Introduction
Cold agglutinin disease (CAD) is a rare form of cold
autoimmune hemolytic anemia. Also referred to as primary CAD, this
chronic condition is characterized by complement-mediated hemolysis
and non-progressive, clinically non-malignant B cell proliferation.
CAD should be considered in the differential diagnosis of patients
presenting with unexplained anemia, with or without associated
cold-related symptoms, such as acrocyanosis, livedo reticularis,
Raynaud's phenomenon and fingertip ulceration. Notably, nearly all
cold agglutinins test positive for the C3d direct antiglobulin test
(1).
Cold autoimmune hemolytic anemia (AIHA) can be
classified into primary CAD and secondary cold agglutinin syndrome
(CAS), with the latter often triggered by underlying conditions,
such as infections (e.g., mycoplasma pneumoniae, Epstein-Barr virus
or influenza A), malignancies (e.g., diffuse large B-cell lymphoma,
Hodgkin lymphoma or metastatic melanoma) and autoimmune diseases
(e.g., systemic lupus erythematosus). In the differential diagnosis
of cold AIHA, other causes of hemolysis should also be considered,
including warm AIHA, hereditary hemolytic anemias (e.g., hereditary
spherocytosis or G6PD deficiency), paroxysmal cold hemoglobinuria
and drug-induced hemolysis. Cold-induced hemolysis can also occur
in cases of hypothermia or mechanical damage to red blood cells
(RBCs), although these do not typically result in cold
agglutination. The diagnosis of cold AIHA involves clinical
evaluation, testing for cold agglutinins, direct antiglobulin tests
and the careful exclusion of other potential causes of hemolysis,
such as infections, malignancies, or hereditary conditions
(2).
For the majority of patients without severe
symptoms, a strategy of watchful waiting is appropriate.
Conventional treatment options, such as corticosteroids,
azathioprine, or cyclophosphamide which are typically effective in
warm antibody-mediated AIHA, are ineffective for CAD. Moreover, a
splenectomy is not beneficial in CAD, as hemolysis predominantly
occurs in the liver. Rituximab monotherapy is considered the
first-line treatment for CAD. Other treatment options include the
use of bendamustine, combinations of rituximab and bendamustine,
ibrutinib, acalabrutinib, venetoclax, eculizumab and sutimlimab
(3,4). Currently, to the best of our knowledge,
there is no specific treatment option available for secondary CAS
other than addressing the underlying disease.
The present study describes the case of a male
patient who sought a second opinion at the authors' clinic due to
newly detected anemia.
Case report
A 53-year-old male patient presented to the
Department of Hematology, Dokuz Eylul University Hospital, Izmir,
Turkey, seeking a second opinion regarding anemia newly detected
during routine check-ups. The patient, who reported no symptoms,
had a medical history of diabetes, hypertension and inactive
chronic hepatitis B virus (HBV) infection for the past 10 years. He
was being treated with metformin, losartan and amlodipine. His
family history did not reveal any significant findings. The patient
had been a smoker for >20 years and consumed alcohol
socially.
A physical examination revealed no abnormalities.
Prior to his visit to the Department of Hematology, Dokuz Eylul
University Hospital, two separate complete blood count (CBC)
analyses were conducted at two different hospitals the previous
week, yielding conflicting results: One report indicated normal
findings, while the other indicated macrocytic anemia.
A CBC, hemolytic parameters (including lactate
dehydrogenase, bilirubin, haptoglobin and reticulocyte count), and
a peripheral blood smear were performed. Routine blood tests,
including liver and kidney function tests, yielded results which
were within normal range. The initial CBC results indicated an
elevated mean corpuscular volume and mean corpuscular hemoglobin
concentration, alongside a decreased RBC count and hematocrit,
while hemoglobin levels remained within the normal range (Table I). The peripheral blood smear
revealed agglutination of the RBCs. Other tests, including
hemolytic parameters, yielded results which were within normal
limits.
 | Table IComplete blood count of unheated and
heated blood samples. |
Table I
Complete blood count of unheated and
heated blood samples.
