Introduction
Meningiomas are the most common tumors of the
primary central nervous system (CNS), accounting for ~37.6% of such
tumors and 50% of all benign brain tumors. They arise from
meningothelial (arachnoid) cells and are classified into three WHO
grades based on histopathological features. Grade I meningiomas,
constituting >80% of cases, are benign, while grades II and III
cases exhibit increased mitotic activity, necrosis and a greater
metastatic potential (1,2). These slow-growing tumors can develop
from any intracranial or spinal dural surface, with symptoms
varying by location, commonly presenting as headaches, seizures, or
focal neurological deficits (3).
Treatment options include observation, surgical
resection and radiotherapy (RT), with chemotherapy reserved for
rare cases. RT is primarily used for inoperable or recurrent
tumors, with doses of ~50 Gy for grade I and ~60 Gy for grade
II/III tumors delivered via external beam radiotherapy or
stereotactic radiosurgery (3,4). While
effective, the use of RT is associated with risks, including
radiation-induced brain edema, cognitive decline, necrosis and
demyelination (5).
Radiation-induced demyelination results from axonal
degeneration, vascular damage and blood-brain barrier disruption,
mimicking multiple sclerosis (MS) in clinical and imaging findings
(6). MS is an immune-mediated
inflammatory disorder causing CNS demyelination and axonal injury,
diagnosed using the McDonald criteria, which emphasize lesion
dissemination in time and space, supported by magnetic resonance
imaging (MRI), cerebrospinal fluid (CSF) analysis and oligoclonal
band detection (7,8). Distinguishing MS from radiation-induced
demyelination is challenging, as both conditions exhibit white
matter abnormalities on MRI. Advanced neuroimaging and CSF analysis
are crucial for accurate diagnosis to avoid delayed MS treatment or
unnecessary interventions (9,10).
The present study describes the case of a
40-year-old male patient who developed demyelinating lesions
following RT for a WHO grade I meningioma, ultimately diagnosed
with MS. This case raises critical questions as regards whether
radiation serves as a potential trigger for MS in predisposed
