Introduction
Subphrenic abscesses are collections of infected
fluid located beneath the diaphragm, typically in the space between
the diaphragm and the upper abdominal organs. These abscesses are
anatomically bordered inferiorly by the transverse colon, mesocolon
and greater omentum (1). Although
they are relatively uncommon, subphrenic abscesses are clinically
significant and often arise as complications of intra-abdominal
infections following surgery, trauma or immunosuppression (2). When typical risk factors are absent,
the diagnosis and management of subphrenic abscesses can be more
complex.
The causative pathogens differ by the type of
surgery, with Staphylococcus aureus infection being common
following gastric procedures and Bacteroides fragilis and
Clostridium species infections being more common following
colon surgery or appendicitis (2).
However, Proteus mirabilis (P. mirabilis), a
Gram-negative bacterium typically associated with urinary tract
infections, is a rare cause of subphrenic abscesses and is
particularly uncommon among immunocompetent patients.
The management of subphrenic abscesses requires a
tailored approach, particularly when atypical or drug-resistant
pathogens are involved. The present study describes a rare case of
an immunocompetent patient with subphrenic abscess caused by P.
mirabilis, without the usual risk factors (3). The prolonged 2-year course and
multidrug-resistant nature of the infection made both diagnosis and
treatment particularly challenging.
The case presented herein underscores the importance
of comprehensive diagnostic imaging and culture-directed antibiotic
therapy in the management of such infections. It further highlights
the critical role of timely intervention, including percutaneous
drainage and the judicious use of antibiotics, to achieve favorable
clinical outcomes in cases involving rare and resistant
pathogens.
Case report
A 77-year-old male patient presented for an
outpatient evaluation by an internist at New Hospitals (Tbilisi,
Georgia), with a 2-year history of recurrent febrile episodes, with
temperatures ranging from 37 to 39˚C, partially alleviated by
acetaminophen and ibuprofen. The fever was accompanied by
generalized weakness, nausea and vertigo. At 1 month prior to the
presentation, the patient developed a nocturnal spasmodic cough
producing white sputum.
His medical history was notable for an episode of
community-acquired pneumonia 2 years prior, complicated by
pleuritis and a right-sided pleural effusion. The pleural fluid was
exudative, with an adenosine deaminase level of 56 U/l. No specific
infectious agent was identified as a cause of pneumonia.
Tuberculosis was ruled out with interferon gamma release assay. The
condition was managed with moxifloxacin and prednisone.
The patient was also treated for a lower urinary
tract infection 2 months prior. At 13 years prior to this, he had
undergone surgical repair for an abdominal aortic rupture, and 5
years prior, a laparoscopic cholecystectomy had been performed.
At the time of the presentation, the vital signs of
the patient were as follows: A temperature of 37.7˚C, heart rate of
65 beats per minute, blood pressure of 130/70 mmHg, respiratory
rate of 22 breaths per minute and an oxygen saturation of 95% in
room air. A physical examination yielded no significant
findings.
Laboratory investigations demonstrated the
following: A red blood cell count of 3.92x106/µl,
hemoglobin level of 10.4 g/dl, mean corpuscular volume of 78.6 fl,
a platelet count of 354x103/µl, white blood cell count
of 22.09x103/µl, neutrophil count of
18.83x103/µl and a lymphocyte count of
1.39x103/µl. Arterial blood gas analysis revealed a pH
of 7.41, partial pressure of carbon dioxide level of 41 mmHg, a
sodium level of 135 mmol/l, a potassium level of 4.3 mmol/l, a
calcium level of 1.14 mmol/l, a glucose level of 111 mg/dl, a
lactate level of 1.7 mmol/l and a bicarbonate level of 26
mmol/l.
