizzanelli C, Biagioni F, Murri
L and Fornai F: The role of norepinephrine in epilepsy: From the
bench to the bedside. Neurosci Biobehav Rev. 28:507–524.
2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Foote SL and Berridge CW: New developments
and future directions in understanding locus
coeruleus-Norepinephrine (LC-NE) function. Brain Res. 1709:81–84.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Amaral-Silva L and Santin JM: Molecular
profiling of CO2/pH-sensitive neurons in the locus coeruleus of
bullfrogs reveals overlapping noradrenergic and glutamatergic cell
identity. Comp Biochem Physiol A Mol Integr Physiol.
283(111453)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Clough RW, Browning RA, Maring ML,
Statnick MA, Wang C and Jobe PC: Effects of intraventricular locus
coeruleus transplants on seizure severity in genetically
epilepsy-prone rats following depletion of brain norepinephrine. J
Neural Transplant Plast. 5:65–79. 1994.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Larsen LE, Caestecker S, Stevens L, van
Mierlo P, Carrette E, Boon P, Vonck K and Raedt R: Hippocampal
seizures differentially modulate locus coeruleus activity and
result in consistent time-locked release of noradrenaline in rat
hippocampus. Neurobiol Dis. 189(106355)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Brawek B, Löffler M, Dooley DJ, Weyerbrock
A and Feuerstein TJ: Differential modulation of K(+)-evoked
(3)H-neurotransmitter release from human neocortex by gabapentin
and pregabalin. Naunyn Schmiedebergs Arch Pharmacol. 376:301–307.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Choi TY, Kwon JE, Durrance ES, Jo SH, Choi
SY and Kim KT: Melatonin inhibits voltage-sensitive Ca(2+)
channel-mediated neurotransmitter release. Brain Res. 4:34–42.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Briere R, Sherwin AL, Robitaille Y,
Olivier A, Quesney LF and Reader TA: Alpha-1 adrenoceptors are
decreased in human epileptic foci. Ann Neurol. 19:26–30.
1986.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nicoletti F, Barbaccia ML, Iadarola MJ,
Pozzi O and Laird HE II: Abnormality ofalpha 1-adrenergic receptors
in the frontal cortex of epileptic rats. J Neurochem. 46:270–273.
1986.PubMed/NCBI View Article : Google Scholar
|
|
24
|
McIntyre DC and Edson N: Effect of
norepinephrine depletion on dorsal hippocampus kindling in rats.
Exp Neuron. 77:700–704. 1982.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kokaia M, Bengzon J, Kalen P and Lindvall
O: Noradrenergic mechanisms in hippocampal kindling with rapidly
recurring seizures. Brain Res. 491:398–402. 1989.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dailey JW and Naritoku DK: Antidepressants
and seizures: Clinical anecdotes overshadow neuroscience. Biochem
Pharmacol. 52:1323–1329. 1996.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fitzgerald PJ: Is elevated norepinephrine
an etiological factor in some cases of epilepsy? Seizure.
19:311–318. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen J, Liang H, Miao M, Su X, Yang F,
Thomsen RW, Yuan W and Li J: In utero beta-2-adrenergic agonists
exposure and risk of epilepsy: A Danish nationwide population-based
cohort study. Pharmacoepidemiol Drug Saf. 27:1200–1208.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Felippotti TT, dos Reis Ferreira CM, de
Freitas RL, de Oliveira RC, de Oliveira R, Paschoalin-Maurin T and
Coimbra NC: Paradoxical effect of noradrenaline-mediated
neurotransmission in the antinociceptive phenomenon that
accompanies tonic-clonic seizures: role of locus coeruleus neurons
and α(2)- and β-noradrenergic receptors. Epilepsy Behav. 22:165–77.
2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hillman KL, Lei S, Doze VA and Porter JE:
Alpha-1A adrenergic receptor activation increases inhibitory tone
in CA1 hippocampus. Epilepsy Res. 84:97–109. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pizzanelli C, Lazzeri G, Fulceri F, Giorgi
FS, Pasquali L, Cifelli G, Murri L and Fornai F: Lack of alpha
1b-adrenergic receptor protects against epileptic seizures.
