Introduction
In recent years, various disease biomarkers have
been discovered, and the development of simple blood tests is
underway to determine the pathological condition, predict the onset
of disease and its prognosis, and identify preventive/therapeutic
targets. In terms of biomarker species, studies have reported
enzyme, antigen, and, in recent years, nucleotide markers (1-3).
However, there are still some reports on antibody markers, which
include heat-shock 60-kD protein 1(4), replication protein A2(5), programmed cell death 11(6), metalloproteinase 1, chromobox homolog
1, chromobox homolog 5(7), DnaJ heat
shock protein family (Hsp40) member C2(8), adaptor-related protein complex 3
subunit delta 1(9), serpin peptidase
inhibitor, clade E member 1(10),
death-inducer obliterator 1, cleavage and polyadenylation
specificity factor 2, forkhead box J2(11) and thiosulfate sulfurtransferase-like
domain-containing 2(12) for acute
ischemic stroke (AIS); ATPase, Ca++ transporting, plasma
membrane 4(10), bone morphogenetic
protein 1 (3,13), deoxyhypusine synthase (14), SH3 domain-binding protein 5(15), prolyl carboxypeptidase (16), low-density lipoprotein
receptor-related protein-associated protein 1(17) and additional sex combs-like
2(18) for atherosclerosis;
nardilysin (19) for acute cardiac
syndrome; and insulin (20),
glutamic acid decarboxylase (21),
adiponectin (22) and growth arrest
and DNA-damage-inducible gene 34 (23,24), and
proprotein convertase subtilisin/kexin type 9(25) for diabetes mellitus (DM).
The anti-p53 antibody is a typical antibody
biomarker for cancer that has been used in clinical practical for
diagnosing, monitoring and predicting the prognosis of esophageal
cancer (EC) and head and neck cancer (26,27).
Further application of the serological identification of antigens
by cDNA expression cloning and the protein array method have
identified autoantibodies against tumor-associated calcium signal
transducer 2(28), solute carrier
family 2/facilitated glucose transporter, member 1(29), tripartite motif-containing
21(30), myomegalin (31), makorin 1(32), esophageal carcinoma SEREX antigen
(33), cyclin L2(34), cofilin, β-actin (35) and WD repeat-containing protein
1(36) for EC; FBP-interacting
repressor for colorectal cancer (CRC) (37) and gastric cancer (GC) (38); SH3 domain, GRB2-like 1(39) and filamin C (40) for glioma; EP300-interacting inhibitor
of differentiation 3 for non-functional pancreatic neuroendocrine
tumors (41); wingless-type MMTV
integration site family, member 7 for biliary cancer (42); and coatomer protein complex subunit
epsilon (43), differential
screening-selected gene aberrant in neuroblastoma (44), and sorting nexins 16(45) for obstructive sleep apnea syndrome
(OSAS). Here, we report on serum antibodies against KIAA0513
(s-KIAA0513-Ab) as a broad-spectrum biomarker applicable to
atherosclerosis-related diseases such as ischemic stroke,
cardiovascular disease (CVD), chronic kidney disease (CKD), DM and
solid cancers.
Materials and methods
Patients and control sera
The present study was conducted according to the
guidelines of the Declaration of Helsinki and approved by the Local
Ethical Review Board of the Chiba University, Graduate School of
Medicine (Chiba, Japan), as well as by the review boards of the
participating hospitals (approval no. 2018-320). The Ethics
Committee of Toho University, Graduate School of Medicine, Tokyo,
Japan (No.
A18103_A17052_A16035_A16001_26095_25024_24038_22047_22047) and Port
Square Kashiwado Clinic, Kashiwado Memorial Foundation, China,
Japan (approval no. 2012-001) also approved the study protocol.
Sera were collected from patients who had provided written informed
consent. Each serum sample was centrifuged at 3,000 x g for 10 min
at 4˚C, and the supernatant was stored at -80˚C until use.
Serum samples collected from patients with AIS,
transient ischemic attack (TIA), asymptomatic ischemic stroke
(Asympt-CI), chronic-phase cerebral infarction (cCI), and deep and
subcortical white matter hyperintensity (DSWMH) were obtained from
Chiba Prefectural Sawara Hospital. The stroke subtypes were
determined according to the criteria of the Trial of Org 10172 in
the Acute Stroke Treatment classification system (46), and large-artery atherosclerosis and
small-artery occlusion (lacune) were included as AIS and ischemic
stroke. Samples from patients with DM, CVD and OSAS were obtained
from the Chiba University Hospital. CVD included acute myocardial
infarction (AMI) and unstable angina. The serum samples of patients
with AIS, TIA and AMI were obtained within 2 weeks following
disease onset. Samples collected from patients with CKD were
obtained from the Kumamoto cohort (47,48),
whereas those collected from patients with EC, GC, CRC, lung cancer
(LC), and breast cancer (BC) were obtained from the Department of
Surgery, Toho University Hospital. Serum samples from healthy
donors (HDs) were obtained from Chiba University, Port Square
Kashiwado Clinic, the National Hospital Organization, Shimoshizu
Hospital (Yotsukaido, Japan), and Chiba Prefectural Sawara Hospital
(Katori, Japan). For comparisons with TIA and AIS, serum samples
from HDs were selected from patients who exhibited no abnormalities
in cranial magnetic resonance imaging.
ProtoArray® screening
The initial screening was performed using
ProtoArray® Human Protein Microarrays v4.0 (Thermo
Fisher Scientific, Inc.), which were loaded with 9,480 species of
proteins, as previously described (11,14,15). In
total, 20 serum samples (10 each from the patients and HDs) were
employed to detect antigens specifically recognized by IgG
antibodies in the patient sera. The complete results from the
ProtoArray® screening are presented in Table SI.
Expression and purification of
KIAA0513 protein
A sequence encoding cDNA of the isoform c of human
KIAA0513 (accession no. NP_001284695.1) was cloned into pGEX-6P
(Cytiva). The expression of the cDNA product was induced by
treating Escherichia coli (E. coli) KRX (Promega
Corp.) cells harboring the pGEX-6P-KIAA0513 and pMINOR with 0.5 mM
isopropyl-β-D-thiogalactoside (FUJIFILM Wako Pure Chemical
Corporation) at 37˚C for 3 h (49).
The cells were lysed by sonication in phosphate-buffered saline
(FUJIFILM Wako Pure Chemical Corporation) containing 1% Triton
X-100 (FUJIFILM Wako Pure Chemical Corporation) and 2 mM
dithiothreitol (FUJIFILM Wako Pure Chemical Corporation). The
glutathione S-transferase (GST)-fused proteins were specifically
bound to Glutathione-Sepharose 4 Fast Flow medium (Cytiva) and
HiPrep 26/10 Desalting column (1 ml) (Cytiva), followed by washing
with 30 ml of phosphate-buffered saline. GST-KIAA0513 protein was
eluted with 10 mM glutathione (Wako Pure Chemicals) and 2 mM
dithiothreitol in phosphate-buffered saline, and concentrated to
3.5 mg/ml in phosphate-buffered saline containing 2 mM
dithiothreitol as previously described (49). Protein concentration was determined
by Bradford Protein Assay (Bio-Rad Laboratories, Inc.).
Western blot analysis
Purified GST-KIAA0513 and the control GST proteins
(0.3 µg/lane) were electrophoresed through sodium dodecyl
sulfate-polyacrylamide (11%) gels, followed by directly staining
with 0.05% Coomassie Brilliant Blue (Nacalai Tesque) in 50%
methanol and 10% acetic acid for 1 h at 25˚C, or blotting onto
nitrocellulose membranes (S045A330R, Advantec). The membranes were
blocked with 0.1% dry milk (Megmilk Snow Brand Co., Ltd.) in 150 mM
NaCl, 20 mM Tris-HCl (pH 7.6) and 0.1% Tween-20 (TBS-T) at 25˚C for
1 h, and then treated with anti-GST (goat, ab6613, Abcam),
anti-KIAA0513 (rabbit, HPA012866, Atlas Antibodies) antibodies at a
final concentration of 0.2 µg/ml, or the patient sera (1/1,000 fold
dilution) at 25˚C overnight. The membranes were washed five times
with TBS-T and treated with HRP-conjugated secondary antibodies
(HRP-conjugated donkey anti-goat IgG, sc-2020, Santa Cruz
Biotechnology, Inc., 1/30,000-fold dilution; HRP-conjugated goat
anti-rabbit IgG, 111-035-003, Jackson ImmunoResearch Laboratories,
Inc, 1/30,000-fold dilution; HRP-conjugated goat anti-human IgG,
A130PD, American Qualex, 1/30,000-fold dilution) at 25˚C for 20
min. Following five washes with TBS-T, the addition of Immobilon
(Merck KGaA) produced luminescence, which was detected using
LuminoGraph II (Atto Co., Ltd.), as previously described (7,8,11,17).
Amplified luminescence proximity
homogeneous assay-linked immunosorbent assay (AlphaLISA)
AlphaLISA was performed in 384-well microtiter
plates (white opaque OptiPlate™, Revvity) containing either 2.5 µl
of 1:100-diluted serum with 2.5 µl of GST or GST-KIAA0513 proteins
(10 µg/ml) in AlphaLISA buffer (25 mM
N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid, pH 7.4, 0.1%
casein, 0.5% Triton X-100, 1 mg/ml dextran-500 and 0.05%
Proclin-300) (Revvity). The reaction mixture was incubated at room
temperature for 6-8 h, followed by the addition of anti-human
IgG-conjugated acceptor beads (2.5 µl at 40 µg/ml) and
glutathione-conjugated donor beads (2.5 µl at 40 µg/ml), and the
mixture was incubated at room temperature in the dark for 1-14
days. The chemical emissions were measured using an EnSpire Alpha
microplate reader (Revvity), as previously described (7-11).