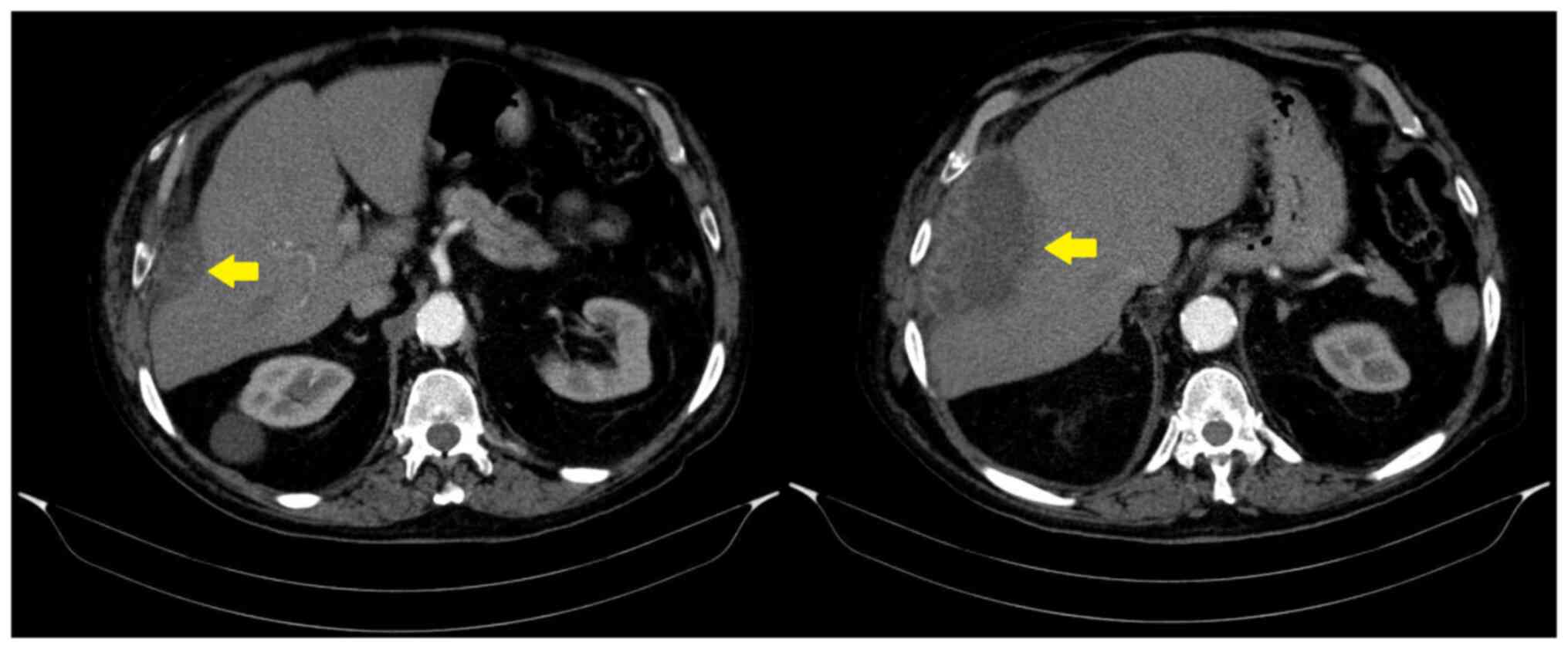
Given the persistent fever of unknown origin, a
chest computed tomography (CT) scan was performed, which revealed a
subphrenic abscess (Fig. 1). To aid
in diagnosis and intervention, an 18 G puncture needle was
initially used to aspirate the abscess and collect a specimen for
analysis. Subsequently, a 12CH pigtail catheter was inserted to
enable continuous drainage and facilitate abscess lavage with
saline four times daily, following local hospital protocols.
The aspirate analysis revealed pinkish-yellow,
turbid, exudative fluid with a high lactate dehydrogenase level of
678 U/l (normal range, 140-280 U/l), an elevated leukocyte count
(normal value, <500 cells/µl) and a predominance of lymphocytes
at 82% (normal value, <50%), while polymorphonuclear cells
accounted for 18% (normal value >50%).
A specimen biopsy was obtained for microscopy and
immunohistochemistry due to concerns about possible malignancy.
