Introduction
Acute respiratory infections (ARIs) pose a
significant burden on healthcare systems worldwide (1). According to epidemiological data, ARIs
account for 20-40% of outpatient visits and 12-35% of hospital
admissions in general healthcare settings (2). Pulmonary infections remain a leading
cause of infant and childhood mortality, contributing to ~15% of
all deaths among children <5 years of age globally (3,4). A
previous cohort study estimated the incidence of ARIs at 1.8
episodes per infant per year, with upper respiratory infections
comprising 95% of the cases (5).
The primary causes of ARIs in infants and young
children include a variety of viral and bacterial pathogens.
Viruses such as respiratory syncytial virus (RSV), influenza,
rhinovirus, adenovirus, parainfluenza and human metapneumovirus are
the most frequent causes of upper and lower respiratory tract
infections in early life. Bacterial pathogens, including
Streptococcus pneumoniae, Haemophilus influenzae and
Staphylococcus aureus, are also implicated, particularly in
more severe or secondary infections. The ability of the host to
mount an effective immune response to these pathogens is essential
in preventing complications. Consequently, any factor that
compromises immune function during critical developmental windows,
such as prenatal exposure to environmental immunotoxins, could
potentially elevate the risk or severity of ARIs (6,7).
Perfluoroalkyl and polyfluoroalkyl substances (PFAS)
constitute a group of synthetic chemicals extensively utilized in
industrial and commercial applications since the mid-20th century.
These compounds serve as surfactants and repellents in various
products, including coatings for paper and packaging, textiles,
leather, firefighting foams, photographic materials, cleaning
products and pesticides (8-10).
Due to their widespread use, PFAS have led to extensive
environmental contamination on a global scale (10).
Human exposure to PFAS occurs through multiple
pathways, including the ingestion of contaminated water and food.
The highest concentrations have been detected in fish and
shellfish, followed by red meat, animal fats, processed snacks
(11,12) and beverages (13). Additionally, the inhalation and
ingestion of dust represent key exposure routes (14). The bioaccumulation of PFAS in the
bloodstream is directly proportional to the duration and level of
exposure (15).
The persistence of PFAS in the environment,
attributed to their chemical and thermal stability and high surface
activity, along with their prolonged biological half-life, raises
significant concerns regarding their potential impact on human
health (8,15,16).
Given their extensive use and resistance to degradation, PFAS have
achieved global geographic distribution, with notable variations in
exposure levels among different regions (15).
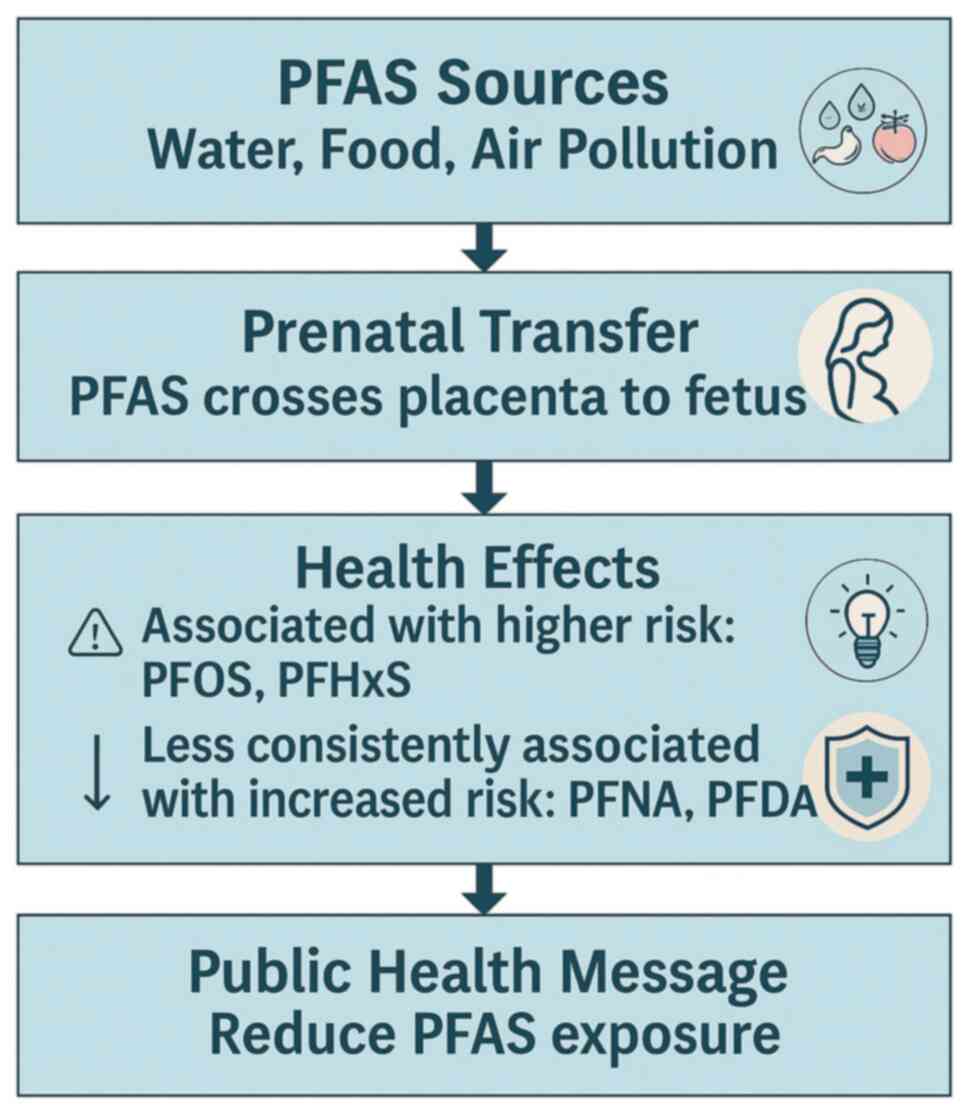
Furthermore, PFAS can cross the placental barrier
and be transferred into breast milk, representing primary exposure
sources for neonates (15). The
fetal and early postnatal periods are critical windows for immune
system development, increasing susceptibility to adverse effects
from environmental exposures (15).
Notably, prolonged breastfeeding has been associated with higher
PFAS concentrations in infants, underscoring the need to elucidate
the role of breastfeeding in the etiology of pediatric infectious
diseases (15).
The European Food Safety Authority (EFSA) has
recently established tolerable intake levels for a subset of PFAS
based on their association with reduced antibody responses in
children and adults, aiming to mitigate potential health risks
(https://www.efsa.europa.eu/en/news/pfas-food-efsa-assesses-risks-and-sets-tolerable-intake).