Epilepsia. 50 (Suppl 1):S59–S64. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shafaroodi H, Moezi L, Bahremand A and
Dehpour AR: The role of α2-adrenoceptors in the anti-convulsant
effects of cannabinoids on pentylenetetrazole-induced seizure
threshold in mice. Eur J Pharmacol. 714:1–6. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shouse MN, Scordato JC, Farber PR and de
Lanerolle N: The alpha2 adrenoreceptor agonist clonidine suppresses
evoked and spontaneous seizures, whereas the alpha2 adrenoreceptor
antagonist idazoxan promotes seizures in amygdala-kindled kittens.
Brain Res. 1137:58–68. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fletcher A and Forster EA: A proconvulsant
action of selective alpha 2-adrenoceptor antagonists. Eur J
Pharmacol. 151:27–34. 1988.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Payandemehr B, Bahremand A, Ebrahimi A,
Nasrabady SE, Rahimian R, Bahremand T, Sharifzadeh M and Dehpour
AR: Protective effects of lithium chloride on seizure
susceptibility: Involvement of α2-adrenoceptor. Pharmacol Biochem
Behav. 133:37–42. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Moezi L, Mansoori E, Niknahad H and
Shafaroodi H: The role of alpha-2 adrenoceptors in the
anticonvulsant effects of adenosine on pentylenetetrazole-induced
seizure threshold in mice. Pharmacol Biochem Behav. 126:36–42.
2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Abraham PA, Xing G, Zhang L, Yu EZ, Post
R, Gamble EH and Li H: beta1- and beta2-adrenoceptor induced
synaptic facilitation in rat basolateral amygdala. Brain Res.
1209:65–73. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
McIntyre DC and Roberts DCS: Long-term
reduction in beta-adrenergic receptor binding after amygdala
kindling in rats. Exp Neurol. 82:17–24. 1983.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Philipp M, Brede M and Hein L:
Physiological significance of alpha(2)-adrenergic receptor subtype
diversity: One receptor is not enough. Am J Physiol Regul Integr
Comp Physiol. 283:R287–R295. 2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wu Y, Zeng L and Zhao S: Ligands of
adrenergic receptors: A structural point of view. Biomolecules.
11(936)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Perez DM: α1-Adrenergic receptors in
neurotransmission, synaptic plasticity, and cognition. Front
Pharmacol. 11(581098)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cavalli A, Lattion AL, Hummler E, Nenniger
M, Pedrazzini T, Aubert JF, Michel MC, Yang M, Lembo G, Vecchione
C, et al: Decreased blood pressure response in mice deficient of
the alpha1b adrenergic receptor. Proc Natl Acad Sci USA.
94:11589–11594. 1997.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Graham RM, Perez DM, Hwa J and Piascik MT:
alpha1-adrenergic receptor subtypes: Molecular structure, function,
and signaling. Circ Res. 78:737–749. 1996.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Perez DM and Doze VA: Cardiac and
neuroprotection regulated by α(1)-adrenergic receptor Subtypes. J
Recept Signal Transduct Res. 31:98–110. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Papay R, Gaivin R, Jha A, McCune DF,
McGrath JC, Rodrigo MC, Simpson PC, Doze VA and Perez DM:
Localization of the mouse alpha1A-adrenergic receptor (AR) in the
brain: Alpha1aar is expressed in neurons, GABAergic interneurons,
and NG2 oligodendrocyte progenitors. J Comp Neurol. 497:209–222.
2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gupta MK, Papay RS, Jurgens CW, Gaivin RJ,
Shi T, Doze VA and Perez DM: Alpha1-Adrenergic receptors regulate
neurogenesis and gliogenesis. Mol Pharmacol. 76:314–326.