| Test/parameter | Unheated sample | Heated sample | Reference range | Units |
|---|
| WBC | 6.6 | 6.2 | 4-10.3 | 10³/µl |
| Neutrophils | 5.7 | 4.6 | 2.1-6.1 | 10³/µl |
| Lymphocytes | 0.4 | 1 | 1.3-3.5 | 10³/µl |
| Monocytes | 0.5 | 0.5 | 0.3-0.9 | 10³/µl |
| Eosinophils | 0 | 0.1 | 0-0.5 | 10³/µl |
| Basophils | 0 | 0 | 0-0.2 | 10³/µl |
| RBC | 1.72 | 4.73 | 4-5.7 |
106/µl |
| Hemoglobin | 14.2 | 14.5 | 12-16 | g/dl |
| Hematocrit | 19.1 | 41.9 | 36-46 | % |
| MCV | 110.8 | 88.6 | 80.7-95.5 | fl |
| MCH | 82.7 | 30.7 | 27.2-33.5 | pg |
| MCHC | 74.6 | 34.7 | 32.7-35.6 | g/dl |
| RDW | 14.5 | 13.6 | 11.8-14.3 | % |
| Platelets | 192 | 289 | 156-373 | 10³/µl |
| MPV | 9.9 | 8.4 | 6.9-10.8 | fl |
| Plateletcrit | 0.189 | 0.249 | 0.20-0.36 | % |
Further analyses revealed negative cryoglobulin and
mycoplasma antibody test results, normal blood
immunoelectrophoresis, a positive direct Coombs test (C3d), and a
positive cold agglutinin titer of 1:256 at 4˚C, leading to a
definitive diagnosis of cold AIHA. A second CBC and peripheral
blood smear conducted with warmed blood samples yielded normal CBC
results (Table I) and the
disappearance of RBC agglutination.
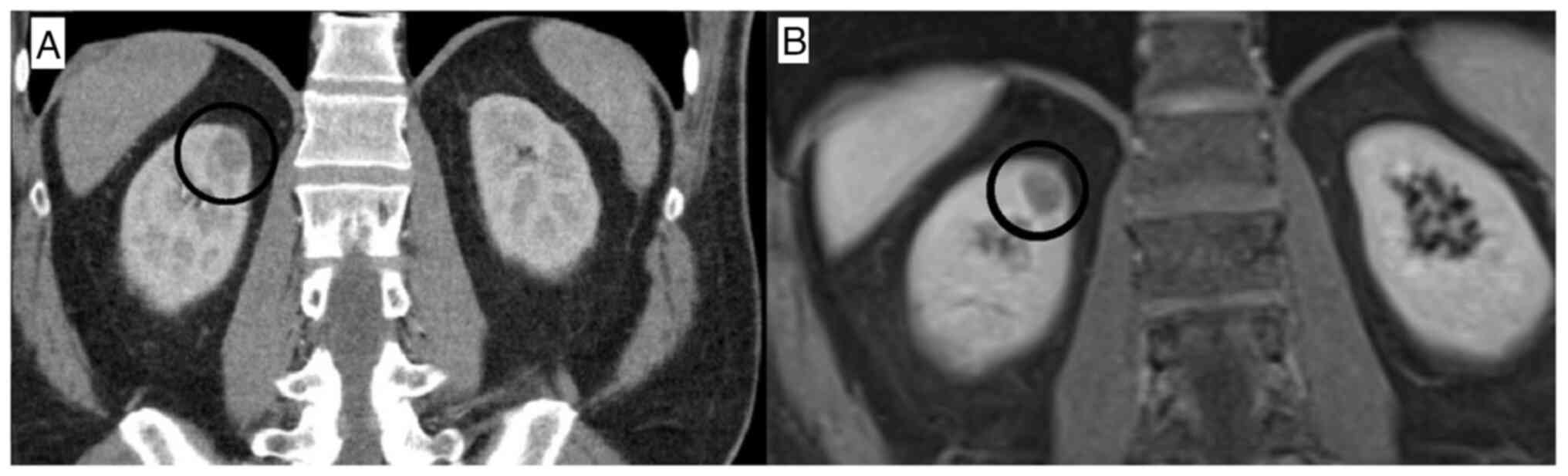
A whole-body computed tomography (CT) scan revealed
a mass 2 cm in size in the right kidney (Fig. 1A). Magnetic resonance imaging of the
mass suggested renal cell carcinoma (RCC) (Fig. 1B). Subsequently, the patient
underwent partial nephrectomy, and a pathological examination
confirmed the diagnosis of RCC. No other abnormalities were noted
on cross-sectional imaging. Notably, no secondary cause of cold
AIHA was identified. A bone marrow biopsy was performed, revealing
no malignancy or evidence of cold agglutinin-associated
lymphoproliferative bone marrow disease.