individuals or if the observed changes reflect radiation-induced
demyelination. Through a detailed clinical, radiological and
pathological assessment, the case described herein highlights the
challenges in distinguishing these conditions and emphasizes the
importance of an accurate diagnosis in guiding treatment
decisions.
Case report
A 40-year-old male with a history of left occipital
WHO grade I meningioma, characterized by extensive bone invasion
and an elevated Ki-67 index, presented to Government Medical
College Omandurar Hospital, Chennai, India. His clinical course was
complicated by the subsequent diagnosis of MS. The patient
initially presented with worsening chronic headaches and associated
visual deficits over a period of several weeks, prompting an MRI of
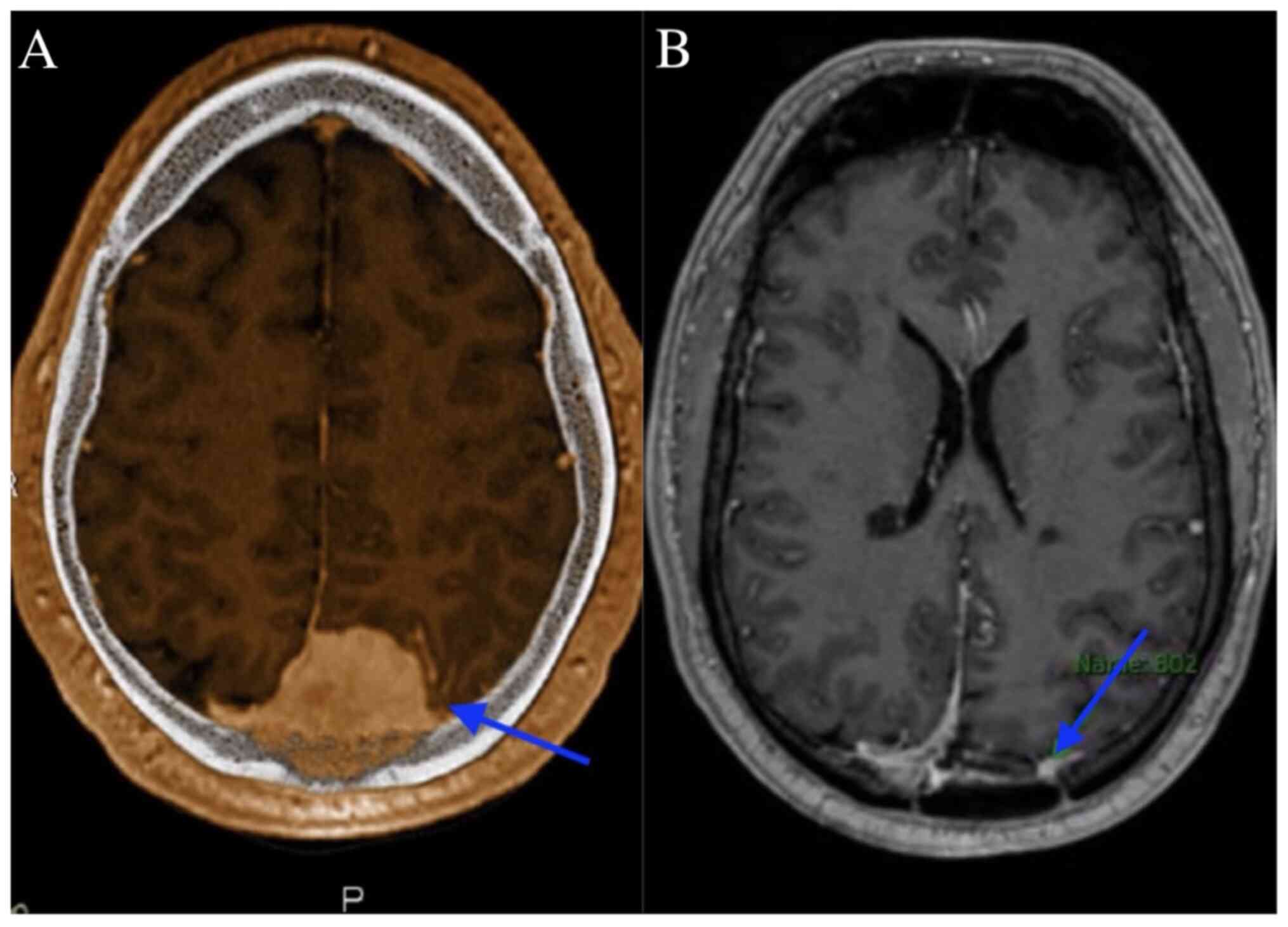
the brain, which revealed a 4.5-cm left occipital dural-based
lesion concerning for meningioma, as shown in Fig. 1A. A pre-operative MRI scan revealed
the progressive enlargement of the lesion along with increasing
vasogenic edema over the following months. The patient underwent a
left occipital craniectomy to debulk the parafalcine tumor.
A pathological examination confirmed a WHO grade I
meningioma with significant bone invasion, a high Ki-67 index and
molecular characteristics indicative of a more aggressive subtype.
Immunohistochemical analysis, performed at the Health Plus
Laboratory, Chennai, India, revealed strong positivity for
epithelial membrane antigen and somatostatin receptor 2A, further
supporting the diagnosis. The patient is under the care of the
Department of Neurology at Government Medical College Omandurar
Hospital, Chennai, India. It should be noted that the
immunohistochemical staining was performed externally at Health
Plus Laboratory, Chennai, which is not affiliated with the authors'
institutions. The findings were reported by the laboratory and
integrated into the patient's diagnostic workup. The authors did
not perform the staining themselves. Representative images are
unavailable, as the laboratory did not provide digital slides or
photomicrographs. However, the results were reviewed and
corroborated with clinical and radiological findings by the
treating team.