Despite increasing evidence of the toxicological effects of PFAS,
data on their impact on infant and childhood respiratory health
remain limited. Further research is essential to address this gap,
particularly concerning the association between alternative PFAS
compounds and respiratory infections in children, as research
focuses on conventional PFAS (17).
Recent evidence has highlighted that PFAS exposure,
particularly during critical periods such as gestation, may disrupt
normal immune development (18).
Several PFAS compounds have been shown to alter cytokine
production, reduce antibody responses to vaccines, and impair
immune cell function (18). Given
that respiratory infections are largely managed through innate and
adaptive immune responses, it is plausible that PFAS-induced
immunotoxicity during fetal development could predispose children
to developing more frequent or severe respiratory infections.
Despite this biologically plausible link, only a limited number of
studies have systematically investigated the association between
prenatal PFAS exposure and respiratory health outcomes in early
life (9-12).
The health implications of prenatal PFAS exposure
are of growing concern, particularly given accumulating evidence
linking PFAS to immunosuppression and altered vaccine responses in
children. However, the respiratory consequences of in utero
PFAS exposure remain inadequately characterized, despite
respiratory infections being a leading cause of global childhood
morbidity and mortality. The present systematic review addresses a
critical gap by systematically evaluating whether prenatal PFAS
exposure may increase susceptibility to respiratory infections
during infancy and early childhood, a period marked by immune
vulnerability. By clarifying these associations, the present study
aimed to inform public health strategies and regulatory policies
targeting maternal exposure to persistent environmental toxins.
Data and methods
The present study was conducted according to the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
(PRISMA) guidelines (19). The
present systematic review has been registered in the International
Prospective Register of Systematic Reviews (PROSPERO) with ID no.
CRD420251001057.
Search strategy
A total of two independent reviewers conducted the
literature search and screened the titles and abstracts for
eligibility. Any disagreements were resolved through discussion or
consultation with a third reviewer. A comprehensive literature
search was conducted across three major electronic databases:
PubMed/Medline, Scopus and the Cochrane Library. The search
strategy was designed to identify relevant studies examining
prenatal exposure to PFAS and their impact of the development of
respiratory infections in children. The search algorithm
incorporated a combination of controlled vocabulary (e.g., MeSH
terms) and free-text keywords related to PFAS, including
‘perfluoroalkyl’ ‘polyfluoroalkyl’ ‘perfluorooctanoic acid (PFOA)’
‘perfluorooctane sulfonate (PFOS)’ ‘perfluorononanoic acid (PFNA)’
‘perfluorohexane sulfonate (PFHxS)’ and ‘perfluorobutane sulfonate
(PFBS)’.
To ensure the identification of studies specifically
addressing prenatal exposure, the search strategy incorporated
terms such as ‘pregnancy’, ‘prenatal’, ‘gestational’, ‘maternal
exposure’ and ‘maternal blood levels’. Boolean operators (AND, OR)
were used to refine the search, and additional filters were applied
where applicable. To enhance the comprehensiveness of the
systematic review, reference lists of included studies were
manually screened for additional relevant articles.
In addition to the primary database search, grey
literature was considered to reduce potential publication bias,
i.e., the tendency for studies with statistically significant or
positive findings to be more likely published in indexed,
peer-reviewed journals. Sources of grey literature included
institutional repositories, governmental or non-governmental
organization-funded cohort reports, conference proceedings, and
preprint servers (e.g., medRxiv).
Inclusion and exclusion criteria
Studies were included if they focused on pregnant
women and women of reproductive age, assessed prenatal exposure to
PFAS, and compared outcomes between exposed and non-exposed
populations. The primary outcome of interest was the association
between prenatal PFAS exposure and infant or childhood respiratory
infections. Only primary research studies published in English were
considered. The timeframe for inclusion was restricted to studies
published between January 1, 2015, and February 1, 2025, and any
studies conducted outside this period were excluded.
The population, intervention,
comparator, outcomes and study design (PICOS) framework
The PICOS framework for the present systematic
review on prenatal exposure to PFAS and childhood respiratory
infections can be outlined as follows: i) Population (P): Pregnant
women and their offspring (infants and children); ii)
intervention/exposure (I): Prenatal exposure to PFAS, including
PFOS, PFOA, PFNA, PFHxS, perfluorodecanoic acid (PFDA), etc.; iii)
comparison (C): Not applicable (no explicit comparison group in the
included studies); iv) outcome (O): Incidence of respiratory
infections in infancy and childhood, including pneumonia, RSV
infections, bronchitis, and other respiratory tract infections; and
v) study design (S): Observational studies (cohort, case-control,
and cross-sectional studies).
Outcomes
The primary outcome of interest in the present
systematic review was the incidence of ARIs in infants and children
associated with prenatal exposure to PFAS. ARIs included upper and
lower respiratory tract infections, such as pneumonia, bronchitis,
RSV infection, the common cold and streptococcal throat infections,
as reported in the included studies.
Secondary outcomes included: i) The severity of
respiratory infections, where available (e.g., number of infection
episodes, presence of fever, co-occurrence of symptoms such as
cough or nasal discharge); ii) lung function parameters [e.g.,
forced vital capacity (FVC), forced expiratory volume in one second
(FEV1)]; c) Immune-related biomarkers, including
alterations in immune cell subpopulations and cytokine profiles;
and iv) hospitalization rates or clinical care utilization related
to respiratory infections, when reported.