The patient was routinely monitored, and the CBC
remained consistent with the initial findings. The persistent
agglutination of RBCs was noted in the peripheral blood smear, with
no notable hemolysis observed during follow-up.
At 2 years following his initial visit, the patient
presented to the hematology department with complaints of a growing
mass in the groin. He did not report any symptoms of B-cell
lymphoma, such as unexplained weight loss, night sweats, or fever.
The initial examination revealed hepatosplenomegaly and a bilateral
enlargement of the inguinal lymph nodes. A CBC indicated
lymphopenia and marked anemia. Similar to the first presentation,
the agglutination of erythrocytes was observed in the peripheral
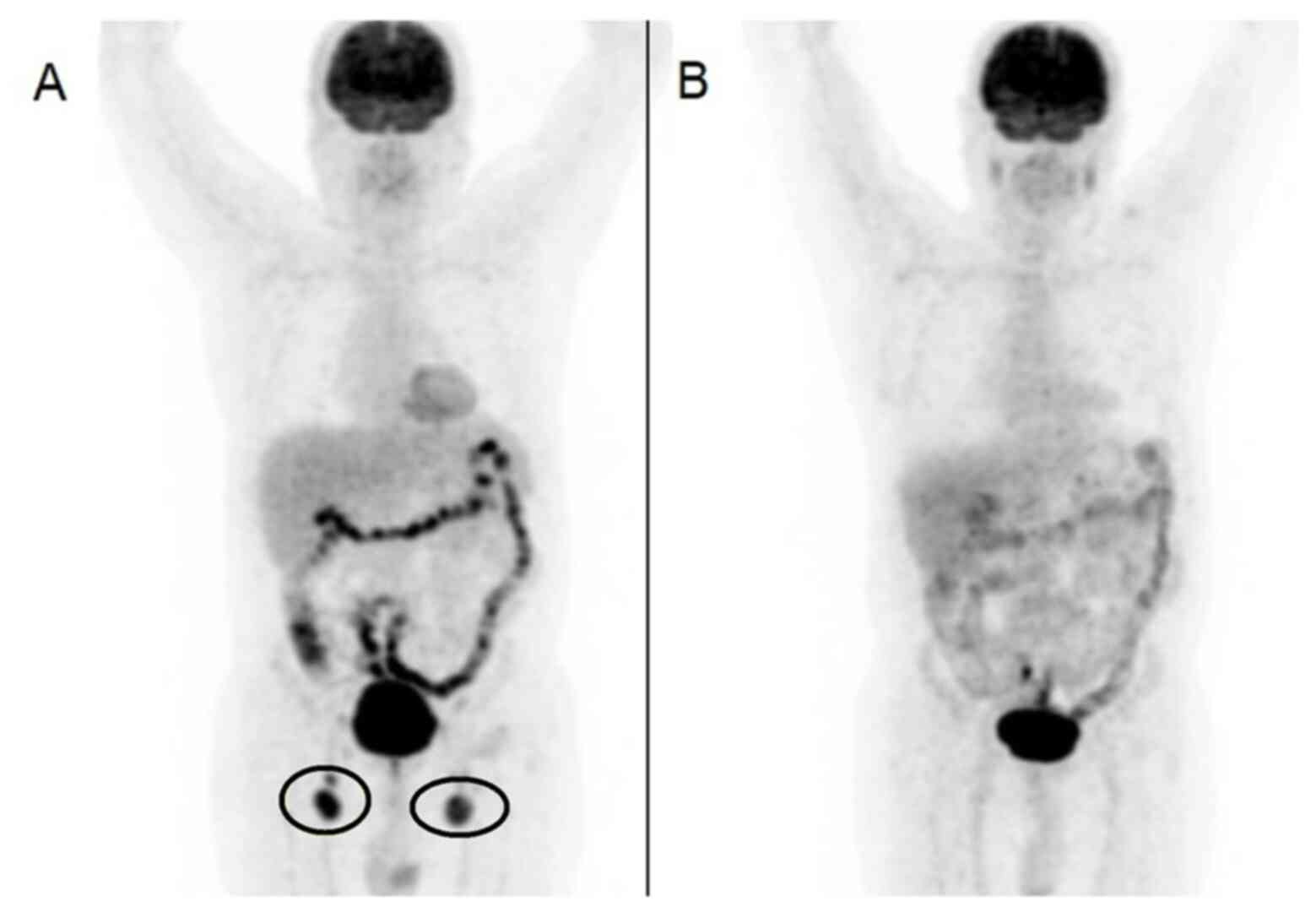
blood smear. A fludeoxyglucose-18 (FDG) positron emission
tomography (PET)CT scan was requested, and the results were
consistent with lymphoma (Fig. 2A).