Post-surgical imaging revealed residual/recurrent
biparietal meningioma with calvarial and sagittal sinus invasion,
along with a stable resection cavity and a small extra-axial fluid
collection, as shown in Fig. 1B. No
new lesions or mass effect were noted. Post-surgery, the patient
completed radiation treatment delivering a total of 54 Gy in 30
fractions, followed by a 6 Gy boost in 3 fractions, in line with
the treatment protocol for WHO grade I meningioma. Following
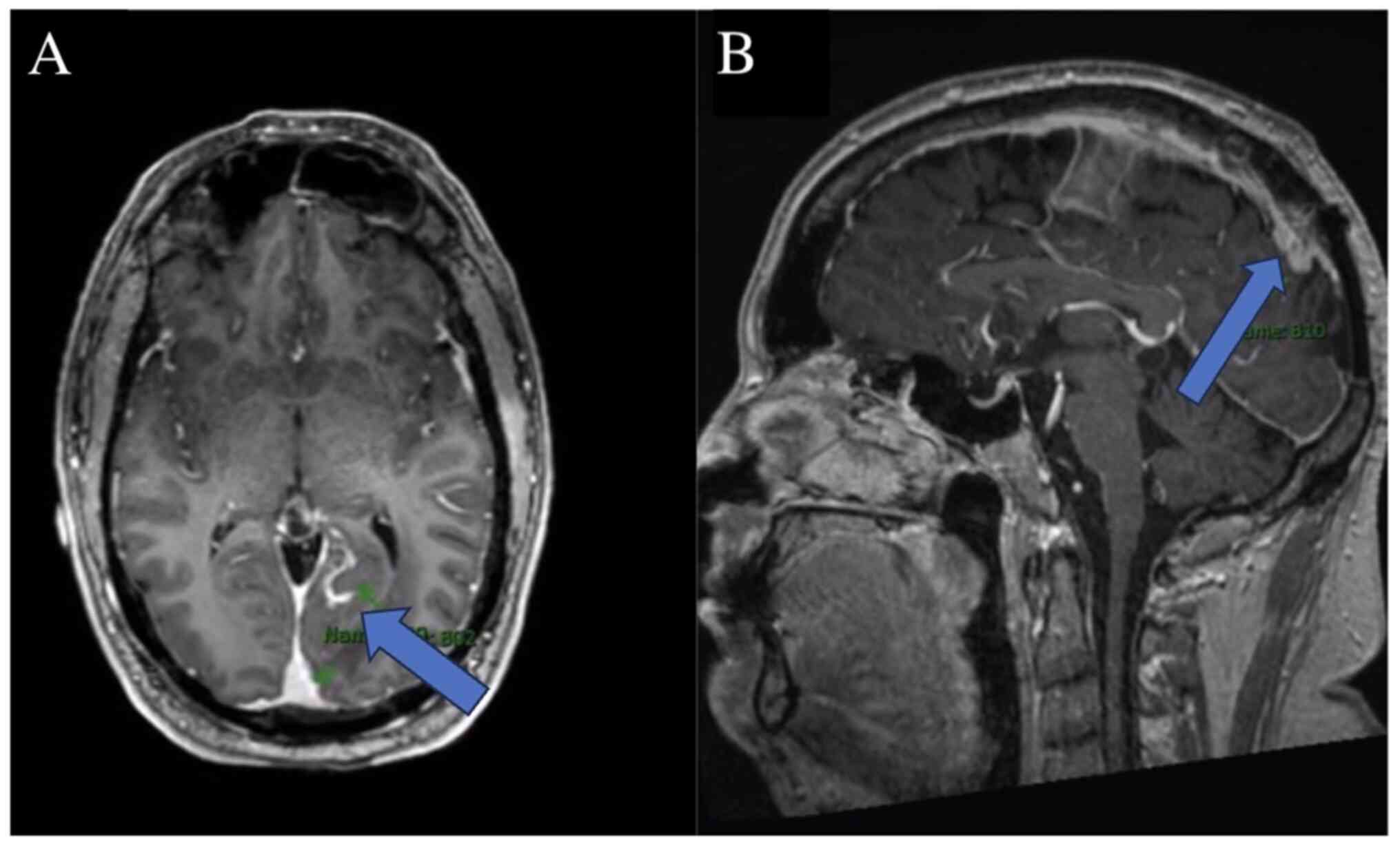
treatment, he experienced worsening left facial numbness, ataxia
and the emergence of new enhancing lesions, leading to a diagnosis
of MS, as shown in Fig. 2.
Further diagnostic evaluation for MS included CSF
analysis, which revealed the presence of IgG oligoclonal bands and
an elevated IgG index, CNS inflammation. These findings indicated
intrathecal IgG synthesis, consistent with the inflammatory and
demyelinating processes characteristic of MS. Visual evoked
potentials (VEPs) demonstrated delayed conduction, further
reinforcing the diagnosis of MS. The detailed CSF analysis results
are summarized in Table I.
 | Table IResults of CSF analysis. |
Table I
Results of CSF analysis.