The specific reactions were calculated by subtracting the emission
counts of the GST control from the counts of GST-fused KIAA0513
protein.
Immunohistochemical staining
The formalin-fixed paraffin-embedded EC tissues were
sectioned into 4-µm-thick slices, which were deparaffinized,
blocked with Hyper Peroxide Block and Protein Block (Rabbit
Specific HRP/DAB Detection Kit, Abcam), reacted with primary
anti-KIAA0513 antibody (rabbit polyclonal antibodies, HPA012866,
Atlas Antibodies) at 0.75 µg/ml for 18 h at 4˚C, incubated with
biotinylated anti-rabbit IgG (biotin-conjugated goat anti-rabbit
IgG, sc-2040, Santa Cruz Biotechinology, Inc.) at 2 µg/ml for 30
min at 25˚C, and reacted with streptavidin conjugated to
horseradish peroxidase reagent (ab7403, Abcam) for 30 min at 25˚C.
Finally, the reaction was visualized with a chromogen
(diaminobenzidine) in DAB substrate (ab64238, Abcam). The sections
were then counterstained with hematoxylin (Mfcd00078111, Merck
KGaA) for 30 sec at 25˚C, dehydrated, mounted as previously
described (11,17). Photomicrographs were obtained using a
light microscope (BA210E, Shimazu) at x100 magnification.
Nested case-control study
A nested case-cohort study was conducted using the
aforementioned AlphaLISA detection antibody levels. The present
study was nested within the Japan Public Health Center-based
Prospective Study (50-52),
which involved ~30,000 Japanese individuals aged 40-69 years at a
baseline period from 1990-1994 whose plasma samples were stored.
The plasma samples employed were from 202 cases of incidental AIS
in the cohort that occurred between baseline and 2008 and from 202
controls whose age (within 2 years), sex, date of blood sampling
(within 3 months), time since last meal (within 4 h), and study
location (Public Health Center area) were matched with those of the
cases. A conditional logistic regression model was used to estimate
the odds ratios (ORs) and 95% confidence intervals (95% CIs). The
study participants were informed of the objectives and methods of
the study, and those who answered the questionnaire and donated
blood were regarded as having given informed consent to
participate.
Statistical analysis
The Mann-Whitney U test was employed to determine
the significant differences between two groups and the
Kruskal-Wallis test (with the Bonferroni correction applied) was
used to evaluate the differences among ≥3 groups. Correlations were
analyzed using Spearman's correlation analysis and logistic
regression analysis. All the statistical analyses were performed
using GraphPad Prism 5 (GraphPad Software, Inc.). The predictive
values of the putative disease markers were assessed via a receiver
operating characteristic (ROC) curve analysis and determined the
sensitivity and specificity. Patient survival was evaluated using
the Kaplan-Meier method and compared using the log-rank test.
X-tile 3.6.1 software (Yale University, New Haven, CT, USA)
(53) was used to determine the
optimal cut-off values for discrimination of the survival rates
between antibody positive and negative groups. All tests were
two-tailed, and P-values <0.05 were considered to indicate
statistically significant differences.
Results
Recognition of KIAA0513 by serum
components from patients with atherosclerosis
The present study employed a ProtoArray loaded with
9,480 protein species to identify the antigens recognized by
antibodies in the sera of patients with atherosclerosis. It was
found that KIAA0513 isoform c (Accession no. BC030280.1) reacted
with 6 of the 10 serum samples from the patients with
atherosclerosis, and with only 1 of the 10 samples from the HDs
(Table SI). Subsequently, GST-fused
full-length KIAA0513 protein was expressed in E. coli and
purified by affinity-chromatography.
Presence of autoantibodies against
KIAA0513 in the sera of patients with AIS, TIA, DM, EC, or CC
The present study examined the presence of
autoantibodies against KIAA0513 in sera using western blot analysis
(Fig. 1). GST-KIAA0513 protein
reacted with commercial anti-GST and anti-KIAA0513 antibodies,
whereas the control, GST, reacted with anti-GST, but not with
anti-KIAA0513 antibodies. GST-KIAA0513 protein was also recognized
by serum IgG antibodies in the patients with AIS (anonymization
nos. #07065 and #070684), TIA (#02337), DM (#22226), EC (#EC-6) and
CRC (#Co-58), but not in the HDs (#09101). GST alone exhibited no
apparent reaction with any serum from the patients or HDs.
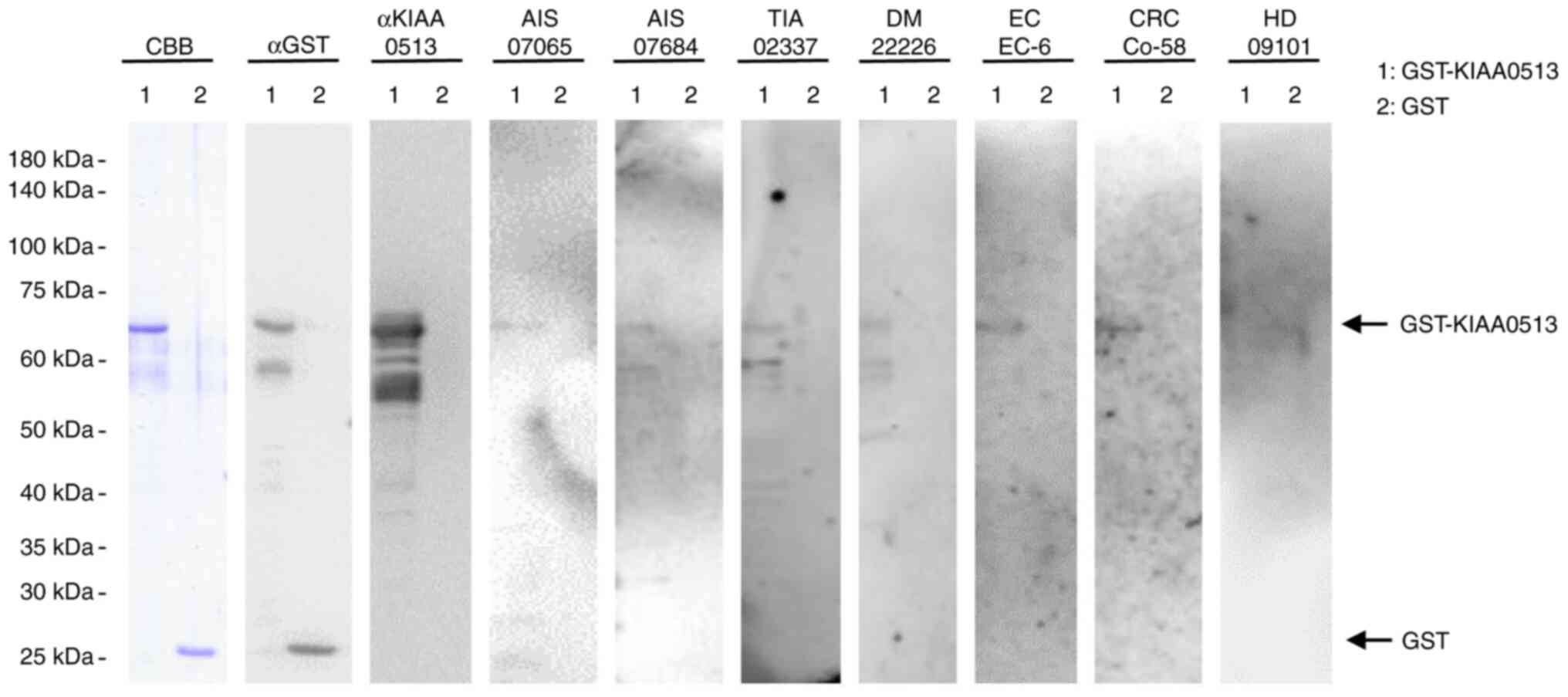 | Figure 1Presence of antibodies against
KIAA0513 in sera from patient with AIS, TIA, DM, EC, or CRC.
Purified proteins of GST-KIA0513 (lane 1) and GST (lane 2) were
electrophoresed through sodium dodecyl sulfate-polyacrylamide gel,
followed by staining with CBB or western blotting using anti-GST
(αGST), anti-KIAA0513 (αKIAA0513), serum IgG from patients with AIS
(AIS-07065, AIS-07684), TIA (TIA-02337), DM (DM-22226), EC (EC-6),
or CRC (Co-58) and from a healthy donor (HD) (HD-09101). Sample
number are subjects' anonymization numbers. AIS, acute ischemic
stroke; TIA, transient ischemic attack; DM, diabetes mellitus; EC,
esophageal cancer; CRC, colorectal cancer; CBB, Coomassie Brilliant
Blue; GST, glutathione S-transferase. |
Elevation of s-KIAA0513-Ab levels in
the patients with AIS and TIA
The s-KIAA0513-Ab levels were then examined in the
patients with AIS or TIA. Sera from HDs, and patients with AIS and
TIA were obtained from the Chiba Prefectural Sawara Hospital. The
results of AlphaLISA revealed that the s-KIAA0513-Ab levels were
significantly higher in the patients with AIS or TIA than in the
HDs (Fig. 2A). Using the cut-off
values of the average plus two standard deviations (SDs) of the HD
values, the s-KIAA0513-Ab positivity rates for the HDs, patients
with AIS, and those with TIA were 0.0, 7.6 and 15.6%, respectively
(Table SII). ROC analysis revealed
that the areas under the ROC curves (AUCs) of s-KIAA0513-Abs were
0.6439 (95% CI, 0.587-0.700) for AIS (Fig. 3A) and 0.6604 (95% CI, 0.563-0.758)
for TIA (Fig. 3B). Thus, TIA (which
can be a prodromal stage of AIS) and AIS were equally associated
with the s-KIAA0513-Ab marker.