A biopsy was performed on the right inguinal lymph node, and
histological examination revealed low-grade B-cell lymphoma. A
repeat bone marrow biopsy was conducted, and histological analysis
reported hypercellular bone marrow without evidence of clonal
disease, lymphoma infiltration, or cold agglutinin-associated
lymphoproliferative bone marrow disease.
Routine laboratory examinations prior to initiating
chemotherapy did not detect any abnormal findings, including
aspartate aminotransferase, alanine aminotransferase, kidney
function tests and liver function tests. Moreover, it is standard
procedure in Turkey to screen for HBV infection prior to
chemotherapy: HBsAg was positive as was expected, and HBV-DNA was
positive, although at an undetectable level. Therefore, tenofovir
alafenamide was initiated as HBV reactivation prophylaxis. No HBV
reactivation was detected before or after chemotherapy.
The patient was subsequently initiated on R-CHOP
therapy (cycle repeats every 21 days), consisting of rituximab (375
mg/m2 intravenously on day 1), cyclophosphamide (750
mg/m2 intravenously on day 1), doxorubicin (50
mg/m2 intravenously on day 1), vincristine (1.4
mg/m2 intravenously on day 1) and prednisone (40
mg/m2 perorally on every day of first week in every
cycle). A second FDG PET/CT was ordered, and the patient exhibited
a complete response after two cycles of R-CHOP treatment (Fig. 2B). It was decided to complete a total
of four cycles of R-CHOP. Following this regimen, the patient began
rituximab maintenance therapy (375 mg/m2 intravenously
every 6 months). Peripheral blood smears were performed after the
second and fourth cycles of chemotherapy, as well as at the 6-month
mark following the completion of chemotherapy. Post-treatment, the
CBC and peripheral blood smear returned to normal limits, and the
erythrocyte agglutinations previously observed in the peripheral
blood smear conducted with unheated blood were no longer
present.
Discussion
In the case described in the present study, there
was no true anemia or associated symptoms at the initial
presentation; however, anemia is the most prevalent finding in cold
AIHA, occurring in 51% of patients at diagnosis (5). The first indication of CAS was the
discrepancy observed between two CBCs performed prior to the
admission of the patients to hospital. Subsequent re-examinations
using heated blood samples revealed the disappearance of
erythrocyte agglutination in the peripheral blood smear, alongside
a return to normal CBC values, which were crucial for the diagnosis
of CAS in this instance. The findings on the CBC were
contradictory, as the degree of RBC agglutination is dependent on
the environmental temperature and the duration of exposure. Various
factors, including the time from obtaining the blood sample to
receiving the laboratory results, could affect the impact of
environmental temperature on the degree of agglutination.
Terán Brage et al (6) presented an intriguing case of CAS in a
patient with renal carcinoma; a male in his 60s was diagnosed with
paraneoplastic CAS, while being evaluated for microcytic anemia
with evident hemolysis. In the case described herein, RCC was
incidentally identified during the investigation of secondary
causes, and while the tumor was excised, the findings of CAS
persisted, suggesting no clear association between RCC and CAS in
this instance. Notably, the patient in the present study exhibited
no signs of anemia or hemolysis, and the initial CBC erroneously
indicated macrocytic anemia due to erythrocyte agglutination.
Furthermore, cold AIHA may also manifest in renal findings;
Taberner et al (7) reported a
case of renal hemosiderosis associated with CAD.
Additionally, there are case reports linking CAS to
lymphoma (8,9). In the present case report, CAS was
identified during the evaluation of conflicting CBC results; the
patient remained asymptomatic, with no true anemia or clear
hemolytic signs. While other case reports have diagnosed lymphoma
during the investigation of CAS, the patient in the present study
was diagnosed with lymphoma 2 years after CAS was detected
(8-10).
CAS may serve as an early indicator of lymphoma, as
supported by previous case reports in the literature (10,11).