These analyses were performed at the Pathology Laboratory of New
Hospitals, and as per routine local practice, only the final report
was provided without image documentation. Microscopy revealed
fibrous and hyalinized stroma with the infiltration of white blood
cells. Immunohistochemistry demonstrated CD3-, CD20- and
PAX5-positive lymphocytes; CD38-positive plasma cells;
CD68-positive macrophages; and CD15-positive granulocytes. CD30, a
lymphoma marker, was negative, thereby excluding an underlying
malignant etiology.
Bacteriological analysis of the aspirated fluid
identified P. mirabilis revealed resistance to gentamicin,
imipenem, cefotaxime, cefazolin, trimethoprim
(TMP)/sulfamethoxazole (SMX), amoxicillin/clavulanate and
tigecycline based on phenotypic testing, while it revealed
sensitivity to fluoroquinolones, tobramycin, amikacin, meropenem,
ceftazidime, cefepime, piperacillin-tazobactam. Genotypic
resistance testing [e.g., for extended-spectrum β-lactamase (ESBL)
or carbapenemase genes] was not performed. Immunohistochemical
analysis revealed inflammation without any evidence of
malignancy.
During hospitalization, the patient was commenced on
empirical treatment with IV meropenem, administered at 2 g every 8
h for 2 weeks. Following the antibiotic sensitivity report, which
revealed susceptibility to fluoroquinolones, the treatment was
adjusted to IV moxifloxacin, at 400 mg once daily, for the
following 6 weeks. This de-escalation was guided by antimicrobial
stewardship principles to reduce unnecessary carbapenem use, once
effective narrower-spectrum therapy was identified. Moxifloxacin
was selected due to its confirmed activity against the isolated
P. mirabilis strain and its excellent intra-abdominal tissue
penetration, which is essential for treating deep-seated
infections, such as subphrenic abscesses (4). Maintaining IV administration ensured
consistent therapeutic levels in this elderly patient during
prolonged treatment.
The patient was closely monitored through regular
follow-up visits over a 6-month period. He remained afebrile and
symptom-free throughout this period, with no recurrence of
abdominal pain, respiratory symptoms, or signs of systemic
infection. Serial laboratory investigations, including analyses of
complete blood count and C-reactive protein levels, yielded results
which were within the normal limits. A follow-up abdominal
ultrasound performed at 3 and 6 months post-discharge revealed the
complete resolution of the abscess, with no residual fluid
collection or new abnormalities. As per local clinical practice,
ultrasound images were not archived or provided to the referring
physician. The patient did not require further antibiotic therapy
and returned to his usual daily activities without restrictions. No
late complications or relapses were reported.
Discussion
A subphrenic abscess, also referred to as a
subdiaphragmatic or infra-diaphragmatic abscess, is characterized
by a localized collection of pus in the subphrenic space. Although
rare, its exact incidence remains unclear. The condition is
typically unilateral, most often occurring on the right side in
~50% of cases, with 40% on the left side and 25% presenting
bilaterally (1-3,5).
In rare instances, both intraperitoneal and extraperitoneal
involvement may be observed, as was the case of the patient
described herein, who presented with a right-sided subphrenic
abscess exhibiting both forms of involvement (5).
Subphrenic abscesses most commonly arise from the
introduction of bacteria into the subphrenic space. Secondary
subphrenic abscesses are most frequently associated with gastric
and biliary surgeries, accounting for 52% of cases. Appendicitis is
responsible for 8%, while colonic surgery and trauma contribute to
19 and 8%, respectively (6).
Localized inflammation in the space between the liver, intestines,
and lungs can also serve as a cause of subphrenic abscess (2). The diagnosis is often delayed or missed
when the abscess is not related to surgery. The majority cases
appear within weeks of abdominal surgery, though some may present
as late as 5 months post-operatively (7). Although the patient in the present
study had a history of intra-abdominal surgeries, including
abdominal aortic aneurysm repair 13 years prior and laparoscopic
cholecystectomy 5 years prior, the timing of these procedures did
not coincide with the onset of symptoms, making them unlikely
contributors to the subphrenic abscess. The source of the P.
mirabilis infection remains uncertain. However, it is plausible
that a prior episode of pneumonia, complicated by a right-sided
pleural effusion ~2 years prior, played a role. Despite the absence
of pathogen isolation from the pleural fluid at that time, the
anatomical proximity between the pleural cavity and subphrenic
space suggests the possibility of transdiaphragmatic spread.