2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Trendelenburg AU, Sutej I, Wahl CA,
Molderings GJ, Rump LC and Starke K: A re-investigation of
questionable subclassifications of presynaptic α2-autoreceptors:
Rat vena cava, rat atria, human kidney and guinea-pig urethra.
Naunyn Schmiedebergs Arch Pharmacol. 356:721–737. 1997.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Rump CL, Bohmann C, Schaible U, Schöllhorn
J and Limberger N: Alpha 2C-adrenoceptor-modulated release of
noradrenaline in human right atrium. Br J Pharmacol. 116:2617–2624.
1995.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Brodde O: Beta-1 and beta-2 adrenoceptor
polymorphisms: Functional importance, impact on cardiovascular
diseases and drug responses. Pharmacol Ther. 117:1–29.
2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Leineweber K and Heusch G: Beta 1- and
beta 2-adrenoceptor polymorphisms and cardiovascular diseases. Br J
Pharmacol. 158:61–69. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kume H, Nishiyama O, Isoya T, Higashimoto
Y, Tohda Y and Noda Y: Involvement of allosteric effect and
KCa channels in crosstalk between
β2-adrenergic and muscarinic M2 receptors in
airway smooth muscle. Int J Mol Sci. 19(1999)2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sawa M and Harada H: Recent developments
in the design of orally bioavailable beta3-adrenergic receptor
agonists. Curr Med Chem. 13:25–37. 2006.PubMed/NCBI
|
|
53
|
Ferrer-Lorente R, Cabot C, Fernández-López
JA and Alemany M: Combined effects of oleoyl-estrone and a
β3-adrenergic agonist (CL316,243) on lipid stores of diet-induced
overweight male Wistar rats. Life Sci. 77:2051–2058.
2005.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Gundlach AL, Burazin TC, Jenkins TA and
Berkovic SF: Spatiotemporal alterations of central alpha
1-adrenergic receptor binding sites following amygdaloid kindling
seizures in the rat: Autoradiographic studies using (3H)prazosin.
Brain Res. 672:214–227. 1995.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Jazrawi SP and Horton RW: Brain
adrenoceptor binding sites in mice susceptible (DBA/2J) and
resistant (C57 Bl/6) to audiogenic seizures. J Neurochem.
47:173–177. 1986.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kulik A, Haentzsch A, Lückermann M,
Reichelt W and Ballanyi K: Neuron-glia signaling via alpha(1)
adrenoceptor-mediated Ca(2+) release in Bergmann glialcells in
situ. J Neurosci. 19:8401–8408. 1999.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Terakado M: Adrenergic regulation of GABA
release from presynaptic terminals in rat cerebral cortex. J Oral
Sci. 56:49–57. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Rutecki PA: Noradrenergic modulation of
epileptiform activity in the hippocampus. Epilepsy Res. 20:125–136.
1995.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Jurgens CWD, Knudson CA, Carr PA, Perez DM
and Doze VA: a1 Adrenergic receptor regulation of interneuron
function. FASEB J. 23 (Suppl 946)(4)2009.
|
|
60
|
Knudson CA, Carr PA, Perez DM and Doze VA:
Alpha-1A adrenergic receptor overexpression protects hippocampal
interneurons. FASEB J. 21(A1209)2007.
|
|
61
|
Zuscik MJ, Sands S, Ross SA, Waugh DJ,
Gaivin RJ, Morilak D and Perez DM: Overexpression of the
alpha1B-adrenergic receptor causes apoptotic neurodegeneration:
Multiple system atrophy. Nat Med. 6:1388–1394. 2000.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kruse SW, Dayton KG, Purnell BS, Rosner JI
and Buchanan GF: Effect of monoamine reuptake inhibition and α1
blockade on respiratory arrest and death following
electroshock-induced seizures in mice. Epilepsia. 60:495–507.
2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kunieda T, Zuscik MJ, Boongird A, Perez
DM, Lüders HO and Najm IM: Systemic overexpression of the alpha
1B-adrenergic receptor in mice: An animal model of epilepsy.