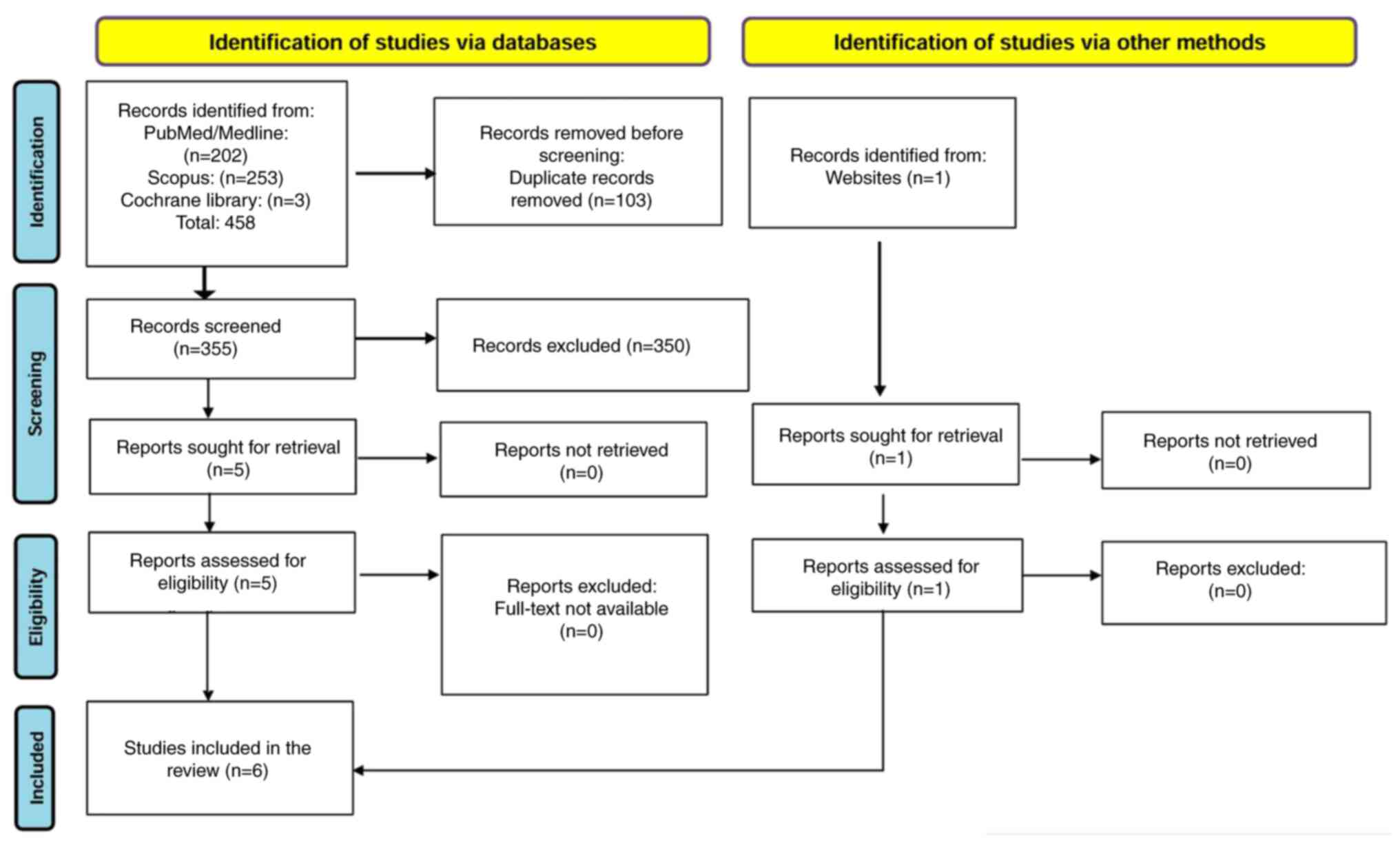
PRISMA process
The initial search in the PubMed, Scopus and
Cochrane Library databases yielded 458 records. Of these, 202
records were identified in PubMed/Medline, 253 in Scopus and three
in the Cochrane Library. After removing 103 duplicate records, 355
unique records remained for further evaluation.
Each retrieved record underwent a detailed screening
process to identify the most relevant studies. The first stage
involved a review of titles and abstracts, eliminating studies that
did not align with the research question, specifically those
unrelated to the association between prenatal PFAS exposure and
infant or childhood respiratory infections. Following this initial
screening, 350 studies were excluded, leaving five studies eligible
for full-text review.
All five studies were retrieved in full text and
assessed for eligibility. Upon detailed examination, all five met
the inclusion criteria and were retained for analysis. One
additional relevant study, not retrieved from PubMed, Scopus, or
the Cochrane Library, was identified through a manual search of
reference lists and an institutional database. Although this record
was not originally indexed in a peer-reviewed journal, it met tge
inclusion criteria as a primary observational study with a defined
methodology and sufficient data transparency. A final dataset of
six studies were included in the present systematic review. The
entire selection process is visually represented in Fig. 1, depicting the identification,
screening, eligibility and final inclusion of studies.
Quality assessment
The selected studies underwent a quality assessment
based on the Caldwell framework, ensuring methodological rigor
(20). The results of quality
assessment are displayed in Data S1. The risk of bias and study
quality were assessed independently by two reviewers. Any
discrepancies in the quality assessment were discussed and resolved
through consensus.
Data extraction
Data extraction was performed independently by two
reviewers using a standardized extraction form. Differences in data
interpretation were addressed through discussion or resolved by a
third reviewer if necessary. From the selected studies, several key
variables were extracted for systematic analysis. These included
the first author, year of publication, study design, sample size
and data collection methods. The core findings of each study were
examined, focusing particularly on their relevance to prenatal PFAS
exposure and respiratory infections in infants and children.
Additional factors, such as follow-up assessments, study
limitations, and the country in which the research was conducted
were also documented.
The findings of the selected studies were
synthesized to provide a comprehensive understanding of the
potential public health implications associated with early-life
exposure to PFAS.
Results
Study selection
A total of six scientific studies were analyzed to
investigate the association between PFAS and the occurrence of
respiratory infections in infants and children (9-11,14,15,21).
Among these studies, one study focused on infant infections, while
the remaining five studies examined childhood infections.
Study and patient characteristics
The six included studies were conducted in China,
Denmark, Japan, Norway and Spain. All were prospective cohort
studies focusing on mother-child pairs, with prenatal PFAS exposure
assessed primarily through maternal blood samples collected during
pregnancy. The majority of the studies employed questionnaires to
gather outcome data, often supplemented by medical or birth
records. Sample sizes varied considerably across studies. The
smallest analytical cohort included just >230 mother-infant
pairs, while the largest involved >1,500 such pairs. Some
studies were nested within national birth cohorts, with original
recruitment populations >6,000 pregnancies. Follow-up periods
ranged from infancy to mid-childhood, extending up to 10 years in
certain cases. Across the studies, measured outcomes included
respiratory infections such as pneumonia, bronchitis, RSV, and
throat infections, as well as fever episodes, lung function
parameters, and general immune-related symptoms.
Sociodemographic characteristics, such as maternal
age, education level and parity were often reported to influence
PFAS levels, and several studies highlighted breastfeeding duration
as a key determinant of ongoing postnatal exposure. However,
differences in exposure assessment methods, follow-up duration and
outcome definitions contributed to heterogeneity among studies.