Additionally, while P. mirabilis is not typically associated
with subphrenic abscesses, it has been documented as a causative
agent in various deep-seated infections in susceptible individuals,
which may support its role as a potential source in this case
(8-14).
While transdiaphragmatic extension from the prior
pleural effusion remains the most plausible explanation, other
potential routes of infection were also considered. These include
hematogenous spread, gastrointestinal translocation, or
reactivation from previous abdominal surgery. However, the patient
had no evidence of bacteremia, gastrointestinal symptoms, or recent
surgical intervention to support these mechanisms. Therefore, the
precise route of P. mirabilis entry remains speculative.
Although a causal link remains unclear, this assumption should be
viewed in light of diagnostic limitations during the earlier
pneumonia episode. In Georgia, routine microbiological testing,
such as bronchoalveolar lavage or pleural fluid culture, is not
commonly performed for community-acquired pneumonia unless the case
is severe or atypical. As a result, no causative organism was
identified at that time. The pleural effusion was unilateral and
exudative, making alternative causes such as cardiac origin less
likely. These limitations reduce diagnostic certainty and are
common in resource-limited settings, where comprehensive microbial
workup is not always feasible. Nevertheless, the timing, anatomical
proximity, and clinical context make a transdiaphragmatic
infectious process a reasonable consideration.
Previous studies indicate that subphrenic abscesses
are often polymicrobial, with aerobic bacteria, such as
Escherichia coli, Enterococcus spp.,
Enterobacter and Staphylococcus aureus being the
predominant isolates. The most common anaerobes include
Peptostreptococcus, Bacteroides fragilis,
Clostridium spp. and Prevotella. Following biliary
surgery, Enterococcus group D infection predominates, while
infections with Fusobacterium and Prevotella species
are common following gastric or duodenal surgery (15). Notably, the case stands out due to
the isolation of P. mirabilis from the abscess aspirate, a
rare finding in subphrenic abscesses.
P. mirabilis belongs to the Proteus
genus, which includes five named species (P. mirabilis,
Proteus penneri, Proteus vulgaris,
Proteusmyxofaciens and Proteus hauseri) and several
unnamed (Proteus 4,5,6) genomospecies (16). Although P. mirabilis is a
common Gram-negative pathogen in clinical settings, it is not
typically associated with severe infections and is more frequently
found in colonizing wounds (17).
However, in immunocompromised individuals, it may cause systemic
infections, including urinary tract infections, biliary infections,
wound infections and peritonitis (17). While P. vulgaris is commonly
identified as a causative agent of visceral intra-abdominal
abscesses, P. mirabilis has been reported as a pathogen in
abscesses of the intracranial region, lungs, kidneys and liver,
breast, iliopsoas muscle (8-14).
However, there is a paucity of detailed reports on P.
mirabilis in subphrenic abscesses, with the majority of reports
focusing on its prevalence rather than its precise role or
characteristics, which renders the present case report particularly
significant (6,8,10,11,13,17,18).
Moreover, the patient described herein immunocompetent and had no
history of nephrolithiasis, which is commonly associated with P.
mirabilis, raising questions about how the pathogen contributed
to the abscess.