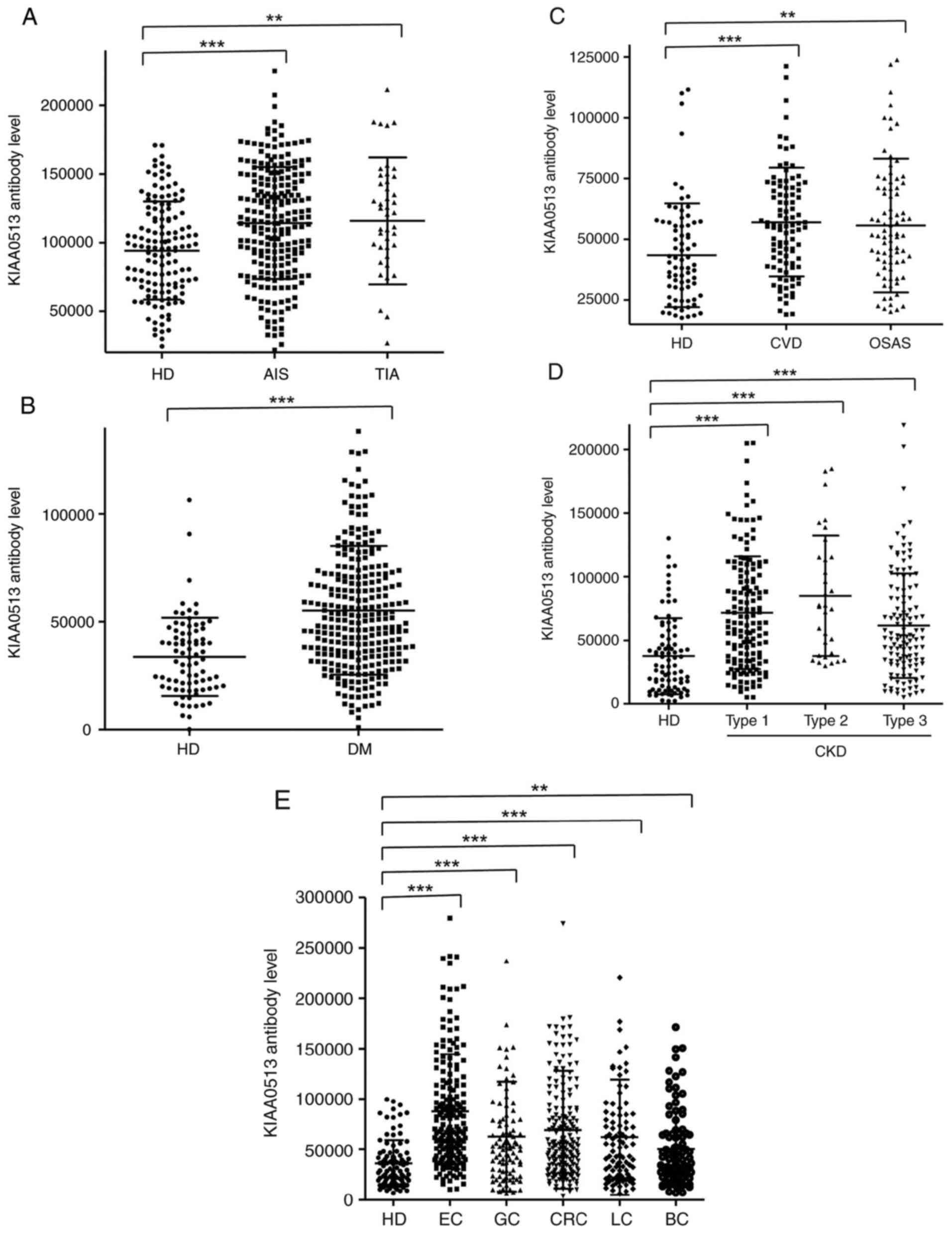 | Figure 2Comparison of serum anti-KIAA0513
antibody (s-KIAA0513-Ab) levels between HDs and patients. The
s-KIAA0513-Ab levels of HDs and patients with (A) AIS or TIA, (B)
DM, (C) CVD or OSAS, (D) CKD, and (E) EC, GC, CRC, LC, or BC were
examined by AlphaLISA using GST-KIAA05132-302 protein as
the antigen, followed by subtraction of the levels against control
GST. Scatter dot plots of the s-KIAA0513-Ab levels are shown. The
bars represent the average and average ± SD. P-values were
calculated using the Mann-Whitney U test to analyze the differences
between two groups, and the Kruskal-Wallis test (with the
Bonferroni correction applied) to evaluate the differences among ≥3
groups. **P<0.01 and ***P<0.001 vs. HD
specimens. Type-1, type-2, and type-3 CKDs represent diabetic
kidney disease, nephrosclerosis, and glomerulonephritis,
respectively. The total (male/female) numbers, average ages ± SDs,
average antibody levels ± SDs, cut-off values, positive numbers,
positive rates (%), and P-values vs. HDs are summarized and shown
in Table SII, Table SIII, Table SIV, Table SV and Table VI. HD, healthy donors; AIS, acute
ischemic stroke; TIA, transient ischemic attack; DM, diabetes
mellitus; EC, esophageal cancer; CVD, cardiovascular disease; OSAS,
obstructive sleep apnea syndrome; CKD, chronic kidney disease; GC,
gastric cancer; LC, lung cancer; BC, breast cancer; SD, standard
deviation. |
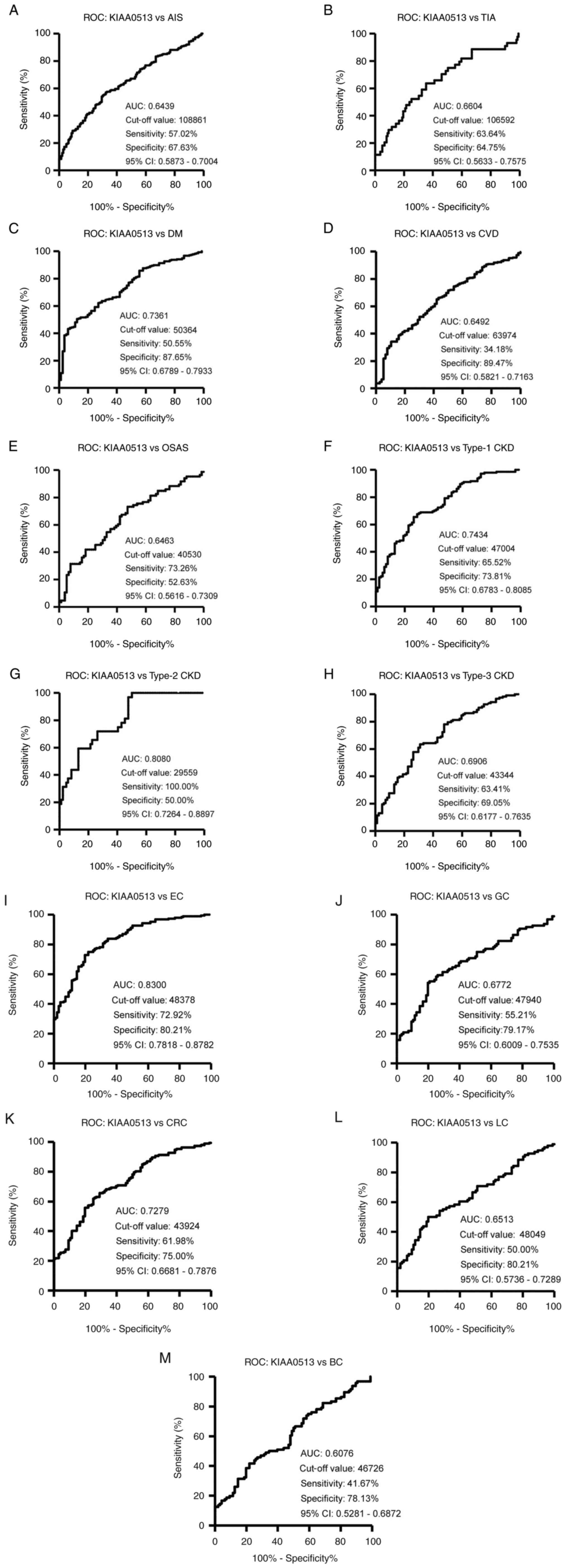 | Figure 3ROC curve analysis. The abilities of
s-KIAA0513-Abs to detect (A) AIS, (B) TIA, (C) DM, (D) CVD, (E)
OSAS, (F) type-1 CKD, (G) type-2 CKD, (H) type-3 CKD, (I) EC, (J)
GC, (K) CRC, (L) LC, and (M) BC were evaluated using ROC analysis.
Numbers in the figures indicate the AUC, cut-off values for
antibody levels, sensitivity, specificity, and 95% CIs. ROC,
receiver operating characteristic; AIS, acute ischemic stroke; TIA,
transient ischemic attack; DM, diabetes mellitus; EC, esophageal
cancer; CVD, cardiovascular disease; OSAS, obstructive sleep apnea
syndrome; CKD, chronic kidney disease; GC, gastric cancer; LC, lung
cancer; BC, breast cancer; AUC, area under the curve; CI,
confidence interval. |
Elevation of serum antibody levels
against KIAA0513 in patients with DM
The present study then examined the s-KIAA0513-Ab
levels in patients with DM. Serum samples from HDs and patients
with DM were obtained from Chiba University and Chiba University
Hospital. The s-KIAA0513-Ab levels were significantly higher in the
samples from the patients with DM than in those from HDs (Fig. 2B). At a cut-off value equivalent to
the average plus two SDs of the HD specimen values, the positive
rates of s-KIAA0513-Abs in the HDs and patients with DM were 2.5
and 26.5%, respectively (Table
SIII). ROC analysis was performed to evaluate the ability of
these antibody markers to indicate the presence of DM. The AUC for
s-KIAA0513-Abs was 0.736, yielding a sensitivity and specificity of
50.55 and 87.65%, respectively (Fig.
