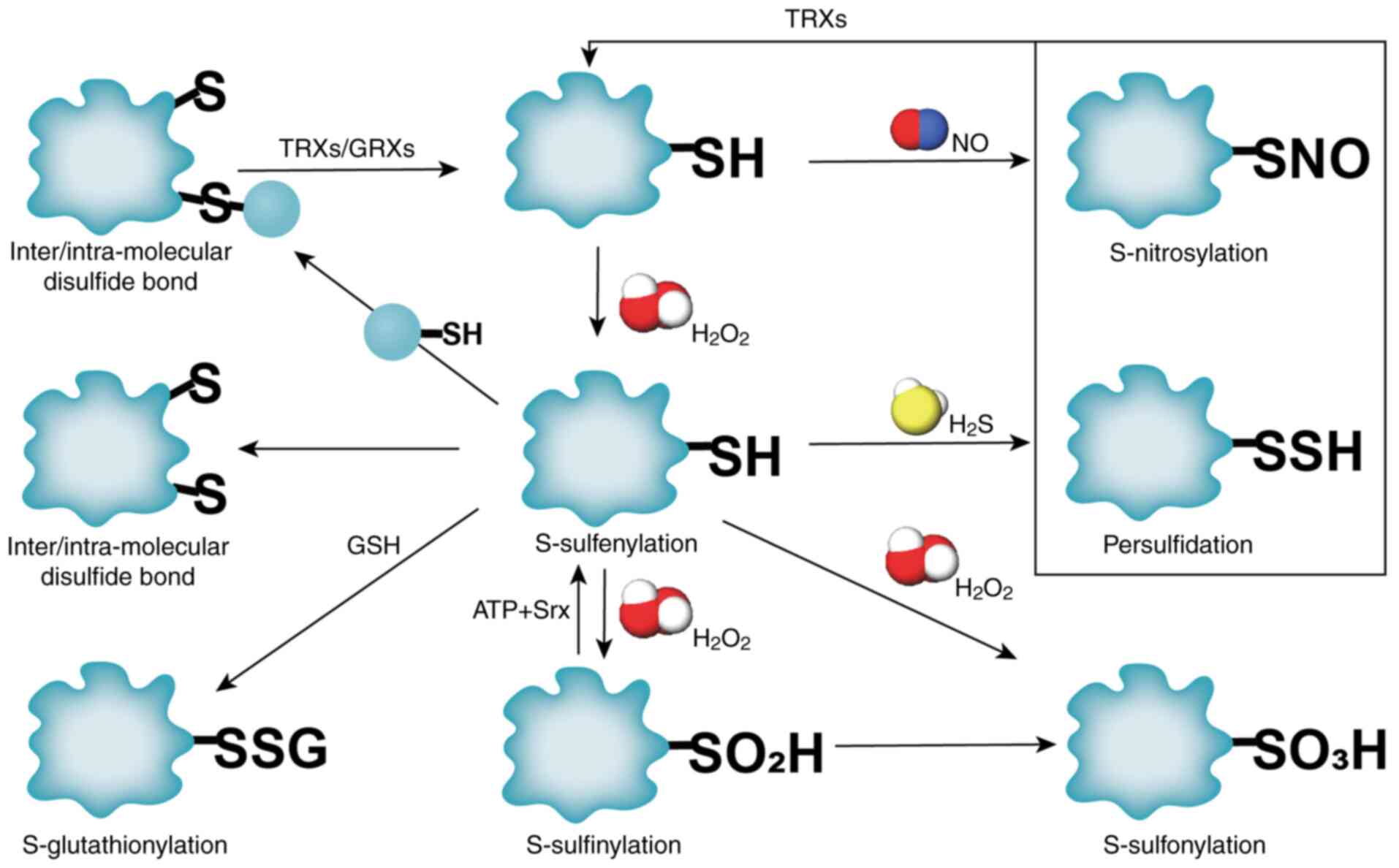
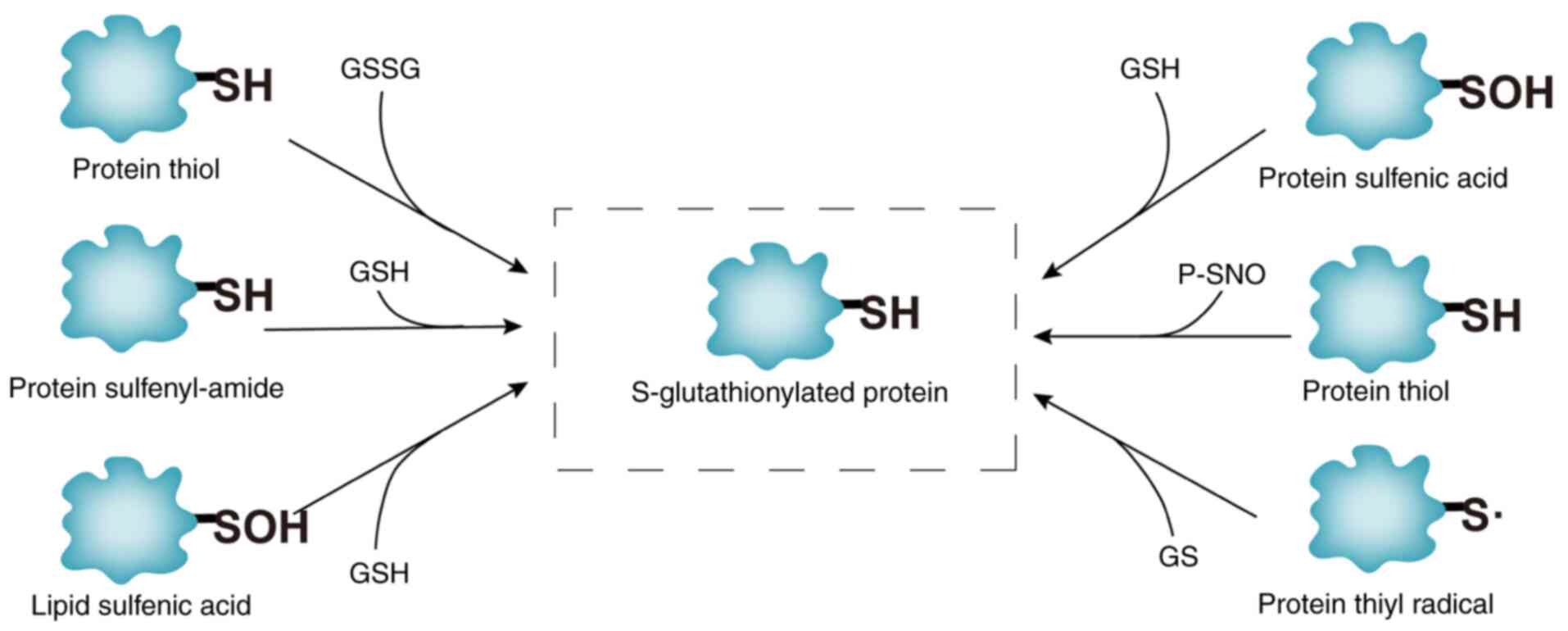
|
1
|
Mohammadi SA, Najafi H, Zolgharnian S,
Sharifian S and Asasian-Kolur N: Biological oxidation methods for
the removal of organic and inorganic contaminants from wastewater:
A comprehensive review. Sci Total Environ.
843(157026)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jakubczyk K, Dec K, Kałduńska J, Kawczuga
D, Kochman J and Janda K: Reactive oxygen species-sources,
functions, oxidative damage. Pol Merkur Lekarski. 48:124–127.