Epilepsia. 43:1324–1329. 2002.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Chen CR, Qu WM, Qiu MH, Xu XH, Yao MH,
Urade Y and Huang ZL: Modafinil exerts a dose-dependent
antiepileptic effect mediated by adrenergic alpha1 and
histaminergic H1 receptors in mice. Neuropharmacology. 53:534–541.
2007.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Niitani K, Ito S, Wada S, Izumi S,
Nishitani N, Deyama S and Kaneda K: Noradrenergic stimulation of α1
adrenoceptors in the medial prefrontal cortex mediates acute
stress-induced facilitation of seizures in mice. Sci Rep.
19(8089)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Ciltas AC, Ozdemir E, Gumus E, Taskiran
AS, Gunes H and Arslan G: The anticonvulsant effects of alpha-2
adrenoceptor agonist dexmedetomidine on pentylenetetrazole-induced
seizures in rats. Neurochem Res. 47:305–314. 2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Nissinen J, Andrade P, Natunen T, Hiltunen
M, Malm T, Kanninen K, Soares JI, Shatillo O, Sallinen J,
Ndode-Ekane XE and Pitkänen A: Disease-modifying effect of
atipamezole in a model of post-traumatic epilepsy. Epilepsy Res.
136:18–34. 2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Jurgens CW, Hammad HM, Lichter JA, Boese
SJ, Nelson BW, Goldenstein BL, Davis KL, Xu K, Hillman KL, Porter
JE and Doze VA: Alpha2A adrenergic receptor activation inhibits
epileptiform activity in the rat hippocampal CA3 region. Mol
Pharmacol. 71:1572–1581. 2007.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Szot P, Lester M, Laughlin ML, Palmiter
RD, Liles LC and Weinshenker D: The anticonvulsant and
proconvulsant effects of alpha2-adrenoreceptor agonists are
mediated by distinct populations of alpha2A-adrenoreceptors.
Neuroscience. 126:795–803. 2004.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Yavuz M, Aydın B, Çarçak N, Akman Ö,
Yananlı HR and Onat F: Atipamezole, a specific α2A antagonist,
suppresses spike-and-wave discharges and alters
Ca2+/calmodulin-dependent protein kinase II in the
thalamus of genetic absence epilepsy rats. Epilepsia. 61:2825–2835.
2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Ferraro L, Tanganelli S, Calo G, Antonelli
T, Fabrizi A, Acciarri N, Bianchi C, Beani L and Simonato M:
Noradrenergic modulation of gamma-aminobutyric acid outflow from
the human cerebral cortex. Brain Res. 629:103–108. 1993.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Louis WJ, Papanicolaou J, Summers RJ and
Vajda FJ: Role of central beta-adrenoceptors in the control of
pentylenetetrazol-induced convulsions in rats. Br J Pharmacol.
75:441–446. 1982.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Nakamura T, Oda Y, Takahashi R, Tanaka K,
Hase I and Asada A: Propranolol increases the threshold for
lidocaine-induced convulsions in awake rats: A direct effect on the
brain. Anesth Analg. 106:1450–1455. 2008.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Santana N and Artigas F: Laminar and
cellular distribution of monoamine receptors in rat medial
prefrontal cortex. Front Neuroanat. 11:1–13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Luo F, Tang H and Cheng ZY: Stimulation of
α1-adrenoceptors facilitates GABAergic transmission onto pyramidal
neurons in the medial prefrontal cortex. Neuroscience. 300:63–74.
2015.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Hillman KL, Knudson CA, Carr PA, Doze VA
and Porter JE: Adrenergic receptor characterization of CA1
hippocampal neurons using real time single cell RT-PCR. Brain Res
Mol Brain Res. 139:267–276. 2005.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Sapa J, Zygmunt M, Kulig K, Malawska B,
Dudek M, Filipek B, Bednarski M, Kusak A and Nowak G: Evaluation of
anticonvulsant activity of novel pyrrolidin-2-one derivatives.