3C).
Association between s-KIAA0513-Ab
levels and CVD and OSAS
Subsequently, the antibody levels in serum samples
from patients with CVD obtained from Chiba University Hospital were
examined. Given that OSAS is related to atherosclerosis and is
associated with a high risk of AIS and CVD (36-39),
the present study also examined the sera of patients with OSAS
obtained from Chiba University Hospital. Compared with those of the
HDs, the s-KIAA0513-Ab levels were significantly higher in the
patients with CVD or OSAS (Fig. 2C),
although the positive rates in the patients with CVD and those with
OSAS were not markedly high (10.3 and 11.6%, respectively)
(Table SIV). ROC analysis revealed
that the AUCs for CVD and OSAS were 0.649 (95% CI, 0.582-0.716)
(Fig. 3D) and 0.646 (95% CI,
0.562-0.731) (Fig. 3E),
respectively. Compared with the low P-value (<0.001) of
s-KIAA0513-Ab for CVD, the P-value for OSAS was <0.01 (Table SIV), suggesting a weaker association
of the s-KIAA0513-Ab marker with OSAS than with CVD.
Elevation of s-KIAA0513-Ab levels in
patients with CKD
The present study then examined the antibody levels
in the sera of patients with CKD, which is also closely related to
atherosclerosis. CKD was divided into three groups as follows: Type
1, diabetic kidney disease; type 2, nephrosclerosis; and type 3,
glomerulonephritis. Samples from patients with CKD were obtained
from the Kumamoto cohort, and samples from HDs were obtained from
Chiba University. Patients from all three CKD groups had
significantly higher s-KIAA0513-Ab levels than the HDs (Fig. 2D). The s-KIAA0513-Ab positivity rates
in the HDs and patients with types 1, 2 and 3 CKD were 6.1, 29.0,
37.5 and 20.3%, respectively (Table
SV), indicating that the highest positive rate was observed in
the patients with type 2 CKD. ROC analysis revealed s-KIAA0513-Ab
AUCs as high as 0.7434 (95% CI, 0.678-0.809) for type 1 CKD
(Fig. 3F), 0.808 (95% CI,
0.726-0.890) for type 2 CKD (Fig.
3G) and 0.691 (95% CI, 0.618-0.764) for type 3 CKD (Fig. 3H).
s-KIAA0513-Ab levels in solid
cancer
Given that atherosclerotic diseases are frequently
related to cancer with certain common biomarkers being reported
(20), the present study examined
the serum samples from patients with EC, GC, CRC, LC and BC
obtained from Toho University Hospital. The s-KIAA0513-Ab levels
were significantly higher in the samples from all patients with
cancer than in those from the HDs (Fig.
2E and Table SVI). The highest
average value and positive rate of s-KIAA0513-Ab levels were
observed for EC. Similarly, the AUC values were highest for EC
(0.830), but lowest for BC among the cancers examined (Fig. 3I-M).
The present study then examined whether the
s-KIAA0513-Ab levels are related to the post-operative survival of
patients with EC or GC. The s-KIAA0513-Ab levels were divided into
the positive and negative groups using the cut-off values obtained
using X-tile software (53). The
s-KIAA-Ab-positive group presented a more unfavorable prognosis
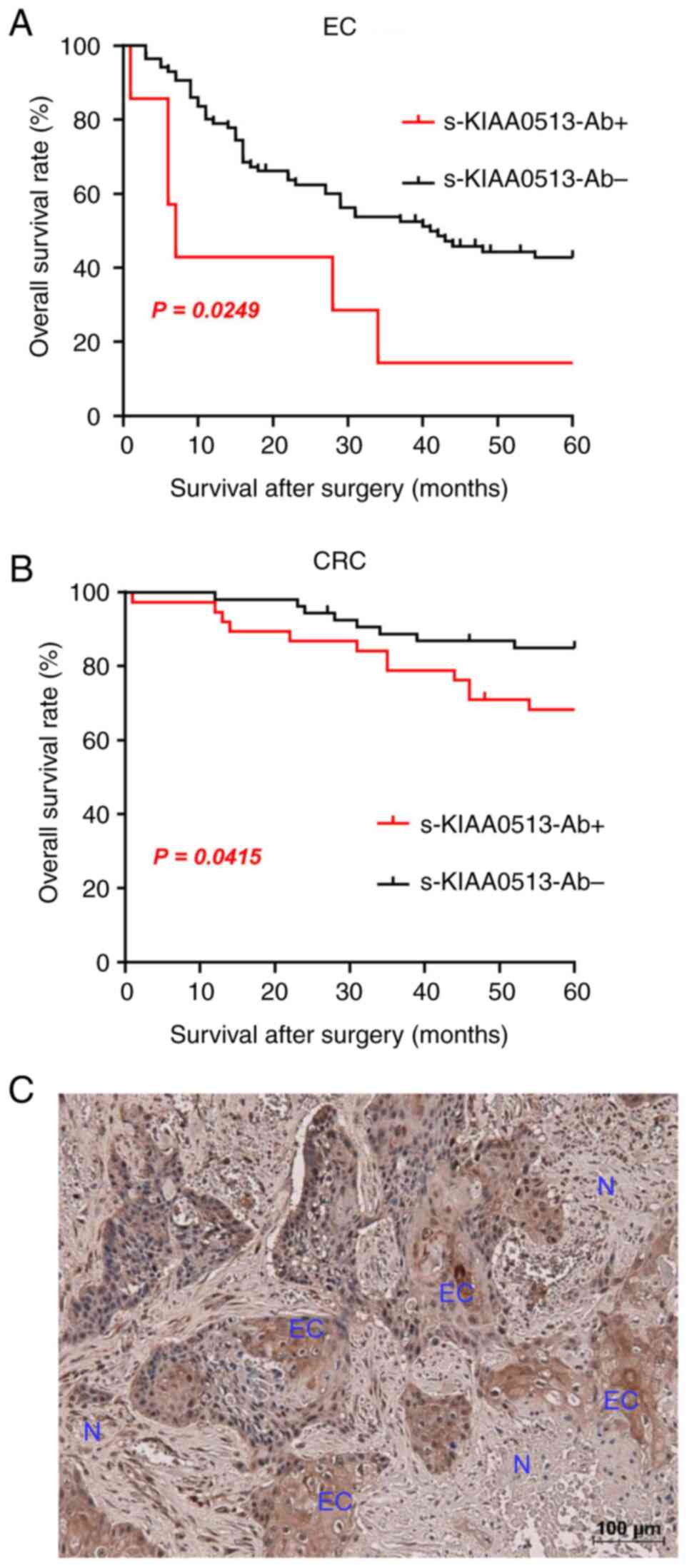
than the negative group in all of EC and CRC (Fig. 4A and B). The X-tile-determined cut-off values are
best ones to distinguish the favorable and poor survivals. The
cut-off value of EC samples (180,487) was much higher than the
average (87,535) (Table SVI),
whereas that of CRC (56,879) was lower than the average of CRC
(69,308) (Table SVI). Thus, the
considerably high levels of s-KIAA-Abs in patients with EC and the
moderately high levels in patients with CRC were associated with
the prognosis.
The expression levels of KIAA0513 antigenic protein
in EC tissues were examined using immunohistochemical staining. A
representative example of the staining is illustrated in Fig. 4C. EC tissues were heavily stained by
anti-KIAA0513 antibody, whereas surrounding healthy esophageal
tissues were not. KIAA0513 protein was localized in the cytoplasm,
which is consistent with the findings in a previous study (54). Thus, the high KIAA0513 expression
levels may account for some, if not all, of the development of
serum KIAA0513-Abs.
Association analysis
An analysis of the association analysis between the
s-KIAA0513-Ab levels and participant data was performed using 665
specimens from Chiba Prefectural Sawara Hospital, including 139
specimens from HDs, 225 from patients with AIS, 44 from patients
with TIA, 17 from patients with Asympt-CI, 122 from patients with
DSWMH, 59 from patients with cCI and 41 from disease controls. The
remaining 18 subjects were excluded because they did not have
disease information. In this analysis, the Mann-Whitney U test we
employed to compare the s-KIAA0513-Ab levels between the male and
female participants, with or without DM, hypertension, CVD,
dyslipidemia and obesity [body mass index (BMI) ≥25] and with or
without smoking and alcohol intake habits. A significant difference
in the s-KIAA0513-Ab levels was observed only between the patients
with hypertension and those without hypertension (Table I).
 | Table IAssociation analysis of antibody
levels against KIAA0513 protein with data of subjects in the Sawara
Hospital cohort. |
Table I
Association analysis of antibody
levels against KIAA0513 protein with data of subjects in the Sawara
Hospital cohort.
| Sex | Average or SD | Male | Female |
|---|
|
No. of
samples | | 396 | 268 |
|
KIAA0513-Ab
level | Average | 100,136 | 104,138 |
| | SD | 39,889 | 40,969 |
|
P-value (vs.
male) | | | 0.1466 |
| Other disease | | DM- | DM+ |
|
No. of
samples | | 525 | 135 |
|
KIAA0513-Ab
level | Average | 101,818 | 101,727 |
| | SD | 40,022 | 41,930 |
|
P-value (vs.