| Parameter | Result | Reference range | Interpretation |
|---|
| IgG oligoclonal
bands | Positive (4
bands) | Negative (0
bands) | Abnormal, suggestive
of MS |
| IgG index | 0.92 | 0.34-0.66 | Elevated, supports
the diagnosis of MS |
| CSF total
protein | 48 mg/dl | 15-45 mg/dl | Mildly elevated |
| CSF glucose | 60 mg/dl | 40-70 mg/dl | Normal |
| White blood cell
count | 8 cells/µl | 0-5 cells/µl | Mild pleocytosis |
The presence of four distinct IgG oligoclonal bands
in the CSF, absent in the serum, strongly supported the diagnosis
of MS, reflecting immune activity within the central nervous
system. The elevated IgG index (0.92) indicated increased IgG
production relative to albumin, a finding observed in patients with
MS. Mildly elevated CSF total protein levels and a slight increase
in white blood cells (pleocytosis) are also consistent with the
inflammatory profile of MS, although these are less specific. These
clinicopathological data, combined with clinical symptoms, the VEP
findings and MRI evidence of demyelinating lesions, align with the
McDonald criteria for the diagnosis of MS.
The most recent MRI, performed at 3 months following
the completion of RT, revealed a stable appearance of demyelinating
disease, with no new lesions or abnormal enhancement indicative of
active demyelination. At his last follow-up with neurosurgery and
neurology, the patient denied any prolonged episodes of
nausea/vomiting, headaches, seizures, diplopia, changes in hearing,
speech disturbances, gait disturbances or falls, focal
motor/sensory deficits, or dysphagia.
Discussion
RT can lead to a range of harmful effects on nervous
tissue, which can be classified into three categories based on the
timing of onset. Acute complications develop within days to weeks,
early delayed complications arise between 1 to 6 months, and
late-delayed complications emerge after >6 months (11).
Demyelination is a known complication of RT which
belongs to the early-delayed category. The possible pathogenesis of
radiation-induced demyelination is due to the direct effects of
radiation on oligodendrocytes, as well as
oligodendrocyte-type-2-astrocyte progenitor cells, which serve as
progenitor cells for oligodendrocytes and type-2 astrocytes.
Radiation also affects vasculature and blood brain barrier and
alters cytokine expression, which together, may play a role in
pathogenesis (12). MS is an
inflammatory immune disorder. Similar to radiation-induced
demyelination, in MS, oligodendrocytes are considered to be the
primary target through which various mechanisms lead to the loss of
myelin and impaired nerve conduction (13).
The diagnosis of MS is primarily based on the
McDonald criteria, which emphasize the demonstration of
dissemination of lesions in both time and space, supported by
clinical symptoms, MRI findings and CSF analysis, particularly the
presence of oligoclonal bands (14).
By contrast, radiation-induced demyelination is characterized by
localized white matter changes in irradiated regions, often without
evidence of dissemination. In the patient in the present study, the
presence of multiple new enhancing lesions, independent of the
irradiated field, alongside clinical progression, was more
suggestive of MS rather than radiation-induced demyelination.
However, additional diagnostic markers, including CSF analysis,
could have further strengthened this distinction.
Several case reports and studies have described
radiation-induced demyelination as a delayed complication of
cranial irradiation, typically presenting months to years
post-treatment (15).
Pathophysiologically, radiation damages oligodendrocytes and
disrupts the blood-brain barrier, leading to a cascade of
inflammatory responses that can mimic MS. While some studies have
suggested that radiation may unmask or accelerate MS in predisposed
individuals, direct causation remains unproven (16). The clinical history of the patient, a
lack of prior demyelinating episodes and the pattern of lesion
development necessitate a cautious approach in determining whether
radiation plays a contributory role in the onset of MS. The patient
described herein was diagnosed with MS after he received RT for
meningioma.
Recent advancements in imaging and biomarker
research have improved the ability to of medical professionals to
differentiate MS from radiation-induced changes. Studies such as
those reported in Visualizing Emerging Worlds (VIEW) 2024 and ACS
Nano (American Chemical Society Nano) have explored novel imaging
modalities and molecular markers that could aid in distinguishing
these conditions (17). Advanced MRI
techniques, including diffusion tensor imaging (DTI) and
susceptibility-weighted imaging (SWI), provide additional insights
into microstructural changes unique to radiation-induced damage vs.
MS-related demyelination. DTI assesses white matter integrity by
measuring water diffusion along axonal tracts, which helps
differentiate MS plaques from radiation-induced lesions, while SWI
enhances the visualization of iron deposition and microvascular
abnormalities, features more commonly observed in MS due to chronic
inflammation and neurodegeneration (18,19). The
future integration of these imaging techniques, along with
molecular biomarkers, could refine diagnostic accuracy and guide
more personalized treatment strategies, ultimately improving
patient outcomes.