Each study was systematically reviewed, and key findings were
extracted and organized in chronological order (Table I).
 | Table ICharacteristics of the included
studies. |
Table I
Characteristics of the included
studies.
| Included studies |
|---|
| Title | Association between
prenatal exposure to perfluorinated compounds and symptoms of
infections at age 1-4 years among 359 children in the Odense Child
Cohort (10) | Prenatal exposure to
perfluoroalkyl acids and prevalence of infectious diseases up to 4
years of age (21) | Prenatal exposure to
perfluoralkyl substances (PFASs) associated with respiratory tract
infections but not allergy- and asthma-related health outcomes in
childhood (11) | Maternal levels of
perfluoroalkyl substances (PFASs) during pregnancy and childhood
allergy and asthma-related outcomes and infections in the Norwegian
Mother and Child (MoBa) cohort (14) | Prenatal exposure
to perfluoroalkyl substances, immune-related outcomes, and lung
function in children from a Spanish birth cohort study (9) | Association between
maternal serum concentration of perfluoroalkyl substances (PFASs)
at delivery and acute infectious diseases in infancy (15) |
| Year of
publication | 2016 | 2017 | 2018 | 2019 | 2019 | 2022 |
| Country | Denmark | Japan | Norway | Norway | Spain | China |
| Study type | Prospective cohort
study | Prospective cohort
study | Prospective cohort
study | Cohort Study | Cohort study | Prospective
study |
| Participants | 6,707 pregnant
women; 1,540 families | 35,000 pregnant
women | 3754 healthy
newborns with a birth weight of at least 2,000 g | 2,000 mother-child
pairs | 2,150 pregnant
women | 773 pregnant women,
>18 years old with singleton pregnancy |
| Focus group | 649 samples, 359
mother-child pairs | 1,558 mother-child
pairs | 641 infants | 792
participants | 1,214 mother-child
pairs | 235 mother-infant
pairs |
| Measurement and
data collection methods | Blood sample,
questionnaires | Questionnaires,
birth medical records, and maternal blood samples | Questionnaires | Questionnaires,
maternal blood samples, and medical records | Questionnaires from
interviews and maternal blood samples in the first trimester | Questionnaires,
logistic and Poisson regression models |
| Measured
outcome | Investigation of
the association between prenatal exposure to PFAS and symptoms of
infections at ages 1-4 years | Examination of the
relationship between prenatal exposure to PFAA and the prevalence
of infectious diseases up to 4 years of age | Determination of
whether prenatal exposure to PFAS is associated with asthma,
allergic diseases, or respiratory infections in childhood | Examination of the
relationship between PFAS measured during pregnancy and childhood
asthma, allergies, and common infectious diseases up to age 7 | Association between
prenatal exposure to PFAS and immune health, respiratory system,
and lung function up to age 7 | Investigation of
associations between prenatal exposure to PFAS and acute infectious
diseases in early childhood |
| Key findings | Positive
association between prenatal exposure to PFOS and PFOA and the
prevalence of fever, which may be a sensitive indicator of
infection. | Prenatal exposure
to PFAA may have immunotoxic effects on the immune system of
offspring. | Prenatal exposure
to PFAS was not associated with atopic or pulmonary conditions up
to age 10, but some PFAS were linked to an increased number of
respiratory infections in the first 10 years of life, suggesting
immunosuppressive effects of PFAS. | i)
Immunosuppressive effects of PFAS on respiratory infections such as
bronchitis/pneumonia and throat infections, as well as
diarrhea/gastric flu. ii) Prenatal exposure to PFAS was inversely
associated with non-communicable urinary tract infections. iii)
Possible role of sex in PFAS-health outcome associations. | i) Different PFAS
may affect the developing immune and respiratory system
differently. ii) Prenatal exposure to PFNA and PFOS may be linked
to a reduced risk of respiratory and immune-related outcomes, while
exposure to PFOA may be linked to reduced lung function in young
children. | PFAS exposure was
associated with an increased risk of diarrhea in the first year of
life, with a higher effect among breastfed infants. |
| Follow-up | Questionnaires at 3
months, 18 months, and 3 years. Repeated text message every 2 weeks
(26 times) over 1 year. | Three questionnaire
completions (first trimester of pregnancy, 4 months postpartum, and
4 years postpartum). | At birth and every
6 months until age 2. | Data collected at
22 and 30 weeks of gestation and when the child was 0.5, 1.5, 3 and
7 years of age. | - | - |
| Study
limitations | i) No information
on PFAS exposure during childhood. ii) No data on fever treatment
or vaccinations during the study period, potentially influencing
fever duration. iii) Unmeasured confounding iv) Possibility of
random findings. | i) Evaluation of
infectious diseases based on maternal reports, not confirmed by
medical records. ii) No studies on the validity of doctor-diagnosed
infections. iii) No assessment of co-exposure to other
environmental chemicals affecting the immune system. iv) Potential
selection bias v) Postnatal PFAA levels and immune system
biomarkers not examined. | i) Recall bias from
questionnaires and interviews. ii) Lack of data on significant
confounding factors. | i) Loss to
follow-up. ii) Outcome dependence on questionnaires. iii) The
number of infection episodes may be difficult for parents to recall
and assess. iv) Uncertain counting of infection episodes. v)
Significant loss to follow-up at age 7. | i) Use of maternal
sample early in pregnancy as an indicator of fetal exposure. ii)
Lack of information on PFAS exposure after birth. iii)
Self-reported questionnaires for outcome assessment bias and
misclassification. iv) The effect estimates of PFAS on lung
function were small. | i) Measurement of
maternal serum PFAS concentrations at delivery used as an indicator
of prenatal exposure. ii) The validity of childhood infection
studies is questioned due to misclassification of infectious
disease causes. iii) Significant loss to follow-up or selection
bias. iv) WQS regression cannot simultaneously determine
associations in different directions for composite index elements.
v) Potential influence of uncontrolled confounding factors. |
PFAS exposure levels in maternal
samples
An analysis of the selected studies confirmed the
presence of PFAS in all maternal blood samples, with PFOS and PFOA
being the most commonly detected compounds, followed by PFNA, PFDA,
PFUA, PFHxS, perfluorododecanoic acid (PFDoA), PFBS,
perfluorooctanesulfonamide (PFOSA), perfluoroheptanoic acid (PFHpA)
and perfluoroundecanoic acid (PFUnDA) (15,21).
Differences in PFAS levels were not observed in cases of
infertility or pre-eclampsia (14).