2020.PubMed/NCBI
|
|
3
|
Jomova K, Raptova R, Alomar SY, Alwasel
SH, Nepovimova E, Kuca K and Valko M: Reactive oxygen species,
toxicity, oxidative stress, and antioxidants: Chronic diseases and
aging. Arch Toxicol. 97:2499–2574. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Noguchi N, Saito Y and Niki E: Actions of
thiols, persulfides, and polysulfides as free radical scavenging
antioxidants. Antioxid Redox Signal. 39:728–743. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tan M, Yin Y, Ma X, Zhang J, Pan W, Tan M,
Zhao Y, Yang T, Jiang T and Li H: Glutathione system enhancement
for cardiac protection: Pharmacological options against oxidative
stress and ferroptosis. Cell Death Dis. 14(131)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xiao W and Loscalzo J: Metabolic responses
to reductive stress. Antioxid Redox Signal. 32:1330–1347.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kalinina EV and Gavriliuk LA: Glutathione
synthesis in cancer cells. Biochemistry (Mosc). 85:895–907.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang F, Yuan Q, Chen F, Pang J, Pan C, Xu
F and Chen Y: Fundamental mechanisms of the cell death caused by
nitrosative stress. Front Cell Dev Biol. 9(742483)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Diaz-Vivancos P, de Simone A, Kiddle G and
Foyer CH: Glutathione-linking cell proliferation to oxidative
stress. Free Radic Biol Med. 89:1154–1164. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Giustarini D, Milzani A, Dalle-Donne I and
Rossi R: How to increase cellular glutathione. Antioxidants
(Basel). 12(1094)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Corpas FJ, González-Gordo S,
Rodríguez-Ruiz M, Muñoz-Vargas MA and Palma JM: Thiol-based
oxidative posttranslational modifications (OxiPTMs) of plant
proteins. Plant Cell Physiol. 63:889–900. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hofmann F: The cGMP system: Components and
function. Biol Chem. 401:447–469. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hasan MM, Khatun MS and Kurata H: A
comprehensive review of in silico analysis for protein
S-sulfenylation sites. Protein Pept Lett. 25:815–821.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Corti A, Franzini M, Scataglini I and
Pompella A: Mechanisms and targets of the modulatory action of
S-nitrosoglutathione (GSNO) on inflammatory cytokines expression.
Arch Biochem Biophys. 562:80–91. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fu L, Liu K, Ferreira RB, Carroll KS and
Yang J: Proteome-wide analysis of cysteine S-sulfenylation using a
benzothiazine-based probe. Curr Protoc Protein Sci.
95(e76)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li C, Chen X, Zhang S, Liang C, Ma X,
Zhang R and Yan H: Glutaredoxin 1 protects lens epithelial cells
from epithelial-mesenchymal transition by preventing casein kinase
1α S-glutathionylation during posterior capsular opacification.
Redox Biol. 62(102676)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang J, Ye ZW, Singh S, Townsend DM and
Tew KD: An evolving understanding of the S-glutathionylation cycle
in pathways of redox regulation. Free Radic Biol Med. 120:204–216.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Holmgren A: Hydrogen donor system for
Escherichia coli ribonucleoside-diphosphate reductase
dependent upon glutathione. Proc Natl Acad Sci USA. 73:2275–2279.
1976.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fernandes AP and Holmgren A:
Glutaredoxins: Glutathione-dependent redox enzymes with functions
far beyond a simple thioredoxin backup system. Antioxid Redox
Signal. 6:63–74. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ogata FT, Branco V, Vale FF and Coppo L:
Glutaredoxin: Discovery, redox defense and much more. Redox Biol.
43(101975)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lillig CH, Berndt C and Holmgren A:
Glutaredoxin systems. Biochim Biophys Acta. 1780:1304–1317.