Radiation has been shown to have increased
neurotoxic effects in patients with MS or patients who are
predisposed to the disease (13,20). RT
alone does not lead to MS, but it can encourage the development of
flares of MS. The reduced expression of ataxia-telangiectasia
serine/threonine kinase is observed in some patients with MS. This
gene is involved in DNA repair pathways; thus, its decreased
expression can enhance the sensitivity to radiation (21). Thus, it may be possible that the
patient in the present study could have had undiagnosed MS prior to
receiving RT, or perhaps he possessed the risk factors that
predisposed him to developing MS. The dose-effect association
between radiation and demyelination in patients with MS was
evaluated in a previous study. It was shown that all MS lesions
occurred in brain regions irradiated with an intermediate dose;
i.e., a mean biologically effective dose (BED2) of 33.9
Gy (27.3-49.6 Gy) (10). Caution
should be taken when considering RT for patients with MS or who
possess risk factors for the disease. MS can appear as
radionecrosis and metastases on imaging; thus, differential
diagnoses should be kept in mind to avoid mistakes in diagnosis and
initiating the correct treatment promptly.
Radiation-induced demyelination can mimic or trigger
MS, posing diagnostic and management challenges. Prevention focuses
on minimizing radiation exposure to healthy brain tissue using
advanced techniques, such as intensity-modulated radiotherapy and
proton therapy (22). Treatment
varies based on severity; corticosteroids help reduce acute
inflammation, while disease-modifying therapies are considered in
confirmed cases of MS. Emerging neuroprotective and
remyelination-promoting agents show promise, and severe cases may
benefit from plasmapheresis or intravenous immunoglobulin (23). Prognosis is variable, with some
patients stabilizing and others experiencing progression.
The present study outlines key histopathological and
immunohistochemical findings; however, it is limited by the lack of
immunohistochemistry microscopy images and surgical or pathological
photos, due to restricted access and unavailable records. Despite
this, it is considered that the diagnosis is well-supported by
detailed histological and molecular data.
In conclusion, RT for meningiomas can lead to
complications, such as radiation-induced demyelination, which
mimics MS in both clinical symptoms and MRI findings.
Distinguishing between these conditions is challenging,
paritcularly in patients with a history of radiation, as radiation
may also trigger or worsen MS in predisposed individuals. Accurate
diagnosis requires a thorough clinical history, advanced imaging,
and careful differential diagnosis. A multidisciplinary approach is
essential for effective management and optimal patient
outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TP conceptualized the study, reviewed the patient's
data, and contributed to the drafting of the manuscript, and
approved the final version for submission. IF provided critical
revisions to the manuscript, contributed to the interpretation of
the patient's clinical data, and supervised the overall project.
SHC assisted with the diagnosis of the patient, contributed to the
medical treatment, and participated in the review of the
manuscript. SSR analyzed the patient's clinical data, provided
insight into the, and offered significant input in finalizing the
manuscript. HM, SA and SSP contributed to the conception and design
of the case report, assisted in the analysis and interpretation of
clinical, radiological, and pathological data, and were involved in
case selection. Although not based in India, they collaborated
remotely with the treating team at Government Medical College
Omandurar Hospital through secure data sharing and virtual
discussions. YQ contributed to the interpretation of the patient's
data and assisted in the drafting and revision of the manuscript.
IF and TP confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient described in the present case report.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the present case report and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alruwaili AA and De Jesus O: Meningioma.
In: StatPearls [Internet]. StatPearls Publishing, Treasure Island,
FL, 2024. http://www.ncbi.nlm.nih.gov/books/NBK560538/. Accessed
on October 6, 2024.
|
|
2
|
Ogasawara C, Philbrick BD and Adamson DC:
Meningioma: A review of epidemiology, pathology, diagnosis,
treatment, and future directions. Biomedicines.
9(319)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Buerki RA, Horbinski CM, Kruser T,
Horowitz PM, James CD and Lukas RV: An overview of meningiomas.