DM-) | | | 0.5031 |
| Other disease | |
Hypertension- |
Hypertension+ |
|
No. of
samples | | 239 | 421 |
|
KIAA0513-Ab
level | Average | 96,476 | 104,821 |
| | SD | 38,988 | 40,905 |
|
P-value (vs.
hypertension-) | | | 0.0091 |
| Other disease | |
CVD- |
CVD+ |
|
No. of
samples | | 623 | 37 |
|
KIAA0513-Ab
level | Average | 101,376 | 108,939 |
| | SD | 39,757 | 49,697 |
|
P-value (vs.
CVD-) | | | 0.1177 |
| Other disease | |
Lipidemia- |
Lipidemia+ |
|
No. of
samples | | 475 | 185 |
|
KIAA0513-Ab
level | Average | 103,028 | 98,647 |
| | SD | 40,591 | 39,808 |
|
P-value (vs.
lipidemia-) | | | 0.2716 |
| Lifestyle | | Non-smoker | Smoker |
|
No. of
samples | | 344 | 319 |
|
KIAA0513-Ab
level | Average | 98,991 | 104,832 |
| | SD | 38,563 | 42,055 |
|
P-value (vs.
non-smoker) | | | 0.0673 |
| Lifestyle | |
Alcohol- |
Alcohol+ |
|
No. of
samples | | 238 | 419 |
|
KIAA0513-Ab
level | Average | 102,663 | 101,364 |
| | SD | 39,883 | 40,759 |
|
P-value (vs.
alcohol-) | | | 0.4595 |
| Obesity | | BMI <25 | BMI >25 |
|
No. of
samples | | 498 | 158 |
|
KIAA0513-Ab
level | Average | 102,071 | 101,390 |
| | SD | 40,475 | 40,488 |
|
P-value (vs.
alcohol-) | | | 0.7490 |
Correlation analysis
Spearman's correlation analysis was performed a to
determine the correlation between the s-KIAA0513-Ab levels and the
continuous variables of participant parameters, including general
information such as age, height, weight and BMI; the degree of
artery stenosis, such as the maximum intima-media thickness
(max-IMT); lifestyle factors such as smoking duration (years) and
alcohol intake frequency (times/week); and blood test data. The
average values of these parameters are listed in Table SVII. There was a significant
correlation between the s-KIAA013-Ab levels and age, max-IMT,
alkaline phosphatase, potassium, C-reactive protein (CRP), blood
sugar and smoking duration (Table
II), and an inverse correlation with height and weight. The
correlation with max-IMT suggests that the s-KIAA0513-Ab levels are
associated with stenosis and atherosclerosis, which was further
confirmed by using other cohorts. Spearman's correlation analysis
of the CKD cohort (300 participants) revealed a significant
correlation with plaque score, max-IMT (55,56) and
cardio-ankle vascular index (CAVI) (right) (57) (Table
SVIII), which are indices of atherosclerosis. CRP was also
associated with s-KIAA0513-Abs in the CKD cohort, suggesting the
involvement of inflammation. By contrast, age, height, weight, BMI
and potassium levels exhibited no significant correlation with
s-KIAA0513-Abs in the CKD cohort. AIS is closely related to age,
which may be indirectly associated with s-KIAA0513-Abs.
 | Table IICorrelation analysis of serum
antibody levels against KIAA0513 with data on subjects in the
Sawara Hospital cohort. |
Table II
Correlation analysis of serum
antibody levels against KIAA0513 with data on subjects in the
Sawara Hospital cohort.
| | KIAA0513-Ab |
|---|
| Parameter | No. of XY
pairs | Rs value | P-value |
|---|
| Agea | 663 | 0.1959 |
<0.0001b |
| Height | 657 | -0.1317 | 0.0007 |
| Weight | 661 | -0.1093 | 0.0049 |
| BMI | 656 | -0.0414 | 0.2899 |
| max-IMT | 457 | 0.1763 | 0.0002 |
| A/G | 628 | -0.0187 | 0.6402 |
| AST (GOT) | 655 | 0.0395 | 0.3134 |
| ALT (GPT) | 655 | -0.0035 | 0.9278 |
| ALP | 600 | 0.0889 | 0.0295 |
| LDH | 631 | 0.0431 | 0.2798 |
| tBil | 637 | 0.0317 | 0.4250 |
| CHE | 506 | -0.0387 | 0.3851 |
| γ-GTP | 609 | -0.0099 | 0.8072 |
| TP | 633 | -0.0184 | 0.6435 |
| ALB | 638 | -0.0207 | 0.6014 |
| BUN | 654 | -0.0454 | 0.2469 |
| CRE | 649 | -0.0310 | 0.4303 |
| eGFR | 552 | 0.0432 | 0.3113 |
| UA | 492 | -0.0138 | 0.7596 |
| AMY | 415 | -0.0901 | 0.0667 |
| T-CHO | 563 | -0.0446 | 0.2913 |
| HDL-C | 435 | -0.0280 | 0.5600 |
| TG | 460 | -0.0198 | 0.6719 |
| Na | 641 | -0.0347 | 0.3808 |
| K | 641 | -0.0899 | 0.0228 |
| Cl | 641 | -0.0392 | 0.3215 |
| Ca | 380 | -0.0607 | 0.2376 |
| IP | 302 | -0.0085 | 0.8824 |
| Fe | 311 | -0.0083 | 0.8838 |
| CRP | 477 | 0.0917 | 0.0453 |
| LDL-C | 344 | -0.0559 | 0.3011 |
| WBC | 650 | 0.0712 | 0.0697 |
| RBC | 650 | -0.0220 | 0.5748 |
| HGB | 650 | 0.0117 | 0.7651 |
| HCT | 650 | -0.0041 | 0.9174 |
| MCV | 650 | 0.0525 | 0.1814 |
| MCH | 650 | 0.0611 | 0.1199 |
| MCHC | 650 | 0.0401 | 0.3080 |
| RDW | 650 | 0.0437 | 0.2661 |
| PLT | 650 | -0.0269 | 0.4942 |
| MPV | 650 | -0.0354 | 0.3683 |
| PCT | 650 | -0.0271 | 0.4900 |
| PDW | 650 | -0.0342 | 0.3838 |
| Blood sugar | 596 | 0.0932 | 0.0229 |
| HbA1c | 505 | -0.0212 | 0.6351 |
| Smoking duration,
years | 656 | 0.1297 | 0.0009 |
| Alcohol frequency,
times/week | 662 | -0.0328 | 0.4000 |
Japan Public Health Center (JPHC)
cohort analysis
A case-control study nested within the Japan Public
Health Center-based Prospective Study was then conducted, which
involved ~30,000 plasma samples (50-52).
The level of antibodies against the KIAA0513 protein was positively
associated with the risk of AIS. The ORs (95% CIs) were 2.11
(1.17-3.81) and 2.23 (1.18-4.21) for those in the third and highest
quartiles of antibody levels, respectively, compared with those in
the lowest quartile (Table III).
These results indicate that the antibody markers against the
KIAA0513 protein are useful for predicting the onset of AIS.
 | Table IIIOdds ratios of incident KIAA0513-Abs
vs. AIS. |
Table III
Odds ratios of incident KIAA0513-Abs
vs. AIS.
| Quartile group | OR and 95% CI | KIAA0513-Ab vs.
AIS |
|---|
| 2nd | Matched OR | 1.69 |
| | 95% CI | 0.91-3.15 |
| 3rd | Matched OR | 2.11 |
| | 95% CI | 1.17-3.81 |
| Max | Matched OR | 2.23 |
| | 95% CI | 1.18-4.21 |
Discussion
In the present study, the initial ProtoArray
screening identified KIAA0513 as an antigen, as recognized by serum
IgG in patients with atherosclerosis, and subsequently recombinant
GST-tagged KIAA0513 protein of 301 amino acids was purified.
Western blot analysis confirmed the presence of autoantibodies
against KIAA0513 (Fig. 1). Using the
KIAA0513 protein as an antigen, the serum antibody levels were
examined using AlphaLISA. The results revealed significantly higher
s-KIAA0513-Ab levels in the patients with AIS, TIA, DM, CVD, OSAS,
CKD, EC, GC, CC, LC and MC than in the HDs (Fig. 2A-E and Table SII, Table SIII, Table SIV, Table SV and Table VI). Among these diseases, the
highest AUC values were observed for EC, type 2 CKD and DM
(Fig. 3A-M). The close association
between s-KIAA0513-Ab levels and hypertension (Table I) could account for the association
with OSAS, which is frequently accompanied by hypertension
(58). Spearman's correlation
analysis revealed a significant correlation between s-KIAA0513-Ab
and max-IMT, plaque score and CAVI, all of which are indices of
atherosclerosis-related lesions (Tables
II and SVIII) (55-57).
By contrast, the s-KIAA0513-Ab levels were weakly correlated with
blood sugar (P=0.023), but were completely unrelated to HbA1c, a
typical DM marker (Table II). Thus,
although the patients with DM exhibited high s-KIAA0513-Ab levels
compared with the HDs, this antibody marker may not primarily
reflect DM lesions, but rather atherosclerotic lesions caused by
DM. Given that angiogenesis is essential for the development of
cancer, vascular malformation may be accompanied by the typical
alterations in atherosclerosis. In fact, DM and arteriosclerotic
diseases are cancer risk factors (59-61).
There are three known splicing variants of KIAA0513:
Isoform a (411 amino acids, NP_055547.1), isoform b (301 amino
acids, NP_001273495.1) and isoform c (301 amino acids,
NP_001284695.1). The full-length 301 amino acids of KIAA0513
isoforms c and b are exactly the same as the first 301 amino acids
of KIAA0513 isoform a. The present study also purified GST-fused
KIAA0513 isoform a and examined the serum antibodies using sera
from HDs and patients with AIS and CVD. Both isoforms a and c of
KIAA0513 exhibited higher antibody levels in the sera from patients
with AIS or CVD than in the sera from HDs (Fig. S1A and B). The reactivity of KIAA0513 isoform c
against serum antibodies was closely associated with that of
KIAA0513 isoform a, although the former was higher than the latter
(Fig. S1C), implying that the major
epitope sites of serum autoantibodies are located in the 301 amino
acids of isoform c.