2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gladyshev VN, Liu A, Novoselov SV, Krysan
K, Sun QA, Kryukov VM, Kryukov GV and Lou MF: Identification and
characterization of a new mammalian glutaredoxin
(thioltransferase), Grx2. J Biol Chem. 276:30374–30380.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abdalla M, Eltayb WA and Yousif A:
Comparison of structures among Saccharomyces cerevisiae Grxs
proteins. Genes Environ. 40(17)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Matsui R, Ferran B, Oh A, Croteau D, Shao
D, Han J, Pimentel DR and Bachschmid MM: Redox regulation via
glutaredoxin-1 and protein S-glutathionylation. Antioxid Redox
Signal. 32:677–700. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sevilla F, Martí MC, De Brasi-Velasco S
and Jiménez A: Redox regulation, thioredoxins, and glutaredoxins in
retrograde signalling and gene transcription. J Exp Bot.
74:5955–5969. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang Y, Liao Z and Xiao Q: Metformin
ameliorates skeletal muscle atrophy in Grx1 KO mice by regulating
intramuscular lipid accumulation and glucose utilization. Biochem
Biophys Res Commun. 533:1226–1232. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li Y, Liang K, Yuan L, Gao J, Wei L and
Zhao L: The role of thioredoxin and glutathione systems in
arsenic-induced liver injury in rats under glutathione depletion.
Int J Environ Health Res. 34:547–563. 2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen X, Chen Y, Li C, Li J, Zhang S, Liang
C, Deng Q, Guo Z, Guo C and Yan H: Glutaredoxin 2 protects lens
epithelial cells from epithelial-mesenchymal transition by
suppressing mitochondrial oxidative stress-related upregulation of
integrin-linked kinase. Exp Eye Res. 234(109609)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ai Y, Meng Y, Yan B, Zhou Q and Wang X:
The biochemical pathways of apoptotic, necroptotic, pyroptotic, and
ferroptotic cell death. Mol Cell. 84:170–179. 2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vignane T and Filipovic MR: Emerging
chemical biology of protein persulfidation. Antioxid Redox Signal.
39:19–39. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Benhar M: Oxidants, antioxidants and thiol
redox switches in the control of regulated cell death pathways.
Antioxidants (Basel). 9(309)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu T, Sun L, Zhang Y, Wang Y and Zheng J:
Imbalanced GSH/ROS and sequential cell death. J Biochem Mol
Toxicol. 36(e22942)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liaghati A, Pileggi CA, Parmar G, Patten
DA, Hadzimustafic N, Cuillerier A, Menzies KJ, Burelle Y and Harper
ME: Grx2 regulates skeletal muscle mitochondrial structure and
autophagy. Front Physiol. 12(604210)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nie C, Tian C, Zhao L, Petit PX, Mehrpour
M and Chen Q: Cysteine 62 of Bax is critical for its conformational
activation and its proapoptotic activity in response to
H2O2-induced apoptosis. J Biol Chem.
283:15359–15369. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Adluri RS, Thirunavukkarasu M, Zhan L,
Dunna NR, Akita Y, Selvaraju V, Otani H, Sanchez JA, Ho YS and
Maulik N: Glutaredoxin-1 overexpression enhances neovascularization
and diminishes ventricular remodeling in chronic myocardial
infarction. PLoS One. 7(e34790)2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Corteselli E, Aboushousha R and
Janssen-Heininger Y: S-glutathionylation-controlled apoptosis of
lung epithelial cells; potential implications for lung fibrosis.
Antioxidants (Basel). 11(1789)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pan S and Berk BC: Glutathiolation
regulates tumor necrosis factor-alpha-induced caspase-3 cleavage
and apoptosis: Key role for glutaredoxin in the death pathway. Circ
Res. 100:213–219. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sun X, Ye C, Deng Q, Chen J and Guo C:
Contribution of glutaredoxin-1 to Fas s-glutathionylation and
inflammation in ethanol-induced liver injury. Life Sci.
264(118678)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang J, Guo J, Yang N, Huang Y, Hu T and
Rao C: Endoplasmic reticulum stress-mediated cell death in liver
injury. Cell Death Dis. 13(1051)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ye ZW, Zhang J, Aslam M, Blumental-Perry
A, Tew KD and Townsend DM: Protein disulfide isomerase family
mediated redox regulation in cancer. Adv Cancer Res. 160:83–106.