The present case report is notable when compared to
other documented instances of P. mirabilis causing abscesses
in atypical locations. For example, P. mirabilis was
previously reported in a 17-year-old patient with an intracranial
abscess, causing rare neurological symptoms, such as seizures and
altered sensorium in an immunocompetent patient (9). Similarly, P. mirabilis was
previously identified in a breast abscess in a 56-year-old female
patient, which is unusual, as breast abscesses are typically caused
by Staphylococcus aureus and Streptococcus spp
(8). Additionally, P.
mirabilis has been found in iliopsoas muscle abscesses, a site
usually affected by Escherichia coli or Staphylococcus
aureus, further highlighting the atypical role of this pathogen
in deep-seated infections (11). In
neonatal cases, P. mirabilis has even been implicated in
brain abscesses, an uncommon occurrence given that such infections
are typically caused by Streptococcus and
Staphylococcus species (10).
By contrast, subphrenic abscesses are often
polymicrobial, and the isolation of P. mirabilis as the sole
pathogen, as in the case in the present study, is highly unusual,
particularly in an immunocompetent patient without the typical
predisposing factors, such as recent surgery, urinary tract
infection, or nephrolithiasis (15).
Therefore, the present case report adds valuable insight into the
understanding of P. mirabilis and its unusual involvement in
abscesses outside its typical pathogenetic scope, particularly in
the absence of common predisposing conditions. The case described
herein also underscores the need to consider this pathogen in the
differential diagnosis of deep-seated infections, even in patients
with no notable past medical history.
Typically, P. mirabilis is intrinsically
resistant to nitrofurantoin and tetracycline, while remaining
susceptible to β-lactams, aminoglycosides, fluoroquinolones and
trimethoprim-sulfamethoxazole. However, in this instance, the
isolated strain exhibited multidrug resistance, including
resistance to gentamicin, imipenem, cefotaxime, cefazolin, TMP/SMX,
amoxicillin/clavulanate and tigecycline. This posed a significant
challenge in selecting an effective antibiotic regimen, as
multidrug-resistant P. mirabilis infections complicate the
management of deep-seated infections such as subphrenic abscesses
(16,17). The resistance of P. mirabilis
to β-lactams, aminoglycosides and quinolones has been increasingly
reported in clinical isolates, raising concerns about the
effectiveness of these agents in treating infections caused by an
ESBL-producing strain (18). As a
result, carbapenems, such as meropenem, have become the drug of
choice for empirical therapy, particularly when resistant strains
are suspected (19). In the case in
the present study, meropenem was initiated as an empiric therapy
for the subphrenic abscess, and the isolate was found to be
sensitive to it, allowing for effective initial management and
resolution of the infection. This highlights the continued
relevance of carbapenems as a critical therapeutic option, despite
the growing resistance patterns seen in P. mirabilis (20). However, the increasing frequency of
resistance to key antibiotics, including fluoroquinolones,
aminoglycosides and β-lactams, underscores the need for alternative
therapeutic approaches, particularly in patients with infections
caused by multidrug-resistant strains.
The clinical presentation of a subphrenic abscess
can vary depending on its anatomical location. Symptoms often
include fever, pain in the upper abdomen or shoulder, costal margin
tenderness, abdominal tenderness and dyspnea (2). Additionally, it may present with cough,
hiccups, or unexplained pulmonary manifestations such as pneumonia,
pleural effusion, or basal atelectasis (2), as observed in the patient in the
present study. Fever of unknown origin is also a common symptom
(2).
If not treated promptly, patients may develop
systemic inflammatory response syndrome, characterized by
tachycardia, hypotension and oliguria, which can ultimately
progress to multiorgan failure and mortality (2). Notably, the patient in the present
study appeared to have had this condition for ~2 years without
developing such severe complications, renderings this case
particularly unusual. This prolonged clinical course raises the
question of why the abscess remained undiagnosed for such a long
period of time. During this period, the patient experienced
intermittent febrile episodes; however, no cross-sectional imaging
was performed, as he declined CT scans due to personal reluctance.
This hindered early diagnostic evaluation and contributed to a
delayed identification of the evolving deep-seated pathology. The
subphrenic abscess was only detected after clinical deterioration
prompted urgent imaging.