However, maternal parity, age and education level appeared to
influence PFAS concentrations. Higher levels of PFOS, PFOA, PFHxS
and PFNA were detected in nulliparous women compared to multiparous
women (10). Additionally, PFAS
concentrations, particularly PFOS and PFOA, were lower in older
women and those with higher education levels (10). A higher pre-pregnancy body mass index
was associated with lower PFDA concentrations, a pattern that was
also observed for other PFAS (10).
Another notable finding was the significant impact of breastfeeding
duration on postnatal PFAS exposure, indicating that lactation may
play a crucial role in ongoing exposure (14).
Association between prenatal PFAS
exposure and infant respiratory infections
In infants during the first year of life, no
associations were identified between prenatal PFAS exposure and the
occurrence of common colds, bronchitis or pneumonia (15). An inverse association between PFBS
and these infections was initially suggested; however, further
analysis did not support this finding (15). Additionally, no sex-based differences
were observed in the association between PFAS exposure and acute
infectious diseases in infancy (15). However, it was noted that a number of
infants exhibited symptoms of infection within their first year,
suggesting the need for additional research on potential
contributing factors (10).
Beyond infancy, prenatal exposure to PFAS,
particularly PFOS and PFHxS, was significantly associated with an
increased risk of respiratory infections in early childhood. By the
age of 4 years, pneumonia and RSV were among the most frequently
reported infections, with similar prevalence in both sexes
(P>0.05) (21). Notably, children
in the highest quartile of PFOS exposure had a 61% increased risk
of developing at least one infectious disease compared to those in
the lowest quartile [odds, ratio (OR), 1.61; 95% confidence
interval (CI), 1.18-2.21; P-value for trend=0.008). Furthermore,
PFHxS exposure was positively associated with infectious diseases
specifically among females, with the highest exposure quartile
showing a 55% increased risk (OR, 1.55; 95% CI, 0.976-2.45; P-value
for trend=0.045), whereas PFOS exposure was associated with
infection risk in both sexes (21).
By the age of 2 years, PFUnDA was positively
associated with the number of common cold episodes (11). By 3 years of age, PFOS and PFOA
concentrations were inversely associated with the number of common
colds, particularly among female children [risk ratio (RR) for
PFOS, 0.94; 95% CI, 0.92-0.97; P<0.001; RR for PFOA, 0.96; 95%
CI, 0.94-0.99; P=0.012] (14).
However, bronchitis and pneumonia were significantly more frequent
in children with higher prenatal exposure to PFOS, PFOA, PFHxS and
PFHpS, with the effect of PFOA (RR, 1.27; 95% CI, 1.12-1.43) and
PFHxS (RR, 1.15; 95% CI, 1.06-1.24) being stronger in girls
(14). Streptococcal throat
infections were positively associated with PFNA exposure (RR, 1.29;
95% CI, 1.11-1.50), while PFOA exhibited a stronger association in
boys and PFUnDA in girls. Other throat infections were linked to
PFHxS exposure in girls (RR, 1.10; 95% CI, 1.02-1.18) (14). Furthermore, children attending
kindergarten at age three were more likely to develop pseudocroup,
which exhibited a positive association with PFUnDA (inverse RR,
0.86; 95% CI, 0.78-0.95) in those attending childcare, highlighting
a potential interaction between environmental exposures and social
behaviors (14).
Associations between PFAS exposure and
fever in young children
Among children aged 1 to 4 years in the Odense Child
Cohort, prenatal PFAS exposure was significantly associated with
increased infection-related symptoms, independent of confounders
such as smoking, breastfeeding duration, and sex (10). Specifically, children in the highest
tertile of prenatal PFOS exposure experienced a 65% higher rate of
fever days compared to those in the lowest tertile [incidence rate
ratio (IRR), 1.65; 95% CI, 1.24-2.18; P<0.001], and the odds of
having fever above the median level more than doubled (OR, 2.35;
95% CI, 1.34-4.11). A similar though slightly weaker association
was observed for PFOA, with an increased odds ratio of 1.97 (95%
CI, 1.07-3.62) for fever days above the median (10). Additionally, the co-occurrence of
fever with cough or nasal discharge increased with higher PFOS and
PFOA exposure. Notably, children in the medium PFOA exposure
tertile had a 38% higher incidence of episodes with both fever and
nasal discharge compared to the low-exposure group (IRR, 1.38; 95%
CI, 1.03-1.86) (10). PFHxS also
contributed modestly to these combined symptom patterns. Overall,
these findings suggest that fever, as a sensitive and frequent
symptom of infection (reported on average during 1.6% of days per
child annually), may serve as a robust marker of increased
infection susceptibility due to prenatal PFAS exposure (10).
Association between PFAS exposure and
respiratory health in children aged 4 to 7 years
In children aged 4 to 7 years, prenatal exposure to
PFAS remained a key determinant of respiratory health outcomes.
Higher concentrations of several PFAS were associated with a
reduced risk of lower respiratory tract infections (LRTIs) and
wheezing, with the exception of PFHxS, which was associated with an
increased risk of LRTIs across all age groups (RR, 1.14; 95% CI,
1.00-1.29) (9). Higher levels of
PFNA were linked to a lower probability of LRTIs at age 4 (OR,
0.85; 95% CI, 0.71-1.01) and wheezing at age 7 (OR, 0.69; 95% CI,
0.54-0.88). A protective association with asthma was also observed
for PFNA (RR, 0.74; 95% CI, 0.57-0.96), while PFOS exposure was
inversely associated with eczema (RR, 0.86; 95% CI, 0.75-0.98)
(9).
However, stratified analysis by breastfeeding
duration revealed an important effect modification: In children who
were breastfed for <4 months, higher prenatal PFNA exposure was
associated with an increased risk of asthma (RR, 2.73; 95% CI,
1.13-6.57). Furthermore, higher prenatal concentrations of PFOA
were associated with lower lung function at age 4, as indicated by
decreased z-scores in FVC (β, -0.17, 95% CI, -0.34 to -0.01) and
FEV1 (β, -0.13; 95% CI. -0.29 to 0.03) These
associations were not significant at age 7. No sex-based
differences were observed in any of the respiratory outcomes
assessed (9).
Discussion
Summary of key findings
The present systematic review synthesized evidence
regarding the association between prenatal exposure to PFAS and the
incidence of respiratory infections in infants and children. The
findings highlight a complex interplay between PFAS exposure and
respiratory health. They also revealed both positive and negative
associations across different compounds and age groups.