KIAA0513 mRNA expression has been observed
predominantly in the neurons and glial cells of the brain, with
low-level expression in most human tissues, whereas the KIAA0513
protein was exclusively found in the brain (54). Among brain regions, the highest
expression was in the cerebellum, cortex, hippocampus, pons,
putamen and amygdala. Using a yeast 2-hybrid analysis of a fetal
brain cDNA library, Lauriat et al (54) found that the N-terminal portion of
KIAA0513 interacted with KIBRA, HAX1 and INTS4. A
coimmunoprecipitation analysis revealed a physical association
between KIAA0513 and KIBRA. Given that KIBRA, HAX1 and INTS4 are
involved in synaptic and apoptotic signaling, KIAA0513 can also
participate in these signaling pathways.
In addition to the KIAA0513-Abs employed in the
present study, autoantibodies against ATPase, Ca++
transporting, plasma membrane 4, bone morphogenetic protein 1,
deoxyhypusine synthase, low-density lipoprotein receptor-related
protein-associated protein 1 and additional sex combs-like 2, which
are markers of atherosclerosis, were also elevated in the sera of
patients with EC (13,14,17,18),
indicating that arterial abnormalities can also affect the
carcinogenic process. In fact, angiogenesis is essential for the
development of solid tumors (62),
and diabetes and obesity, which induce arteriosclerosis, are risk
factors for CRC and EC (63-65).
Given that all tissues and organs require oxygen and nutrition
provided by arteries, the alteration of arterial structure and/or
function can affect numerous tissues and organs. All tissues and
organs present in a body can affect each other to a certain degree
(66). In other words, the AIS, CVD
and CKD caused by atherosclerosis, the atherosclerosis induced by
DM, and the solid cancer caused by arterial lesions can be
interrelated with each other via arterial abnormalities. Markers
associated with such abnormalities could therefore detect all of
the above disorders.
Cancer, heart disease, cerebrovascular disease and
renal failure are the first, second, fourth and eighth leading
causes of mortality in Japan, respectively (Ministry of Health,
Labor and Welfare 2018 vital statistics; https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei18/dl/10_h6.pdf).
The majority of the other causes of death are unavoidable, such as
senility and accidents. In other words, the onset and progression
of cancer, heart disease, cerebrovascular disease and renal failure
(as well as their risk factor DM) can be suppressed by proper
health management, such as early diagnosis and intervention.
Notably, cancer, heart disease, cerebrovascular disease, renal
failure and DM can be detected by the s-KIAA0513-Ab marker, making
it applicable for diagnostic purposes and providing appropriate
treatment, lifestyle guidance, etc., leading to improved quality of
life.
As of 2020, numerous reports have shown that the
presence of underlying diseases, such as DM, heart disease,
cerebrovascular disease, cancer, OSAS and kidney disease aggravate
the coronavirus disease 2019 (COVID-19) (67-70).
The s-KIAA0513-Ab marker is therefore a highly useful tool for
detecting patients with COVID-19 who are at a higher risk of
mortality. Antibody markers are generally more sensitive than
antigen markers. Given that the KIAA0513 protein has particularly
high antigenicity, this KIAA0513-Ab marker is extremely sensitive.
Given the major life-threatening diseases can be detected by this
marker, the KIAA0513-Ab marker could be referred to as a
‘supermarker’.
The present study has certain limitations, which
should be mentioned. First, although the increase in s-KIAA0513-Ab
levels could be attributable to the high KIAA0513 expression levels
(Fig. 4C) as suggested above, the
association between the expression of the antigen and the antibody
has not been completely verified. The antigen levels can be
examined by immunohistochemistry, western blot analysis and mass
spectrometry. However, accurately quantifying protein amounts
across many specimens using these methods is still challenging. The
introduction of the AlphaLISA method for the quantification of
antigenic proteins may be another approach. Second, the significant
differences of the prognosis between the s-KIAA-Ab-positive and
-negative groups were observed in EC and CRC (Fig. 4A and B) but not GC, LC, or BC. Further
accumulation of the latter specimens may clarify the association
between s-KIAA0513-Ab levels and their prognosis.
In conclusion, the serum anti-KIAA0513 antibody
marker appears to be useful for diagnosing the progress of
atherosclerosis, which can lead to the onset of life-threatening
AIS, CVD and cancer.
Supplementary Material
Comparison of reactivity between
isoform c (amino acids 2-302) and isoform a (amino acids 1-411) of
KIAA0513 as antigens for evaluation of serum antibody levels. The
s-KIAA0513-Ab levels of HDs and patients with AIS or CVD were
examined by AlphaLISA using GST-KIAA05132-302 (A) and
GST-KIAA05131-411 (B) proteins as the antigens. A scatter dot plot
of the antibody levels is shown. Results are presented as described
in the legend of Fig. 2.
***P<0.001 vs. HD specimens. The bars represent the
average ± SD. (C) Correlation plot of antibody levels against
KIAA0513 isoform a vs. isoform c.
ProtoArray® screening for
autoantibodies in atherosclerosis.
Comparison of the serum antibody
levels of HDs vs. those of patients with AIS or TIA.
Comparison of the serum antibody
levels of HDs vs. those of patients with DM.
Comparison of the serum antibody
levels of HDs vs. those of patients with CVD or OSAS.
Comparison of s-KIAA0513-Ab levels of
HDs vs. those of patients with CKD.
Comparison of s-KIAA0513-Ab levels
between HDs and patients with cancer.
Information of subjects in the Sawara
Hospital cohort used for correlation analysis.
Correlation analysis between serum
KIAA0513-Ab levels and the data of CKD cohort.
Acknowledgements
The authors would like to thank Professor Masaki
Takiguchi (Chiba University), Professor Hao Wang (Jinan
University), Professor Kenichiro Kitamura (Yamanashi University),
Dr Xiao-Meng Zhang (Chiba University), Dr Kazushige Katsura
(RIKEN), Dr Hideyuki Kuroda (Fujikura Kasei Co.), Dr Rika Nakamura
(Fujikura Kasei Co.), Dr Natsuko Shinmen (Fujikura Kasei, Co.) and
Dr Noboru Ohsawa (RIKEN) for supporting this research, as well as
Ms. Seiko Otsuka, Masae Suzuki, Chiho Kusaka, Satoko Ishibashi,
Chiemi Mishima-Tsumagari, Risa Kimura, Akiko Kimura, Ryo Fukushima,
Yuko Ohta, and Aki Furuya for providing technical assistance.
Funding
Funding: The present study was supported, in part, by research
grants from the Japan Science and Technology Agency (JST:
Exploratory Research No. 14657335), and JSPS KAKENHI Grant no.
20K17953, 22K07273, 20K07810, 21K19437 and 21K08695. The Japan
Public Health Center-based Prospective Study was supported by
National Cancer Center Research and Development Fund (since 2011)
and a Grant-in-Aid for Cancer Research from the Ministry of Health,
Labour and Welfare of Japan (from 1989 to 2010).
Availability of data and materials
All data of the ProtoArray v4.0 human protein
microarray system are available in the public Figshare database
(https://figshare.com/articles/dataset/Results_of_protein_array_for_atherosclerosis/25906330).
The other datasets used and/or analyzed during the current study
are available from the corresponding author on reasonable
request.
Authors' contributions
TH, MT, KYo, KM, KT, HS and YH conceived and
designed the study. YY, MI, SYo, MT, YK and HT collected the blood
samples and the clinicopathological data. MK, SYL, BSZ and GT
performed the experiments and acquired the data. SM, TMac, MSh, SYa
and GT contributed the reagents, materials, analysis tools or
patient data. BSZ, TMat, TMac, MSa and HI analyzed and interpreted
the data. MK, SYL, MI, MSa, KYa, NS, ST and AH performed the
statistical analyses. TH, YY, KYa, NS and MSh drafted the
manuscript. TH, TMatsutani, HI and ST confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki and approved by the Local
Ethical Review Board of the Chiba University, Graduate School of
Medicine (Chiba, Japan), as well as by the review boards of the
participating hospitals (approval no. 2018-320). The Ethics
Committee of Toho University, Graduate School of Medicine (No.
A18103_A17052_A16035_A16001_26095_25024_24038_22047_22047) and Port
Square Kashiwado Clinic, Kashiwado Memorial Foundation (approval
no. 2012-001) also approved the study protocol. Sera were collected
from patients who had provided written informed consent. Serum
samples were collected from patients who had provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The present study was performed in collaboration
with Fujikura Kasei Co., Ltd. GT is an employee of Fujikura Kasei
Co., Ltd. The other authors have no competing interests.
References
|
1
|
Goo YA and Goodlett DR: Advances in
proteomic prostate cancer biomarker discovery. J Proteomics.
73:1839–1850. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Imafuku Y, Omenn GS and Hanash S:
Proteomics approaches to identify tumor antigen directed
autoantibodies as cancer biomarkers. Dis Markers. 20:149–153.
2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ho PTB, Clark IM and Le LTT:
MicroRNA-based diagnosis and therapy. Int J Mol Sci.
23(7167)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kramer J, Harcos P, Prohászka Z, Horváth
L, Karádi I, Singh M, Császár A, Romics L and Füst G: Frequencies
of certain complement protein alleles and serum levels of
anti-heat-shock protein antibodies in cerebrovascular diseases.