Pharmacol Rep. 66:708–711. 2014.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Clinckers R, Zgavc T, Vermoesen K, Meurs
A, Michotte Y and Smolders I: Pharmacological and neurochemical
characterization of the involvement of hippocampal adrenoreceptor
subtypes in the modulation of acute limbic seizures. J Neurochem.
115:1595–1607. 2010.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Gellman RL, Kallianos JA and McNamara JO:
Alpha-2 receptors mediateendogenous noradrenergic suppression of
kindling development. J Pharmacol Exp Ther. 241:891–898.
1987.PubMed/NCBI
|
|
80
|
Amabeoku GJ: The involvement of
noradrenaline, 5-hydroxytryptamine and acetylcholine in
imipramine-induced seizures in mice. Experientia. 49:859–864.
1993.PubMed/NCBI View Article : Google Scholar
|
|
81
|
MacDonald E, Kobilka BK and Scheinin M:
Gene targeting-homing in on alpha 2-adrenoceptor-subtype function.
Trends Pharmacol Sci. 18:211–219. 1997.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Weinshenker D, Szot P, Miller NS and
Palmiter RD: Alpha1 and beta2 adrenoreceptor agonists inhibit
pentylenetetrazole-induced seizures in mice lacking norepinephrine.
J Pharmacol Exp Ther. 298:1042–1048. 2001.PubMed/NCBI
|
|
83
|
Xiao Z, Deng PY, Rojanathammanee L, Yang
C, Grisanti L, Permpoonputtana K, Weinshenker D, Doze VA, Porter JE
and Lei S: Noradrenergic depression of neuronal excitability in the
entorhinal cortex via activation of TREK-2K+ channels. J Biol Chem.
284:10980–10991. 2009.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Sitnikova E, Pupikina M and Rutskova E:
Alpha2 adrenergic modulation of spike-wave epilepsy: Experimental
study of pro-epileptic and sedative effects of dexmedetomidine. Int
J Mol Sci. 24(9445)2023.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Biggane JP, Xu K, Goldenstein BL, Davis
KL, Luger EJ, Davis BA, Jurgens CWD, Perez DM, Porter JE and Doze
VA: Pharmacological characterization of the α2A-adrenergic receptor
inhibiting rat hippocampal CA3 epileptiform activity: Comparison of
ligand efficacy and potency. J Recept Signal Transduct Res.
42:580–587. 2022.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Ahmadirad N, Fathollahi Y, Janahmadi M,
Ghasemi Z, Shojaei A, Rezaei M, Barkley V and Mirnajafi-Zadeh J:
The role of α adrenergic receptors in mediating the inhibitory
effect of electrical brain stimulation on epileptiform activity in
rat hippocampal slices. Brain Res. 1765(147492)2021.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Rezaei M, Ahmadirad N, Ghasemi Z, Shojaei
A, Raoufy MR, Barkley V, Fathollahi Y and Mirnajafi-Zadeh J: Alpha
adrenergic receptors have role in the inhibitory effect of
electrical low frequency stimulation on epileptiform activity in
rats. Int J Neurosci. 133:496–504. 2023.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Wu HQ, Tullii M, Samanin R and Vezzani A:
Norepinephrine modulates seizures induced by quinolinic acid in
rats: Selective and distinct roles of alpha adrenoceptor subtypes.
Eur J Pharmacol. 138:309–318. 1987.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Eason MG, Kurose H, Holt BD, Raymond JR
and Liggett SB: Simultaneous coupling of alpha 2-adrenergic
receptors to two G-proteins with opposing effects.
Subtype-selective coupling of alpha 2C10, alpha 2C4, and alpha 2C2
adrenergic receptors to Gi and Gs. J Biol Chem. 267:15795–15801.
1992.PubMed/NCBI
|
|
90
|
Atzori M, Cuevas-Olguin R, Esquivel-Rendon
E, Garcia-Oscos F, Salgado-Delgado RC, Saderi N, Miranda-Morales M,
Treviño M, Pineda JC and Salgado H: Locus ceruleus norepinephrine
release: A central regulator of CNS spatio-temporal activation.