The present systematic review of six studies
confirmed the presence of PFAS in maternal blood samples, with PFOS
and PFOA being the most detected compounds. Of note, four out of
the six studies included in the present systematic review
identified significant associations between prenatal PFAS exposure
and an increased risk of respiratory infections in early childhood,
with some evidence suggesting stronger associations in female
children. These associations were particularly evident in female
children. However, the findings were not entirely consistent across
all age groups and PFAS compounds, suggesting that additional
factors may modulate the observed associations.
Interpretation by age
group/outcome
In infants <1 year of age, no significant
associations were detected between prenatal PFAS exposure and
common respiratory infections such as colds, bronchitis, or
pneumonia. However, by the age of 2 to 4 years, increased PFHxS and
PFOS exposure was linked to higher susceptibility to respiratory
infections, including pneumonia and RSV infections. These findings
are consistent with experimental and epidemiological evidence
outside the scope of the present systematic review, which indicate
that PFAS may interfere with immune function and increase
susceptibility to infections (22,23).
Notably, some PFAS compounds, including PFNA and
PFDA, were found to be associated with a reduced risk of lower
respiratory tract infections and wheezing, particularly in children
aged 4 to 7 years. The observed inverse associations between PFNA
and PFDA and certain respiratory outcomes, such as the reduced
incidence of wheezing and lower respiratory tract infections,
warrant cautious interpretation. These findings are
counterintuitive given the known immunotoxic potential of PFAS and
may reflect compound-specific immunomodulatory properties,
differences in toxicokinetics, or residual confounding. For
instance, PFNA and PFDA are longer-chain PFAS with different
biological behaviors compared to PFOS and PFOA, potentially leading
to differing impacts on immune cell differentiation or cytokine
regulation. Additionally, these ‘protective’ associations may be
influenced by factors, such as breastfeeding duration,
socioeconomic status, or reverse causation, where healthier
children are inadvertently more exposed due to differences in
maternal behavior or environmental context. Given the small number
of studies and inconsistent findings, further mechanistic and
epidemiological studies are warranted to clarify whether these
associations represent true protective effects or are the result of
bias or uncontrolled confounding.
Biological mechanisms
The biological mechanisms underlying the association
between prenatal PFAS exposure and childhood respiratory infections
remain incompletely understood. However, several hypotheses have
been proposed. PFAS are known to interfere with immune function.
They do so by altering cytokine production, reducing
vaccine-induced antibody responses and impairing T-cell
differentiation (22). These
immunotoxic effects may increase vulnerability to infectious
diseases during early childhood, a critical period for immune
system development (23).
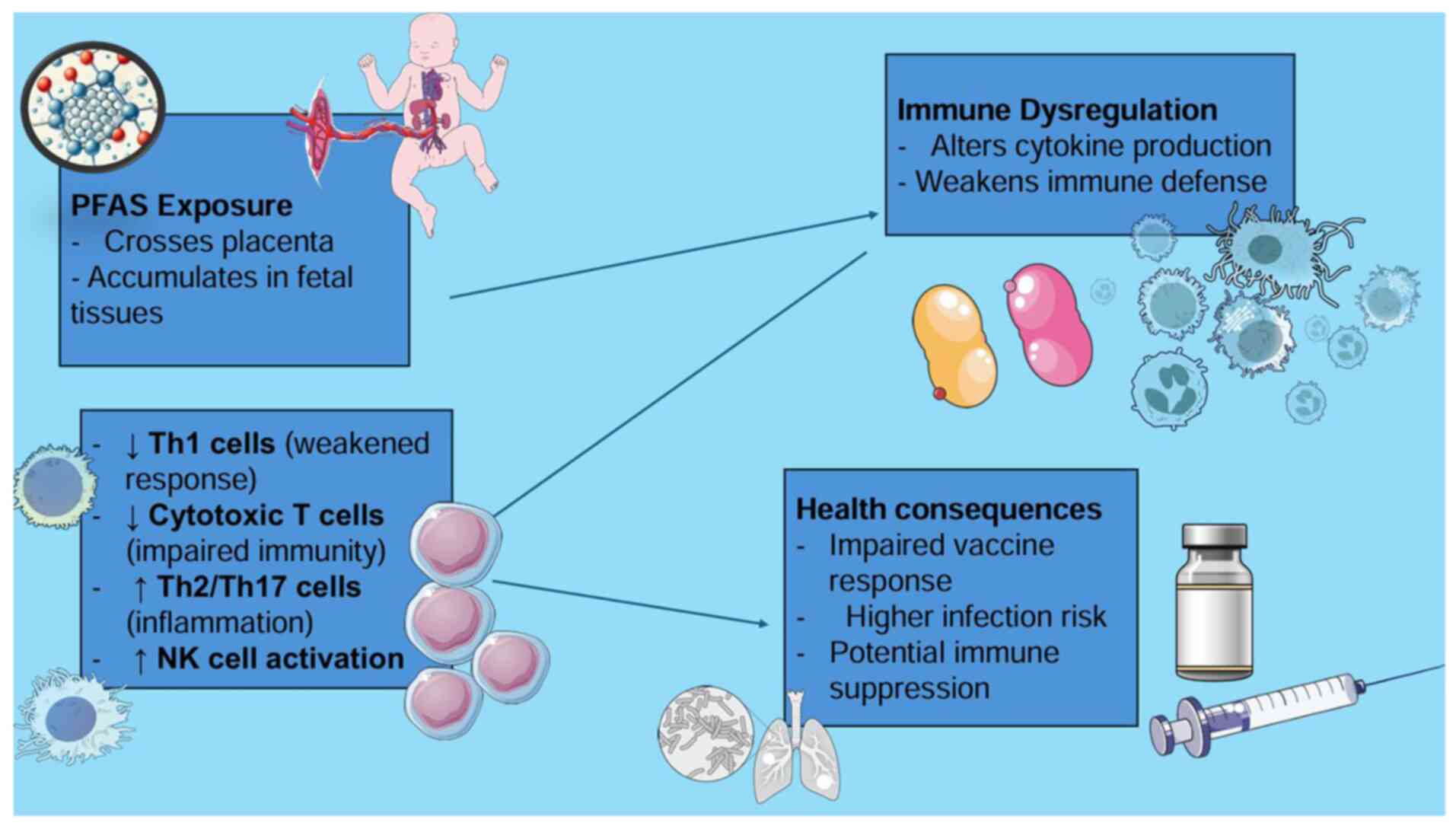
PFAS are considered to interfere with immune system
development through several pathways. They may alter cytokine
production, reduce antibody responses to vaccines and disrupt the
balance of immune cell types. For example, prenatal exposure to
PFOS, PFOA, PFNA and PFHxS has been shown to be associated with
increases in certain immune cells called natural killer (NK) cells
and a shift toward immune responses typically involved in allergies
and inflammation (Th2 and Th17 pathways). At the same time, there
appears to be a reduction in immune responses that fight viral and
bacterial infections (Th1 responses). Some evidence also suggests
that PFAS may weaken immune surveillance by lowering the number of
cytotoxic T-cells involved in long-term protection and increasing
markers of immune overactivation. These changes could help explain
why higher PFAS exposure is linked to reduced vaccine effectiveness
and greater vulnerability to infections in early childhood
(22).