Stroke. 31:2648–2652. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Machida T, Kubota M, Kobayashi E, Iwadate
Y, Saeki N, Yamaura A, Nomura F, Takiguchi M and Hiwasa T:
Identification of stroke-associated-antigens via screening of
recombinant proteins from the human expression cDNA library
(SEREX). J Translat Med. 13(71)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yoshida Y, Wang H, Hiwasa T, Machida T,
Kobayashi E, Mine S, Tomiyoshi G, Nakamura R, Shinmen N, Kuroda H,
et al: Elevation of autoantibody level against PDCD11 in patients
with transient ischemic attack. Oncotarget. 9:8836–8848.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang H, Zhang XM, Tomiyoshi G, Nakamura R,
Shinmen N, Kuroda H, Kimura R, Mine S, Kamitsukasa I, Wada T, et
al: Association of serum levels of antibodies against MMP1, CBX1,
and CBX5 with transient ischemic attack and cerebral infarction.
Oncotarget. 9:5600–5613. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yoshida Y, Zhang XM, Wang H, Machida T,
Mine S, Kobayashi E, Adachi A, Matsutani T, Kamitsukasa I, Wada T,
et al: Elevated levels of autoantibodies against DNAJC2 in sera of
patients with atherosclerotic diseases. Heliyon.
6(e04661)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li SY, Yoshida Y, Kobayashi E, Kubota M,
Matsutani T, Mine S, Machida T, Maezawa Y, Takemoto M, Yokote K, et
al: Serum anti-AP3D1 antibodies are risk factors for acute ischemic
stroke related with atherosclerosis. Sci Rep.
11(13450)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kubota M, Yoshida Y, Kobayashi E,
Matsutani T, Li SY, Zhang BS, Mine S, Machida T, Takizawa H, Hiwasa
T and Iwadate Y: Serum anti-SERPINE1 antibody as a potential
biomarker of acute cerebral infarction. Sci Rep.
11(21772)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hiwasa T, Wang H, Goto KI, Mine S, Machida
T, Kobayashi E, Yoshida Y, Adachi A, Matsutani T, Sata M, et al:
Serum anti-DIDO1, anti-CPSF2, and anti-FOXJ2 antibodies as
predictive risk markers for acute ischemic stroke. BMC Med.
19(131)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kubota M, Zhang BS, Li SY, Yoshida Y, Wang
H, Adachi A, Matsutani T, Mine S, Machida T, Kamitsukasa I, et al:
Serum anti-TSTD2 antibody as a biomarker for
atherosclerosis-induced ischemic stroke and chronic kidney disease.
Med Int (Lond). 3(4)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hiwasa T, Machida T, Zhang XM, Kimura R,
Wang H, Iwase K, Ashino H, Taira A, Arita E, Mine S, et al:
Elevated levels of autoantibodies against ATP2B4 and BMP-1 in sera
of patients with atherosclerosis-related diseases. Immunome Res.
11(097)2015.
|
|
14
|
Nakamura R, Tomiyoshi G, Shinmen N, Kuroda
H, Kudo T, Doi H, Mine S, Machida T, Kamitsukasa I, Wada T, et al:
An anti-deoxyhypusine synthase antibody as a marker of
atherosclerosis-related cerebral infarction, myocardial infarction,
diabetes mellitus, and chronic kidney disease. SM Atheroscler J.
1(1001)2017.
|
|
15
|
Hiwasa T, Tomiyoshi G, Nakamura R, Shinmen
N, Kuroda H, Kunimatsu M, Mine S, Machida T, Sato E, Takemoto M, et
al: Serum SH3BP5-specific antibody level is a biomarker of
atherosclerosis. Immunome Res. 13(132)2017.
|
|
16
|
Zhang XM, Wang H, Mine S, Takemoto M,
Yokote K, Kitamura K, Kobayashi Y, Machida T, Kobayashi E, Yoshida
Y, et al: Association of serum anti-prolylcarboxypeptidase antibody
marker with atherosclerotic diseases accompanied by hypertension. J
Mol Biomark Diagn. 8(361)2017.
|
|
17
|
Sumazaki M, Shimada H, Ito M, Shiratori F,
Kobayashi E, Yoshida Y, Adachi A, Matsutani T, Iwadate Y, Mine S,
et al: Serum anti-LRPAP1 is a common biomarker for digestive organ
cancers and atherosclerotic diseases. Cancer Sci. 111:4453–4464.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li SY, Yoshida Y, Kobayashi E, Adachi A,
Hirono S, Matsutani T, Mine S, Machida T, Ohno M, Nishi E, et al:
Association between serum anti-ASXL2 antibody levels and acute
ischemic stroke, acute myocardial infarction, diabetes mellitus,
chronic kidney disease and digestive organ cancer, and their
possible association with atherosclerosis and hypertension. Int J
Mol Med. 46:1274–1288. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen PM, Ohno M, Hiwasa T, Nishi K, Saijo
S, Sakamoto J, Morita Y, Matsuda S, Watanabe S, Kuwabara Y, et al:
Nardilysin is a promising biomarker for the early diagnosis of
acute coronary syndrome. Int J Cardiol. 243:1–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Palmer JP, Asplin CM, Clemons P, Lyen K,
Tatpati O, Raghu PK and Paquette TL: Insulin antibodies in
insulin-dependent diabetics before insulin treatment. Science.
222:1337–1339. 1983.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Baekkeskov S, Aanstoot HJ, Christgau S,
Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H and De
Camilli P: Identification of the 64K autoantigen in insulin
dependent diabetes as the GABA-synthesizing enzyme glutamic acid
decarboxylase. Nature. 347:151–156. 1990.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hiwasa T, Zhang XM, Kimura R, Ohno M, Chen
PM, Nishi E, Ono K, Kimura T, Kamitsukasa I, Wada T, et al:
Elevated adiponectin antibody levels in sera of patients with
atherosclerosis-related coronary artery disease, cerebral
infarction, and diabetes mellitus. J Circ Biomark.
5(8)2016.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Sugimoto K, Tomiyoshi G, Mori M, Kuwabara
S, Hirano S, Sawai S, Beppu M, Muto M, Uzawa A, Kitamura K, et al:
Identification of serum anti-GADD34 antibody as a common marker of
diabetes mellitus and parkinson disease. J Alzheimers Dis
Parkinsonism. 7(358)2017.
|
|
24
|
Tomiyoshi G, Nakamura R, Shinmen N,
Yoshida Y, Mine S, Machida T, Iwase K, Iwadate Y, Hiwasa T and
Kuroda H: GADD34 activates p53 and may have utility as a marker of
atherosclerosis. Front Med (Lausanne). 10(1128921)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yamagata H, Hayashi A, Yoshida Y,
Koshizaka M, Onishi S, Yoshida T, Hiwasa T and Takemoto M:
Association of high proprotein convertase subtilisin/kexin type 9
antibody level with poor prognosis in patients with diabetes: A
prospective study. Sci Rep. 13(5391)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shimada H, Takeda A, Arima M, Okazumi S,
Matsubara H, Nabeya Y, Funami Y, Hayashi H, Gunji Y, Suzuki T, et
al: Serum p53 antibody is a useful tumor marker in superficial
esophageal squamous cell carcinoma. Cancer. 89:1677–1683.
2000.PubMed/NCBI
|
|
27
|
Shimada H, Ochiai T and Nomura F: Japan
p53 Antibody Research Group. Titration of serum p53 antibodies in
1,085 patients with various types of malignant tumors: A
multiinstitutional analysis by the Japan p53 antibody research
group. Cancer. 97:682–689. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nakashima K, Shimada H, Ochiai T,
Kuboshima M, Kuroiwa N, Okazumi S, Matsubara H, Nomura F, Takiguchi
M and Hiwasa T: Serological identification of TROP2 by recombinant
cDNA expression cloning using sera of patients with esophageal
squamous cell carcinoma. Int J Cancer. 112:1029–1035.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kuboshima M, Shimada H, Liu TL, Nakashima
K, Nomura F, Takiguchi M, Hiwasa T and Ochiai T: Identification of
a novel SEREX antigen, SLC2A1/GLUT1, in esophageal squamous cell
carcinoma. Int J Oncol. 28:463–468. 2006.PubMed/NCBI
|
|
30
|
Kuboshima M, Shimada H, Liu TL, Nomura F,
Takiguchi M, Hiwasa T and Ochiai T: Presence of serum tripartite
motif-containing 21 antibodies in patients with esophageal squamous
cell carcinoma. Cancer Sci. 97:380–386. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shimada H, Kuboshima M, Shiratori T,
Nabeya Y, Takeuchi A, Takagi H, Nomura F, Takiguchi M, Ochiai T and
Hiwasa T: Serum anti-myomegalin antibodies in patients with
esophageal squamous cell carcinoma. Int J Oncol. 30:97–103.
2007.PubMed/NCBI
|
|
32
|
Shimada H, Shiratori T, Yasuraok M, Kagaya
A, Kuboshima M, Nomura F, Takiguchi M, Ochiai T, Matsubara H and
Hiwasa T: Identification of makorin 1 as a novel SEREX antigen of
esophageal squamous cell carcinoma. BMC Cancer.
9(232)2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kagaya A, Shimada H, Shiratori T,
Kuboshima M, Nakashima-Fujita K, Yasuraoka M, Nishimori T, Kurei S,
Hachiya T, Murakami A, et al: Identification of a novel SEREX
antigen family, ECSA, in esophageal squamous cell carcinoma.
Proteome Sci. 9(31)2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shimada H, Ito M, Kagaya A, Shiratori T,
Kuboshima M, Suzuki M, Liu TL, Nabeya Y, Matsubara H, Matsushita K,
et al: Elevated serum antibody levels against cyclin L2 in patients
with esophageal squamous cell carcinoma. J Cancer Sci Ther.