2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
He M, Hu J, Fang T, Tang W, Lv B, Yang B
and Xia J: Protein convertase subtilisin/Kexin type 9 inhibits
hepatocellular carcinoma growth by interacting with GSTP1 and
suppressing the JNK signaling pathway. Cancer Biol Med. 19:90–103.
2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Feng Y, Chen Y, Wu X, Chen J, Zhou Q, Liu
B, Zhang L and Yi C: Interplay of energy metabolism and autophagy.
Autophagy. 20:4–14. 2024.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jeong SJ and Oh GT: Unbalanced redox with
autophagy in cardiovascular disease. J Lipid Atheroscler.
12:132–151. 2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Li N, Wang J, Zang X, Wang Z, Zhang T,
Zhao B, Miao J and Lin Z: H2S probe CPC inhibits
autophagy and promotes apoptosis by inhibiting glutathionylation of
Keap1 at Cys434. Apoptosis. 26:111–131. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mancilla H, Maldonado R, Cereceda K,
Villarroel-Espíndola F, Montes de Oca M, Angulo C, Castro MA, Slebe
JC, Vera JC, Lavandero S and Concha II: Glutathione depletion
induces spermatogonial cell autophagy. J Cell Biochem.
116:2283–2292. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cianfruglia L, Perrelli A, Fornelli C,
Magini A, Gorbi S, Salzano AM, Antognelli C, Retta F, Benedetti V,
Cassoni P, et al: KRIT1 loss-of-function associated with cerebral
cavernous malformation disease leads to enhanced
S-glutathionylation of distinct structural and regulatory proteins.
Antioxidants (Basel). 8(27)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Armeni T, Ercolani L, Urbanelli L, Magini
A, Magherini F, Pugnaloni A, Piva F, Modesti A, Emiliani C and
Principato G: Cellular redox imbalance and changes of protein
S-glutathionylation patterns are associated with senescence induced
by oncogenic H-ras. PLoS One. 7(e52151)2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mallén-Ponce MJ and Pérez-Pérez ME:
Redox-mediated activation of ATG3 promotes ATG8 lipidation and
autophagy progression in Chlamydomonas reinhardtii. Plant Physiol.
194:359–375. 2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Foerster EG, Mukherjee T, Cabral-Fernandes
L, Rocha JDB, Girardin SE and Philpott DJ: How autophagy controls
the intestinal epithelial barrier. Autophagy. 18:86–103.
2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lee SR, Yang KS, Kwon J, Lee C, Jeong W
and Rhee SG: Reversible inactivation of the tumor suppressor PTEN
by H2O2. J Biol Chem. 277:20336–20342.
2002.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zhang J, Tripathi DN, Jing J, Alexander A,
Kim J, Powell RT, Dere R, Tait-Mulder J, Lee JH, Paull TT, et al:
ATM functions at the peroxisome to induce pexophagy in response to
ROS. Nat Cell Biol. 17:1259–1269. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang H, Wang N, Xu D, Ma Q, Chen Y, Xu S,
Xia Q, Zhang Y, Prehn JHM, Wang G and Ying Z: Oxidation of multiple
MiT/TFE transcription factors links oxidative stress to
transcriptional control of autophagy and lysosome biogenesis.
Autophagy. 16:1683–1696. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
López-Grueso MJ, Lagal DJ, García-Jiménez
ÁF, Tarradas RM, Carmona-Hidalgo B, Peinado J, Requejo-Aguilar R,
Bárcena JA and Padilla CA: Knockout of PRDX6 induces mitochondrial
dysfunction and cell cycle arrest at G2/M in HepG2 hepatocarcinoma
cells. Redox Biol. 37(101737)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Murata H, Ihara Y, Nakamura H, Yodoi J,
Sumikawa K and Kondo T: Glutaredoxin exerts an antiapoptotic effect
by regulating the redox state of Akt. J Biol Chem. 278:50226–50233.