Laboratory findings typically include leukocytosis
with neutrophilia and an elevated erythrocyte sedimentation rate
(2). Blood gas analysis may
initially reveal respiratory alkalosis due to hyperventilation,
which can progress to metabolic acidosis if left untreated
(2). Blood cultures showing
polymicrobial growth are highly suggestive of subphrenic abscess
(2).
Imaging plays a pivotal role in diagnosing
subphrenic abscesses and guiding management decisions. Chest X-rays
may reveal diaphragmatic elevation, pleural effusion, or lung
abnormalities (2,21). In the case described herein, a chest
X-ray performed 7 months prior incidentally revealed a small
right-sided pleural effusion. The development of pleural effusion
in association with a subphrenic abscess is considered to result
from diaphragmatic inflammation, increasing capillary permeability
and leading to the accumulation of pleural fluid.
Abdominal ultrasound is commonly used for initial
detection, particularly for right-sided abscesses, due to its high
sensitivity for fluid collections and real-time imaging
capabilities. It aids in the assessment of abscess size and
location, and is particularly useful for guiding percutaneous
drainage. However, the limitations of an ultrasound include reduced
efficacy for left-sided abscesses due to bowel gas interference,
and its operator-dependence, meaning the quality of results can
vary. Additionally, ultrasound may not be able to visualize deep or
complex abscesses (22,23). When ultrasound is inconclusive or for
left-sided abscesses, contrast-enhanced CT is considered the gold
standard. CT provides detailed cross-sectional images that allow
for precise localization of the abscess, assessment of surrounding
structures, and planning of drainage procedures. It is particularly
beneficial for left-sided abscesses, where ultrasound is less
effective. However, CT does involve radiation exposure and may not
be as readily accessible in all clinical settings (24,25). In
cases of deep or challenging abscesses, endoscopic ultrasound (EUS)
can be used to access difficult-to-reach areas, offering real-time
imaging and minimizing the risk of intervening structures during
drainage. While EUS provides a valuable alternative, it is
invasive, requires specialized skills, and is not universally
available (26,27).
The management of a subphrenic abscess typically
involves antibiotic therapy and drainage. Empiric therapy with
broad-spectrum antibiotics should be initiated immediately to cover
both aerobic and anaerobic organisms. Once culture results are
available, the antibiotics should be adjusted accordingly. In
immunocompromised patients, antifungal coverage may also be
necessary, particularly for Candida species (2). For drainage, percutaneous CT-guided
drainage is considered the gold standard due to its high success
rate, minimal invasiveness, and lower complication rates compared
to surgical drainage. It allows for real-time visualization and
avoids the need for general anesthesia, which is particularly
beneficial for elderly patients with multiple comorbidities.
CT-guided drainage is effective in controlling sepsis and can be
used both for diagnostic and therapeutic purposes (28,29).
If percutaneous drainage is unsuccessful or if the
abscess is loculated, surgical drainage may be required.
Laparoscopic drainage is less invasive than open surgery and allows
for direct visualization and drainage of the abscess. However, if
laparoscopic methods fail, open surgery may be necessary, although
this approach carries more risks, such as injury to surrounding
organs. In severe cases, particularly in trauma or abdominal
compartment syndrome, open abdomen therapy may be employed as a
damage control strategy. Timely drainage and appropriate antibiotic
therapy are crucial for preventing sepsis, and intensive care may
be required for patients who develop multiorgan failure (30).
In conclusion, the present case report underscores
the uncommon presentation of a subphrenic abscess caused by P.