7:60–66. 2015.
|
|
35
|
Ito M, Hiwasa T, Yajima S, Suzuki T,
Oshima Y, Nanami T, Sumazaki M, Shiratori F, Li SY, Iwadate Y, et
al: Low anti-CFL1 antibody with high anti-ACTB antibody is a poor
prognostic factor in esophageal squamous cell carcinoma. Esophagus.
19:617–625. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ito M, Yajima S, Suzuki T, Oshima Y,
Nanami T, Sumazaki M, Shiratori F, Wang H, Hu L, Takizawa H, et al:
The combination of positive anti-WDR1 antibodies with negative
anti-CFL1 antibodies is a poor prognostic factor for patients with
esophageal carcinoma. Med Int (Lond). 3(11)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kobayashi S, Hoshino T, Hiwasa T, Satoh M,
Rahmutulla B, Tsuchida S, Komukai Y, Tanaka T, Matsubara H, Shimada
H, et al: Anti-FIRs (PUF60) auto-antibodies are detected in the
sera of early-stage colon cancer patients. Oncotarget.
7:82493–82503. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kobayashi S, Hiwasa T, Ishige T,
Rahmutulla B, Kano M, Hoshino T, Minamoto T, Shimada H, Nomura F,
Matsubara H and Matsushita K: Anti-FIRΔexon2, a splicing variant
form of PUF60, auto-antibody is detected in the sera of esophageal
squamous cell carcinoma. Cancer Sci. 110:2004–2013. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Matsutani T, Hiwasa T, Takiguchi M, Oide
T, Kunimatsu M, Saeki N and Iwadate Y: Autologous antibody to
src-homology 3-domain GRB2-like 1 specifically increases in the
sera of patients with low-grade gliomas. J Exp Clin Cancer Res.
31(85)2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Adachi-Hayama M, Adachi A, Shinozaki N,
Matsutani T, Hiwasa T, Takiguchi M, Saeki N and Iwadate Y:
Circulating anti-filamin C antibody as a potential serum biomarker
for low-grade gliomas. BMC Cancer. 14(452)2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hontani K, Tsuchikawa T, Hiwasa T,
Nakamura T, Ueno T, Kushibiki T, Takahashi M, Inoko K, Takano H,
Takeuchi S, et al: Identification of novel serum autoantibodies
against EID3 in non-functional pancreatic neuroendocrine tumors.
Oncotarget. 8:106206–106221. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Takahashi M, Tsuchikawa T, Hiwasa T,
Nakamura T, Hontani K, Kushibiki T, Inoko K, Takano H, Hatanaka Y,
Matsushita K, et al: Identification of antibody against
wingless-type MMTV integration site family member 7B as a biliary
cancer tumor marker. Oncol Rep. 49(34)2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Matsumura T, Terada J, Kinoshita T,
Sakurai Y, Yahaba M, Ema R, Amata A, Sakao S, Nagashima K, Tatsumi
K and Hiwasa T: Circulating anti-coatomer protein complex subunit
epsilon (COPE) autoantibodies as a potential biomarker for cardio-
and cerebro-vascular events in patients with obstructive sleep
apnea. J Clin Sleep Med. 13:393–400. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Matsumura T, Terada J, Kinoshita T,
Sakurai Y, Yahaba M, Tsushima K, Sakao S, Nagashima K, Iwata Y,
Ozaki T, et al: Autoantibody against NBL1 in obstructive sleep
apnea patients with cardiovascular disease. PLoS One.
13(e0195015)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Katsumata Y, Terada J, Matsumura T,
Koshikawa K, Sakao S, Tomiyoshi G, Shinmen N, Nakamura R, Kuroda H,
Nagashima K, et al: Circulating anti-sorting nexins 16 antibodies
as an emerging biomarker of coronary artery disease in patients
with obstructive sleep apnea. Diagnostics (Basel).
10(71)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Adams HP Jr, Bendixen BH, Kappelle LJ,
Biller J, Love BB, Gordon DL and Marsh EE III: Classification of
subtype of acute ischemic stroke. Definitions for use in a
multicenter clinical trial. TOAST. Trial of Org 10172 in acute
stroke treatment. Stroke. 24:35–41. 1993.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nishiura R, Fujimoto S, Sato Y, Yamada K,
Hisanaga S, Hara S, Nakao H and Kitamura K: Elevated
osteoprotegerin levels predict cardiovascular events in new
hemodialysis patients. Am J Nephrol. 29:257–263. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Komatsu H, Fujimoto S, Hara S, Fukuda A,
Fukudome K, Yamada K, Sato Y and Kitamura K: Recent therapeutic
strategies improve renal outcome in patients with IgA nephropathy.
Am J Nephrol. 30:19–25. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chumpolkulwong N, Sakamoto K, Hayashi A,
Iraha F, Shinya N, Matsuda N, Kiga D, Urushibata A, Shirouzu M, Oki
K, et al: Translation of ‘rare’ codons in a cell-free protein
synthesis system from escherichia coli. J Struct Funct Genomics.
7:31–36. 2006.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Tsugane S and Sawada N: The JPHC study:
Design and some findings on the typical Japanese diet. Jpn J Clin
Oncol. 44:777–782. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ikeda A, Iso H, Sasazuki S, Inoue M and
Tsugane S: JPHC Study Group. The combination of Helicobacter
pylori- and cytotoxin-associated gene-A seropositivity in relation
to the risk of myocardial infarction in middle-aged Japanese: The
Japan public health center-based study. Atherosclerosis. 230:67–72.
2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Iso H, Noda H, Ikeda A, Yamagishi K, Inoue
M, Iwasaki M and Tsugane S: The impact of C-reactive protein on
risk of stroke, stroke subtypes, and ischemic heart disease in
middle-aged Japanese: The Japan public health center-based study. J
Atheroscler Thromb. 19:756–766. 2012.PubMed/NCBI
|
|
53
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lauriat TL, Dracheva S, Kremerskothen J,
Duning K, Haroutunian V, Buxbaum JD, Hyde TM, Kleinman JE and
McInnes LA: Characterization of KIAA0513, a novel signaling
molecule that interacts with modulators of neuroplasticity,
apoptosis, and the cytoskeleton. Brain Res. 1121:1–11.
2006.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Tran LT, Park HJ and Kim HD: Is the
carotid intima-media thickness really a good surrogate marker of
atherosclerosis? J Atheroscler Thromb. 19:680–690. 2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zureik M, Ducimetière P, Touboul PJ,
Courbon D, Bonithon-Kopp C, Berr C and Magne C: Common carotid
intima-media thickness predicts occurrence of carotid
atherosclerotic plaques: Longitudinal results from the aging
vascular study (EVA) study. Arterioscler Thromb Vasc Biol.
20:1622–1629. 2000.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Shirai K, Utino J, Otsuka K and Takata M:
A novel blood pressure-independent arterial wall stiffness
parameter; cardio-ankle vascular index (CAVI). J Atheroscler
Thromb. 13:101–107. 2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Floras JS: Hypertension and sleep apnea.
Can J Cardiol. 31:889–897. 2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Gallagher EJ and LeRoith D: Obesity and
diabetes: The increased risk of cancer and cancer-related
mortality. Physiol Rev. 95:727–748. 2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Goto A, Yamaji T, Sawada N, Momozawa Y,
Kamatani Y, Kubo M, Shimazu T, Inoue M, Noda M, Tsugane S and
Iwasaki M: Diabetes and cancer risk: A mendelian randomization
study. Int J Cancer. 146:712–719. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Tapia-Vieyra JV, Delgado-Coello B and
Mas-Oliva J: Atherosclerosis and cancer; A resemblance with
far-reaching implications. Arch Med Res. 48:12–26. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Makrilia N, Lappa T, Xyla V, Nikolaidis I
and Syrigos K: The role of angiogenesis in solid tumours: An
overview. Eur J Intern Med. 20:663–671. 2009.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Jarvandi S, Davidson NO and Schootman M:
Increased risk of colorectal cancer in type 2 diabetes is
independent of diet quality. PLoS One. 8(e74616)2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Fujihara S, Kato K, Morishita A, Iwama H,
Nishioka T, Chiyo T, Nishiyama N, Miyoshi H, Kobayashi M, Kobara H,
et al: Antidiabetic drug metformin inhibits esophageal
adenocarcinoma cell proliferation in vitro and in
vivo. Int J Oncol. 46:2172–2180. 2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Berger NA: Young adult cancer: Influence
of the obesity pandemic. Obesity (Silver Spring). 26:641–650.
2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Hiwasa T and Shimada H: Autoantibody in
cancer. In: Biomarkers in Cancer Therapy. Shimada H (ed). Springer
Nature, Singapore, 25-40, 2018.
|
|
67
|
Zhou Y, Yang Q, Chi J, Dong B, Lv W, Shen
L and Wang Y: Comorbidities and the risk of severe or fatal
outcomes associated with coronavirus disease 2019: A systematic
review and meta-analysis. Int J Infect Dis. 99:47–56.
2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wu T, Zuo Z, Kang S, Jiang L, Luo X, Xia
Z, Liu J, Xiao X, Ye M and Deng M: Multi-organ dysfunction in
patients with COVID-19: A systematic review and meta-analysis.
Aging Dis. 11:874–894. 2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Ye Q, Lu D, Shang S, Fu J, Gong F, Shu Q
and Mao J: Crosstalk between coronavirus disease 2019 and
cardiovascular disease and its treatment. ESC Heart Fail.
7:3464–3472. 2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Maas MB, Kim M, Malkani RG, Abbott SM and
Zee PC: Obstructive sleep apnea and risk of COVID-19 infection,
hospitalization and respiratory failure. Sleep Breath.
25:1155–1157. 2021.PubMed/NCBI View Article : Google Scholar
|