2003.PubMed/NCBI View Article : Google Scholar
|
|
56
|
López-Grueso MJ, González-Ojeda R,
Requejo-Aguilar R, McDonagh B, Fuentes-Almagro CA, Muntané J,
Bárcena JA and Padilla CA: Thioredoxin and glutaredoxin regulate
metabolism through different multiplex thiol switches. Redox Biol.
21(101049)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ye K, Chen Z and Xu Y: The double-edged
functions of necroptosis. Cell Death Dis. 14(163)2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Che L, Yang CL, Chen Y, Wu ZL, Du ZB, Wu
JS, Gan CL, Yan SP, Huang J, Guo NJ, et al: Mitochondrial
redox-driven mitofusin 2 S-glutathionylation promotes neuronal
necroptosis via disrupting ER-mitochondria crosstalk in
cadmium-induced neurotoxicity. Chemosphere.
262(127878)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Bozonet SM, Magon NJ, Schwartfeger AJ,
Konigstorfer A, Heath SG, Vissers MCM, Morris VK, Göbl C, Murphy
JM, Salvesen GS and Hampton MB: Oxidation of caspase-8 by
hypothiocyanous acid enables TNF-mediated necroptosis. J Biol Chem.
299(104792)2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Gorelenkova Miller O, Behring JB, Siedlak
SL, Jiang S, Matsui R, Bachschmid MM, Zhu X and Mieyal JJ:
Upregulation of glutaredoxin-1 activates microglia and promotes
neurodegeneration: Implications for Parkinson's disease. Antioxid
Redox Signal. 25:967–982. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Thawkar BS and Kaur G: Inhibitors of NF-κB
and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic
opportunities in neuroinflammation induced early-stage Alzheimer's
disease. J Neuroimmunol. 326:62–74. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Shao R, Lou X, Xue J, Yang Y, Ning D, Chen
G and Jiang L: Thioredoxin-1 regulates IRE1α to ameliorate
sepsis-induced NLRP3 inflammasome activation and oxidative stress
in Raw 264.7 cell. Immunopharmacol Immunotoxicol. 45:277–286.
2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wang Y, Shi P, Chen Q, Huang Z, Zou D,
Zhang J, Gao X and Lin Z: Mitochondrial ROS promote macrophage
pyroptosis by inducing GSDMD oxidation. J Mol Cell Biol.
11:1069–1082. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Newton K, Strasser A, Kayagaki N and Dixit
VM: Cell death. Cell. 187:235–256. 2024.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Chen T, Liang L, Wang Y, Li X and Yang C:
Ferroptosis and cuproptposis in kidney diseases: Dysfunction of
cell metabolism. Apoptosis. 29:289–302. 2024.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Lee J, You JH, Shin D and Roh JL:
Inhibition of glutaredoxin 5 predisposes cisplatin-resistant head
and neck cancer cells to Ferroptosis. Theranostics. 10:7775–7786.
2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Seco-Cervera M, González-Cabo P, Pallardó
FV, Romá-Mateo C and García-Giménez JL: Thioredoxin and
glutaredoxin systems as potential targets for the development of
new treatments in Friedreich's ataxia. Antioxidants (Basel).
9(1257)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Liu X, Zhuang L and Gan B: Disulfidptosis:
Disulfide stress-induced cell death. Trends Cell Biol. 34:327–337.
2024.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Elftmaoui Z and Bignon E: Robust AMBER
force field parameters for glutathionylated cysteines. Int J Mol
Sci. 24(15022)2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Guan L, Mao Z, Yang S, Wu G, Chen Y, Yin
L, Qi Y, Han L and Xu L: Dioscin alleviates Alzheimer's disease
through regulating RAGE/NOX4 mediated oxidative stress and
inflammation. Biomed Pharmacother. 152(113248)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Rani P, Krishnan S and Rani Cathrine C:
Study on analysis of peripheral biomarkers for Alzheimer's disease
diagnosis. Front Neurol. 8(328)2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Akterin S, Cowburn RF, Miranda-Vizuete A,
Jiménez A, Bogdanovic N, Winblad B and Cedazo-Minguez A:
Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid
toxicity and Alzheimer's disease. Cell Death Differ. 13:1454–1465.