Following R-CHOP treatment, both the peripheral smear and CBC
returned to normal limits, leading the authors to conclude that the
cold-type AIHA of the patient was attributable to lymphoma. It can
thus be hypothesized that CAS was the earliest manifestation of the
lymphoma in the patient described herein.
It may be suspected that chronic HBV infection was a
risk factor for the patient developing non-Hodgkin lymphoma (NHL).
There is an established link between chronic HBV infection and NHL.
Furthermore, the risk is higher in Asian populations, such as in
Turkey (12).
In conclusion, it is imperative to investigate the
underlying causes in patients newly diagnosed with CAD. Cold AIHA
may serve as an early indicator of lymphoma that can manifest years
prior, necessitating routine monitoring for these patients. In the
case that hemoglobin levels are stable in the CBC, and significant
fluctuations are observed in the erythrocyte count, hematocrit,
mean corpuscular volume and mean corpuscular hemoglobin
concentration, particularly if they do not match the clinical
presentation of the patient, then CAD should be considered.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HD was a major contributor to the writing of the
manuscript, and both authors (HD and GHÖ) commented on subsequent
versions. Both authors (HD and GHÖ) contributed to data collection,
treatment and patient follow-up. HD and GHÖ confirm the
authenticity of all the raw data. Both authors (HD and GHÖ) have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study followed international and
national regulations and was in agreement with the Declaration of
Helsinki, and ethical principles. Dokuz Eylul University Hospital
does not require ethical approval for reporting individual cases or
case series. Written informed consent was obtained from the patient
for his anonymized information to be published in this article.
Patient consent for publication
Written informed consent was obtained from the
patient for his anonymized information and any related images to be
published in this article.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Gabbard AP and Booth GS: Cold agglutinin
disease. Clin Hematol Int. 2:95–100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Berentsen S: Cold agglutinin disease.
Hematology Am Soc Hematol Educ Program. 2016:226–231.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jäger U, Barcellini W, Broome CM, Gertz
MA, Hill A, Hill QA, Jilma B, Kuter DJ, Michel M, Montillo M, et
al: Diagnosis and treatment of autoimmune hemolytic anemia in
adults: Recommendations from the First International Consensus
Meeting. Blood Rev. 41(100648)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Röth A, Barcellini W, D'Sa S, Miyakawa Y,
Broome CM, Michel M, Kuter DJ, Jilma B, Tvedt THA, Fruebis J, et
al: Sutimlimab in cold agglutinin disease. N Engl J Med.
384:1323–1334. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pham HP, Wilson A, Adeyemi A, Miles G,
Kuang K, Carita P and Joly F: An observational analysis of disease
burden in patients with cold agglutinin disease: Results from a
large US electronic health record database. J Manag Care Spec
Pharm. 28:1419–1428. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Terán Brage E, Fonseca Santos M, Lozano
Mejorada R, García Domínguez R, Olivares Hernández A, Amores Martín
A, Vidal Tocino R and Fonseca Sánchez E: Autoimmune haemolytic
anaemia due to cold antibodies in a renal cancer patient. Case Rep
Oncol. 15:507–514. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Taberner K, House AA, Haig A and Hsia CC:
A case of renal iron overload associated with cold agglutinin
disease successfully managed by rituximab. Clin Hematol Int.
5:62–66. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Portich JP, Blos B, Sekine L and Franz
JPM: Cold agglutinin syndrome secondary to splenic marginal zone
lymphoma: A case report. Hematol Transfus Cell Ther. 45:403–405.
2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lin H, Feng D, Tao S, Wu J, Shen Y and
Wang W: A patient with the highly suspected B cell lymphoma
accompanied by the erythrocytes cold agglutination: Case report.
Medicine (Baltimore). 102(e34076)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Goyal K, Sharma R and Garg S: Intestinal
diffuse large B-cell lymphoma preceding cold agglutinin disease. J
Appl Hematol. 11:137–140. 2020.
|
|
11
|
Ranjan V, Dhingra G, Gupta N, Khillan K
and Rana R: Cold agglutinin autoimmune haemolytic anaemia as an
initial presentation of diffuse large B cell lymphoma: A case
study. Curr Med Res Pract. 12:183–187. 2022.
|
|
12
|
Gentile G, Arcaini L, Antonelli G and
Martelli M: Editorial: HBV and lymphoma. Front Oncol.
13(1236816)2023.PubMed/NCBI View Article : Google Scholar
|