Front Synaptic Neurosci. 8(25)2016.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Świąder M, Zakrocka I, Świąder K, Zawadzki
A, Łuszczki JJ, Czuczwar SJ and Munir D: Influence of salbutamol on
the anticonvulsant potency of the antiepileptic drugs in the
maximal electroshock-induced seizures in mice. Pharmacol Rep.
71:466–472. 2019.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Gross RA and Ferrendelli JA: Relationships
between norepinephrine and cyclic nucleotides in brain and seizure
activity. Neuropharmacology. 21:655–661. 1982.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Anlezark G, Horton R and Meldrum B: The
anticonvulsant action of the (-)- and (+)-enantiomers of
propranolol. J Pharm Pharmacol. 31:482–483. 1979.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Levy A, Ngai SH, Finck AD, Kawashima K and
Spector S: Disposition of propranolol isomers in mice. Eur J
Pharmacol. 40:93–100. 1976.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Fischer W: Anticonvulsant profile and
mechanism of action of propranolol and its two enantiomers.
Seizure. 11:285–302. 2002.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Mueller AL and Dunwiddie TV:
Anticonvulsant and proconvulsant actions of alpha- and
beta-noradrenergic agonists on epileptiform activity in rat
hippocampus in vitro. Epilepsia. 24:57–64. 1983.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Lipski WJ and Grace AA: Activation and
inhibition of neurons in the hippocampal ventral subiculum by
norepinephrine and locus coeruleus stimulation.
Neuropsychopharmacology. 38:285–292. 2013.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Fassio A, Rossi F, Bonanno G and Raiteri
M: GABA induces norepinephrine exocytosis from hippocampal
noradrenergic axon terminals by a dual mechanism involving
different voltage-sensitive calcium channels. J Neurosci Res.
57:324–331. 1999.PubMed/NCBI
|
|
99
|
Tully K, Li Y, Tsvetkov E and Bolshakov
VY: Norepinephrine enables the induction of associative long-term
potentiation at thalamo-amygdala synapses. Proc Natl Acad Sci USA.
104:14146–14150. 2007.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Gellman RL and Aghajanian GK: Pyramidal
cells in piriform cortex receive a convergence of inputs from
monoamine activated GABAergic interneurons. Brain Res. 600:63–73.
1993.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Bergles DE, Doze VA, Madison DV and Smith
SJ: Excitatory actions of norepinephrine on multiple classes of
hippocampal CA1 interneurons. J Neurosci. 16:572–585.
1996.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Braga MF, Aroniadou-Anderjaska V, Manion
ST, Hough CJ and Li H: Stress impairs alpha(1A)
adrenoceptor-mediated noradrenergic facilitation of GABAergic
transmission in the basolateral amygdala. Neuropsychopharmacology.
29:45–58. 2004.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Prager EM, Bergstrom HC, Wynn GH and Braga
MFM: The basolateral amygdala -γ aminobutyric system in health and
disease. J Neurosci Res. 94:548–567. 2016.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Dazzi L, Matzeu A and Biggio G: Role of
ionotropic glutamate receptors in the regulation of hippocampal
norepinephrine output in vivo. Brain Res. 1386:41–49.
2011.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Stanton PK: Noradrenergic modulation of
epileptiform bursting and synaptic plasticity in the dentate gyrus.
Epilepsy Res. 7:135–150. 1992.PubMed/NCBI
|
|
106
|
Stanton PK, Jones RS, Mody I and Heinemann
U: Epileptiform activity induced by lowering extracellular (Mg2+)
in combined hippocampal-entorhinal cortex slices: Modulation by
receptors for norepinephrine and N-methyl-D-aspartate. Epilepsy
Res. 1:53–62. 1987.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Paladini CA, Fiorillo CD, Morikawa H and
Williams JT: Amphetamine selectively blocks inhibitory glutamate
transmission in dopamine neurons. Nat Neurosci. 4:275–281.
2001.PubMed/NCBI View
Article : Google Scholar
|