2006.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Kommaddi RP, Tomar DS, Karunakaran S,
Bapat D, Nanguneri S, Ray A, Schneider BL, Nair D and Ravindranath
V: Glutaredoxin1 diminishes amyloid beta-mediated oxidation of
F-actin and reverses cognitive deficits in an Alzheimer's disease
mouse model. Antioxid Redox Signal. 31:1321–1338. 2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Dionísio PA, Amaral JD and Rodrigues CMP:
Oxidative stress and regulated cell death in Parkinson's disease.
Ageing Res Rev. 67(101263)2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Johnson WM, Yao C, Siedlak SL, Wang W, Zhu
X, Caldwell GA, Wilson-Delfosse AL, Mieyal JJ and Chen SG:
Glutaredoxin deficiency exacerbates neurodegeneration in C.
elegans models of Parkinson's disease. Hum Mol Genet.
24:1322–1335. 2015.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Xu J, Kao SY, Lee FJ, Song W, Jin LW and
Yankner BA: Dopamine-dependent neurotoxicity of alpha-synuclein: A
mechanism for selective neurodegeneration in Parkinson disease. Nat
Med. 8:600–606. 2002.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Johnson WM, Golczak M, Choe K, Curran PL,
Miller OG, Yao C, Wang W, Lin J, Milkovic NM, Ray A, et al:
Regulation of DJ-1 by glutaredoxin 1 in vivo: Implications for
Parkinson's disease. Biochemistry. 55:4519–4532. 2016.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Tokarew JM, El-Kodsi DN, Lengacher NA,
Fehr TK, Nguyen AP, Shutinoski B, O'Nuallain B, Jin M, Khan JM, Ng
ACH, et al: Age-associated insolubility of parkin in human midbrain
is linked to redox balance and sequestration of reactive dopamine
metabolites. Acta Neuropathol. 141:725–754. 2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Mailloux RJ, Xuan JY, McBride S, Maharsy
W, Thorn S, Holterman CE, Kennedy CR, Rippstein P, deKemp R, da
Silva J, et al: Glutaredoxin-2 is required to control oxidative
phosphorylation in cardiac muscle by mediating deglutathionylation
reactions. J Biol Chem. 289:14812–14828. 2014.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Zima AV and Mazurek SR: Functional impact
of ryanodine receptor oxidation on intracellular calcium regulation
in the heart. Rev Physiol Biochem Pharmacol. 171:39–62.
2016.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Wegener JW, Wagdi A, Wagner E, Katschinski
DM, Hasenfuss G, Bruegmann T and Lehnart SE: The RyR2-R2474S
mutation sensitizes cardiomyocytes and hearts to catecholaminergic
stress-induced oxidation of the mitochondrial glutathione pool.
Front Physiol. 12(777770)2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Rashdan NA, Shrestha B and Pattillo CB:
S-glutathionylation, friend or foe in cardiovascular health and
disease. Redox Biol. 37(101693)2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Mizuno M, Matsuzaki T, Ozeki N, Katano H,
Koga H, Takebe T, Yoshikawa HY and Sekiya I: Cell membrane fluidity
and ROS resistance define DMSO tolerance of cryopreserved synovial
MSCs and HUVECs. Stem Cell Res Ther. 13(177)2022.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Duan R, Pan H, Li D, Liao S and Han B:
Ergothioneine improves myocardial remodeling and heart function
after acute myocardial infarction via S-glutathionylation through
the NF-ĸB dependent Wnt5a-sFlt-1 pathway. Eur J Pharmacol.
950(175759)2023.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Fan X, Dong T, Yan K, Ci X and Peng L:
PM2.5 increases susceptibility to acute exacerbation of COPD via
NOX4/Nrf2 redox imbalance-mediated mitophagy. Redox Biol.