Additionally, PFAS can cross the placental barrier
and accumulate in fetal tissues. This may disrupt normal immune
maturation. Their long biological half-life leads to ongoing
postnatal exposure, especially through breastfeeding. A previous
study highlighted the role of breastfeeding duration in modulating
PFAS-related immune effects, with prolonged breastfeeding
associated with higher PFAS concentrations in infants (15). This raises critical public health
questions regarding the risks and benefits of breastfeeding in
PFAS-contaminated environments. The proposed pathway linking
prenatal PFAS exposure to childhood respiratory infections,
highlighting key mechanisms, such as placental transfer, immune
system modulation and the increased risk of infections is
illustrated in Fig. 2. Furthermore,
provides a visual representation of how prenatal PFAS exposure
alters immune cell function, including shifts in Th1/Th2/Th17
balance and NK cell activity, contributing to increased infection
risk in early life is provided in Fig.
3.
Public health relevance
Given the widespread environmental contamination by
PFAS, our findings have significant public health implications.
Current regulations primarily focus on conventional PFAS compounds
such as PFOA and PFOS, while emerging PFAS alternatives remain
largely unregulated (24). Further
research is required to assess the impact of these newer compounds
on respiratory health outcomes. Moreover, public health
interventions could consider strategies to reduce PFAS exposure
during pregnancy and early childhood, especially in high-risk
areas. Strategies such as regulating PFAS production and use,
enhancing water filtration systems, and promoting dietary awareness
may help mitigate potential health risks, although further evidence
is needed to guide such measures (25).
Limitations
While the present systematic review provides
valuable insight into the association between prenatal PFAS
exposure and respiratory infections, several limitations should be
acknowledged. First, the included studies exhibit heterogeneity in
study design, exposure assessment methods, and outcome definitions,
which may contribute to inconsistency in findings. Second, the
observational nature of the studies limits causal inferences, as
residual confounding by socioeconomic status, environmental
factors, and genetic predisposition cannot be ruled out. Third,
potential publication bias may be present, given the limited number
of eligible studies and the tendency for positive findings to be
preferentially published in peer-reviewed journals. Although grey
literature was included to mitigate this bias, the small sample
size remains a constraint. Fourth, there was considerable
variability in PFAS measurement approaches, including differences
in the biological matrices used (e.g., maternal serum, cord blood),
timing of sample collection, and analytical techniques, which may
affect comparability of exposure estimates. Finally, ARIs were not
uniformly defined across studies, and outcome assessment relied on
varying clinical criteria or parental reports, which may introduce
misclassification and reduce comparability.
Critical appraisal of included
studies
In evaluating the methodological quality of the six
included studies using the Caldwell framework, several strengths
and limitations were identified. All studies employed appropriate
prospective cohort designs, and most recruited large and
demographically diverse populations, enhancing generalizability.
However, notable methodological weaknesses were observed. All
studies relied heavily on self-reported outcomes, often obtained
through parental questionnaires, introducing potential recall and
misclassification bias. None of the studies explicitly described
comparator groups, and only a few controlled for co-exposures or
provided detailed adjustment for confounding factors such as
environmental variables, socioeconomic status, or vaccination
history.
The exposure assessment was generally based on
maternal blood samples, but variation existed in the timing of
sample collection, biomarkers used, and the analytical methods
applied, affecting consistency across studies. Ethical approval and
participant consent procedures were often assumed but not clearly
stated. Only a few studies (9,14,21)
combined self-reported outcomes with medical record validation,
reducing the reliability of reported infections in most cases.
Furthermore, substantial loss to follow-up was reported in at least
two studies, potentially introducing attrition bias.
Recommendations for future
research
Future research should aim to address these
limitations by conducting large-scale prospective cohort studies
with standardized exposure and outcome assessments. Additionally,
mechanistic studies are needed to elucidate the specific pathways
through which PFAS influence immune function and respiratory
health. Research should also explore potential sex-specific
differences in PFAS susceptibility, as some studies suggest
differential effects in males and females.
In conclusion, the present systematic review
summarized current evidence on the association between prenatal
exposure to PFAS and respiratory infections in infants and
children. While several studies report associations between higher
prenatal PFAS exposure, particularly to PFHxS and PFOS, and
increased risk of respiratory infections in early childhood,
findings are not entirely consistent across compounds or age
groups. PFAS compounds, such as PFHxS and PFOS have been associated
with an increased risk of respiratory infections, while others such
as PFNA and PFDA may exert different immunomodulatory effects,
which in some cases appear to correlate with reduced incidence of
certain infections. These findings underscore the complex and
potentially compound-specific nature of PFAS-related immune
effects. However, the evidence remains limited by heterogeneity in
study design, exposure assessment, and outcome definitions. Most
studies are observational in nature, precluding causal inferences,
and residual confounding cannot be excluded. Despite these
limitations, the potential for PFAS to disrupt immune development
during critical periods raises important concerns. Future research
should prioritize longitudinal cohort studies with standardized
exposure and outcome measures, as well as mechanistic
investigations to clarify immunological pathways. From a public
health perspective, reducing prenatal PFAS exposure through
regulatory policies and environmental interventions may help
mitigate potential risks, particularly among vulnerable populations
such as pregnant women and young children.
Supplementary Material
Caldwell Framework Quality Assessment
for Included Studies (for the reference citations, please see the
reference list in the main manuscript).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ML and AS conceptualized the study. ML, DM, AS, GK,
VJ, KG, VEG, CRA and DAS made a substantial contribution to data
interpretation and analysis and wrote and prepared the draft of the
manuscript. ML and GK analyzed the data and provided critical
revisions. All authors contributed to manuscript revision and have
read and approved the final version of the manuscript. ML and AS
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The other authors declare that they have no
competing interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Georgakopoulou VE: Insights from
respiratory virus co-infections. World J Virol.
13(98600)2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jain N, Lodha R and Kabra SK: Upper
respiratory tract infections. Indian J Pediatr. 68:1135–1138.