Future Oncol. 14:2161–2177. 2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Goldbrunner R, Minniti G, Preusser M,
Jenkinson MD, Sallabanda K, Houdart E, von Deimling A, Stavrinou P,
Lefranc F, Lund-Johansen M, et al: EANO guidelines for the
diagnosis and treatment of meningiomas. Lancet Oncol. 17:e383–e391.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Barisano G, Bergamaschi S, Acharya J,
Rajamohan A, Gibbs W, Kim P, Zada G, Chang E and Law M:
Complications of radiotherapy and radiosurgery in the brain and
spine. Neurographics (2011). 8:167–187. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Karunamuni RA, White NS, McDonald CR,
Connor M, Pettersson N, Seibert TM, Kuperman J, Farid N, Moiseenko
V, Dale AM and Hattangadi-Gluth JA: Multi-component diffusion
characterization of radiation-induced white matter damage. Med
Phys. 44:1747–1754. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Dighriri IM, Aldalbahi AA, Albeladi F,
Tahiri AA, Kinani EM, Almohsen RA, Alamoudi NH, Alanazi AA,
Alkhamshi SJ, Althomali NA, et al: An overview of the history,
pathophysiology, and pharmacological interventions of multiple
sclerosis. Cureus. 15(e33242)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ghasemi N, Razavi S and Nikzad E: Multiple
sclerosis: Pathogenesis, symptoms, diagnoses and cell-based
therapy. Cell J. 19:1–10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Borges A, Garcez D, Pedro C and Passos J:
Chemoradiation induced multiple sclerosis-like demyelination.
eNeurologicalSci. 22(100315)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guillemin F, Biau J, Conde S, Clavelou P
and Dupic G: Multiple sclerosis as differential diagnosis of
radionecrosis for post-irradiation brain lesions: A case report.
Clin Transl Radiat Oncol. 21:44–48. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Béhin A and Delattre JY: Complications of
radiation therapy on the brain and spinal cord. Semin Neurol.
24:405–417. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Belka C, Budach W, Kortmann RD and Bamberg
M: Radiation induced CNS toxicity-molecular and cellular
mechanisms. Br J Cancer. 85:1233–1239. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Miller RC, Lachance DH, Lucchinetti CF,
Keegan BM, Gavrilova RH, Brown PD, Weinshenker BG and Rodriguez M:
Multiple sclerosis, brain radiotherapy, and risk of neurotoxicity:
The Mayo Clinic experience. Int J Radiat Oncol Biol Phys.
66:1178–1186. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Polman CH, Reingold SC, Banwell B, Clanet
M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M,
Kappos L, et al: Diagnostic criteria for multiple sclerosis: 2010
revisions to the McDonald criteria. Ann Neurol. 69:292–302.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Greene-Schloesser D, Robbins ME, Peiffer
AM, Shaw EG, Wheeler KT and Chan MD: Radiation-induced brain
injury: A review. Front Oncol. 2(73)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shaygannejad V, Zare M, Maghzi H and Emami
P: Brain radiation and possible presentation of multiple sclerosis.
J Res Med Sci. 18 (Suppl 1):S93–S95. 2013.PubMed/NCBI
|
|
17
|
Maggi P and Absinta M: Emerging MRI
biomarkers for the diagnosis of multiple sclerosis. Mult Scler.
30:1704–1713. 2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ranzenberger LR, Das JM and Snyder T:
Diffusion Tensor Imaging. In: StatPearls [Internet]. StatPearls
Publishing, Treasure Island, FL, 2025. https://www.ncbi.nlm.nih.gov/books/NBK537361/.
|
|
19
|
Shenton ME, Hamoda HM, Schneiderman JS,
Bouix S, Pasternak O, Rathi Y, Vu MA, Purohit MP, Helmer K, Koerte
I, et al: A review of magnetic resonance imaging and diffusion
tensor imaging findings in mild traumatic brain injury. Brain
Imaging Behav. 6:137–192. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Murphy CB, Hashimoto SA, Graeb D and
Thiessen BA: Clinical exacerbation of multiple sclerosis following
radiotherapy. Arch Neurol. 60:273–275. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu EK, Chen JJ and Braunstein S:
Management of adverse radiation effect associated with stereotactic
radiosurgery of brain metastasis in multiple sclerosis. Adv Radiat
Oncol. 8(101150)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Barazzuol L, Coppes RP and van Luijk P:
Prevention and treatment of radiotherapy-induced side effects. Mol
Oncol. 14:1538–1554. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Talanki Manjunatha R, Habib S, Sangaraju
SL, Yepez D and Grandes XA: Multiple sclerosis: Therapeutic
strategies on the horizon. Cureus. 14(e24895)2022.PubMed/NCBI View Article : Google Scholar
|