mirabilis in an immunocompetent patient without typical risk
factors or a clear timeline linking the infection to prior surgical
interventions. The prolonged 2-year symptom duration, the absence
of severe complications, and the multidrug-resistant nature of the
isolated strain posed significant diagnostic and therapeutic
challenges. This highlights the critical role of comprehensive
diagnostic imaging and culture-guided therapy in managing complex
and atypical abscesses. Early, decisive interventions, such as
percutaneous drainage combined with the strategic use of advanced
antibiotics such as meropenem, proved instrumental in achieving a
successful outcome. The case described herein emphasizes the need
for heightened clinical awareness, meticulous treatment planning
and adaptability in addressing rare, resistant infections
effectively.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LC and EC contributed to reviewing the patient's
medical chart, processing images and drafting the manuscript. LC
specifically focused on the case description, while EC led the
discussion section. NK, an internal medicine physician, was
responsible for the patient's management, including examination,
diagnosis, postoperative care, and follow-up. NK also provided all
the necessary data for manuscript preparation and participated in
the revision of the final manuscript. All authors have read and
approved the final manuscript. LC, EC and NK confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Ethical approval was not required for this
single-patient case report in accordance with local institutional
policies. However, all ethical principles outlined in the
Declaration of Helsinki were followed. Particular care was taken to
protect the patient's privacy, and all potentially identifying
details have been omitted. Informed consent was obtained from the
patient described herein. The present case report has removed all
identifying information to protect patient privacy.
Patient consent for publication
Informed consent for the publication of the present
case report was obtained from the patient. The present case report
has removed all identifying information to protect patient
privacy.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Carter R and Brewer LA III: Subphrenic
Abscess: A Thoracoabdominal Clinical Complex: The Changing Picture
with Antibiotics. Am J Surg. 108:165–174. 1964.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ravi GK and Puckett Y: Subphrenic Abscess.
In: StatPearls. StatPearls Publishing, Treasure Island, FL,
2024.
|
|
3
|
Jamil RT, Foris LA and Snowden J: Proteus
mirabilis Infections. In: StatPearls. StatPearls Publishing,
Treasure Island, FL, 2024.
|
|
4
|
Rink AD, Stass H, Delesen H, Kubitza D and
Vestweber KH: Pharmacokinetics and tissue penetration of
moxifloxacin in intervention therapy for intra-abdominal abscess.
Clin Drug Investig. 28:71–79. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
DeCrosse JJ, Poulin TL, Fox PS and Condon
RE: Subphrenic abscess. Surg Gynecol Obstet. 138:841–846.
1974.PubMed/NCBI
|
|
6
|
Wang SM and Wilson SE: Subphrenic Abscess:
The new epidemiology. Arch Surg. 112:934–936. 2025.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Karki A, Riley L, Mehta HJ and Ataya A:
Abdominal etiologies of pleural effusion. Dis Mon. 65:95–103.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Parnasa E, Cohen A, Avital B, Parnasa YS
and Oster Y: An unusual case of breast abscess caused by Proteus
mirabilis and Prevotella buccalis. J Clin Images Med Case Rep.
2(1406)2021.
|
|
9
|
Muddassir R, Khalil A, Singh R, Taj S and
Khalid Z: Intracranial abscess and proteus mirabilis: A case report
and literature review. Cureus. 12(e12326)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chung MH, Kim G, Han A and Lee J: Case
report of neonatal proteus mirabilis meningitis and brain abscess
with negative initial image finding: Consideration of serial
imaging studies. Neonatal Med. 24:187–191. 2017.
|
|
11
|
Moskaļenko KJ, Aveniņa S, Jeršovs K,
Malzubris M and Golubovska I: Iliopsoas muscle abscess and Proteus
mirabilis caused sepsis: a case report. Int J Sci Rep. 9:1–4.
2023.
|
|
12
|
Umar BM: Intraabdominal Abscesses. In:
Abscess-Types, Causes and Treatment. IntechOpen, London, 2024.
Accessed on January 3, 2025.
|
|
13
|
Kakuchi Y: Multiple renal abscesses due to
Proteus mirabilis. Clin Exp Nephrol. 25:322–323. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vakil N, Hayne G, Sharma A, Hardy DJ and
Slutsky A: Liver abscess in Crohn's disease. Am J Gastroenterol.
89:1090–1095. 1994.PubMed/NCBI
|
|
15
|
Frazier EH: Microbiology of subphrenic
abscesses: a 14-year experience. Am Surg. 65:1049–1053.
1999.PubMed/NCBI
|
|
16
|
O'Hara CM, Brenner FW and Miller JM:
Classification, identification, and clinical significance of
proteus, providencia, and morganella. Clin Microbiol Rev.