2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Everard ML: Paediatric respiratory
infections. Eur Respir Rev. 25:36–40. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
World Health Organization (WHO): The
Global Health Observatory. Children aged <5 years with ARI
symptoms taken to a health facility (%). Indicator metadata
registry: IMR details 70. https://www.who.int/data/gho/indicator-metadata-registry/imr-details/70.
Accessed Feb 17, 2025.
|
|
5
|
Kumar P, Medigeshi GR, Mishra VS, Islam M,
Randev S, Mukherjee A, Chaudhry R, Kapil A, Ram Jat K, Lodha R and
Kabra SK: Etiology of acute respiratory infections in infants: A
prospective birth cohort study. Pediatr Infect Dis J. 36:25–30.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Thompson MG, Hunt DR, Arbaji AK, Simaku A,
Tallo VL, Biggs HM, Kulb C, Gordon A, Khader IA, Bino S, et al:
Influenza and respiratory syncytial virus in infants study (IRIS)
of hospitalized and non-ill infants aged <1 year in four
countries: Study design and methods. BMC Infect Dis.
17(222)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kubale J, Kujawski S, Chen I, Wu Z, Khader
IA, Hasibra I, Whitaker B, Gresh L, Simaku A, Simões EAF, et al:
Etiology of acute lower respiratory illness hospitalizations among
infants in 4 countries. Open Forum Infect Dis.
10(ofad580)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zeng XW, Bloom MS, Dharmage SC, Lodge CJ,
Chen D, Li S, Guo Y, Roponen M, Jalava P, Hirvonen MR, et al:
Prenatal exposure to perfluoroalkyl substances is associated with
lower hand, foot and mouth disease virus antibody response in
infancy: findings from the Guangzhou Birth Cohort Study. Sci Total
Environ. 663:60–67. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Manzano-Salgado CB, Granum B,
Lopez-Espinosa MJ, Ballester F, Iñiguez C, Gascón M, Martínez D,
Guxens M, Basterretxea M, Zabaleta C, et al: Prenatal exposure to
perfluoroalkyl substances, immune-related outcomes, and lung
function in children from a Spanish birth cohort study. Int J Hyg
Environ Health. 222:945–954. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dalsager L, Christensen N, Husby S, Kyhl
H, Nielsen F, Høst A, Grandjean P and Jensen TK: Association
between prenatal exposure to perfluorinated compounds and symptoms
of infections at age 1-4 years among 359 children in the odense
child cohort. Environ Int. 96:58–64. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Impinen A, Nygaard UC, Lødrup Carlsen KC,
Mowinckel P, Carlsen KH, Haug LS and Granum B: Prenatal exposure to
perfluoroalkyl substances (PFASs) associated with respiratory tract
infections but not allergy- and asthma-related health outcomes in
childhood. Environ Res. 160:518–523. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang H, Yu K, Zeng X, Chen Q, Liu Q, Zhao
Y, Zhang J, Zhang X and Huang L: Association between prenatal
exposure to perfluoroalkyl substances and respiratory tract
infections in preschool children. Environ Res.
191(110156)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Van Leeuw V, Malysheva SV, Fosseprez G,
Murphy A, El Amraoui Aarab C, Andjelkovic M, Waegeneers N, Van
Hoeck E and Joly L: Per- and polyfluoroalkyl substances in food and
beverages: Determination by LC-HRMS and occurrence in products from
the Belgian market. Chemosphere. 366(143543)2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Impinen A, Longnecker MP, Nygaard UC,
London SJ, Ferguson KK, Haug LS and Granum B: Maternal levels of
perfluoroalkyl substances (PFASs) during pregnancy and childhood
allergy and asthma-related outcomes and infections in the norwegian
mother and child (MoBa) cohort. Environ Int. 124:462–472.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang Z, Shi R, Ding G, Yao Q, Pan C, Gao Y
and Tian Y: Association between maternal serum concentration of
perfluoroalkyl substances (PFASs) at delivery and acute infectious
diseases in infancy. Chemosphere. 289(133235)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aeraki MM, Metallinou D, Diamanti A,
Georgakopoulou VE and Sarantaki A: Exposure to poly- and
perfluoroalkyl substances during pregnancy and asthma in childhood:
A systematic review. Cureus. 16(e73568)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang H, Li X, Deng Y, San S, Qiu D, Guo
X, Xu L and Li Y, Zhang H and Li Y: The association between
prenatal exposure to per- and polyfluoroalkyl substances and
respiratory tract infections in preschool children: A Wuhan cohort
study. Toxics. 11(897)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bline AP, DeWitt JC, Kwiatkowski CF, Pelch
KE, Reade A and Varshavsky JR: Public health risks of PFAS-related
immunotoxicity are real. Curr Environ Health Rep. 11:118–127.
2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Caldwell K, Henshaw L and Taylor G:
Developing a framework for critiquing health research: An early
evaluation. Nurse Educ Today. 31:e1–e7. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Goudarzi H, Miyashita C, Okada E, Kashino
I, Chen CJ, Ito S, Araki A, Kobayashi S, Matsuura H and Kishi R:
Prenatal exposure to perfluoroalkyl acids and prevalence of
infectious diseases up to 4 years of age. Environ Int. 104:132–138.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tursi AR, Lindeman B, Kristoffersen AB,
Hjertholm H, Bronder E, Andreassen M, Husøy T, Dirven H, Andorf S
and Nygaard UC: Immune cell profiles associated with human exposure
to perfluorinated compounds (PFAS) suggest changes in natural
killer, T helper, and T cytotoxic cell subpopulations. Environ Res.
256(119221)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fenton SE, Ducatman A, Boobis A, DeWitt
JC, Lau C, Ng C, Smith JS and Roberts SM: Per- and polyfluoroalkyl
substance toxicity and human health review: Current state of
knowledge and strategies for informing future research. Environ
Toxicol Chem. 40:606–630. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
European Environment Agency: An assessment
on PFAS in textiles in Europe's circular economy. WSP E&IS
GmbH, Brussels, 2024. https://www.eea.europa.eu/en/analysis/publications/pfas-in-textiles-in-europes-circular-economy/an-assessment-on-pfas-in-textiles-in-europes-circular-economy/@@download/file.
|
|
25
|
Alazaiza MYD, Alzghoul TM, Ramu MB, Abu
Amr SS and Abushammala MFM: PFAS contamination and mitigation: A
comprehensive analysis of research trends and global contributions.
Case Stud Chem Environ Eng. 11(101127)2025.
|