13:534–546. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu JJ, Chen HM, Ko WC, Wu HM, Tsai SH and
Yan JJ: Prevalence of extended-spectrum beta-lactamases in Proteus
mirabilis in a Taiwanese university hospital, 1999 to 2005:
Identification of a novel CTX-M enzyme (CTX-M-66). Diagn Microbiol
Infect Dis. 60:169–175. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Valentin T, Feierl G, Masoud-Landgraf L,
Kohek P, Luxner J and Zarfel G: Proteus mirabilis harboring
carbapenemase NDM-5 and ESBL VEB-6 detected in Austria. Diagn
Microbiol Infect Dis. 91:284–286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bedenić B, Pospišil M, Nad M and Pavlović
DB: Evolution of β-Lactam Antibiotic Resistance in Proteus Species:
From Extended-Spectrum and Plasmid-Mediated AmpC β-Lactamases to
Carbapenemases. Microorganisms. 13(508)2025.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alqurashi E, Elbanna K, Ahmad I and
Abulreesh HH: Antibiotic Resistance in Proteus mirabilis:
Mechanism, Status, and Public Health Significance. J Pure Appl
Microbiol. 1–12. 2022.
|
|
21
|
Saha A, Chakraborty R, Sain B, Basak S and
Saha S: A Case of Bilateral Subphrenic Abscess Mimicking Bilateral
Postoperative Pleural Effusion. Bengal Phys J. 9:68–72. 2023.
|
|
22
|
Shavrina NV, Ermolov AS, Yartsev PA,
Kirsanov II, Khamidova LT, Oleynik MG and Tarasov SA: [Ultrasound
in the diagnosis and treatment of abdominal abscesses]. Khirurgiia
(Mosk). (11):29–36. 2019.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
23
|
Cho KH, Jung KH, Hwang MS, Chang JC, Kwun
KB and Min HS: Ultrasonographic Features of Intra-abdominal
Abscess. J Yeungnam Med Sci. 2(87)1985.
|
|
24
|
Stevens C, Scott D, Ramesh P, Ragland A,
Johnson C, Strobel J, Malone K, D'Agostino H, Ahuja C and De Alba
L: The evolution of percutaneous abdominal abscess drainage: A
review. Medicine (Baltimore). 104(e41799)2025.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fry DE: Noninvasive imaging tests in the
diagnosis and treatment of intra-abdominal abscesses in the
postoperative patient. Surg Clin North Am. 74:693–709.
1994.PubMed/NCBI
|
|
26
|
Liu S, Tian Z, Jiang Y, Mao T, Ding X and
Jing X: Endoscopic ultrasound-guided drainage to abdominal abscess:
A systematic review and meta-analysis. J Minimal Access Surg.
18:489–496. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
De Filippo M, Puglisi S, D'Amuri F,
Gentili F, Paladini I, Carrafiello G, Maestroni U, Del Rio P,
Ziglioli F and Pagnini F: CT-guided percutaneous drainage of
abdominopelvic collections: A pictorial essay. Radiol Med.
126:1561–1570. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Betsch A, Wiskirchen J, Trübenbach J,
Manncke KH, Belka C, Claussen CD and Duda SH: CT-guided
percutaneous drainage of intra-abdominal abscesses: APACHE III
score stratification of 1-year results. Acute Physiology, Age,
Chronic Health Evaluation. Eur Radiol. 12:2883–2889.
2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hemming A, Davis NL and Robins RE:
Surgical versus percutaneous drainage of intra-abdominal abscesses.
Am J Surg. 161:593–595. 1991.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mazuski JE, Tessier JM, May AK, Sawyer RG,
Nadler EP, Rosengart MR, Chang PK, O'Neill PJ, Mollen KP, Huston
JM, et al: The surgical infection society revised guidelines on the
management of intra-abdominal infection. Surg Infect (Larchmt).
18:1–76. 2017.PubMed/NCBI View Article : Google Scholar
|