59(102587)2023.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Kuipers I, Guala AS, Aesif SW, Konings G,
Bouwman FG, Mariman EC, Wouters EF, Janssen-Heininger YM and
Reynaert NL: Cigarette smoke targets glutaredoxin 1, increasing
s-glutathionylation and epithelial cell death. Am J Respir Cell Mol
Biol. 45:931–937. 2011.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Chia SB, Nolin JD, Aboushousha R, Erikson
C, Irvin CG, Poynter ME, van der Velden J, Taatjes DJ, van der
Vliet A, Anathy V, et al: Glutaredoxin deficiency promotes
activation of the transforming growth factor beta pathway in airway
epithelial cells, in association with fibrotic airway remodeling.
Redox Biol. 37(101720)2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Guo Y, Liu Y, Zhao S, Xu W, Li Y, Zhao P,
Wang D, Cheng H, Ke Y and Zhang X: Oxidative stress-induced FABP5
S-glutathionylation protects against acute lung injury by
suppressing inflammation in macrophages. Nat Commun.
12(7094)2021.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Zhang D, Wang X, Chen S, Chen S, Yu W, Liu
X, Yang G, Tao Y, Tang X, Bu D, et al: Endogenous hydrogen sulfide
sulfhydrates IKKβ at cysteine 179 to control pulmonary artery
endothelial cell inflammation. Clin Sci (Lond). 133:2045–2059.
2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Reynaert NL, van der Vliet A, Guala AS,
McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS,
Matthews DE, et al: Dynamic redox control of NF-kappaB through
glutaredoxin-regulated S-glutathionylation of inhibitory kappaB
kinase beta. Proc Natl Acad Sci USA. 103:13086–13091.
2006.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Zhang X, Liu P, Zhang C, Chiewchengchol D,
Zhao F, Yu H, Li J, Kambara H, Luo KY, Venkataraman A, et al:
Positive regulation of interleukin-1β bioactivity by physiological
ROS-mediated cysteine S-glutathionylation. Cell Rep. 20:224–235.
2017.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Anathy V, Lahue KG, Chapman DG, Chia SB,
Casey DT, Aboushousha R, van der Velden JLJ, Elko E, Hoffman SM,
McMillan DH, et al: Reducing protein oxidation reverses lung
fibrosis. Nat Med. 24:1128–1135. 2018.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Shahmarvand N, Nagy A, Shahryari J and
Ohgami RS: Mutations in the signal transducer and activator of
transcription family of genes in cancer. Cancer Sci. 109:926–933.
2018.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Anastasiou D, Poulogiannis G, Asara JM,
Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW,
Auld DS, et al: Inhibition of pyruvate kinase M2 by reactive oxygen
species contributes to cellular antioxidant responses. Science.
334:1278–1283. 2011.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Li X, Ma Y, Wu J, Ni M, Chen A, Zhou Y,
Dai W, Chen Z, Jiang R, Ling Y, et al: Thiol oxidative
stress-dependent degradation of transglutaminase2 via protein
S-glutathionylation sensitizes 5-fluorouracil therapy in
5-fluorouracil-resistant colorectal cancer cells. Drug Resist
Updat. 67(100930)2023.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Zhang L, Zhang J, Ye Z, Manevich Y,
Townsend DM, Marshall DT and Tew KD: S-glutathionylated serine
proteinase inhibitors as biomarkers for radiation exposure in
prostate cancer patients. Sci Rep. 9(13792)2019.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Zhang J, Ye ZW, Chen W, Culpepper J, Jiang
H, Ball LE, Mehrotra S, Blumental-Perry A, Tew KD and Townsend DM:
Altered redox regulation and S-glutathionylation of BiP contribute
to bortezomib resistance in multiple myeloma. Free Radic Biol Med.
160:755–767. 2020.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Abo M and Weerapana E: Chemical probes for
redox signaling and oxidative stress. Antioxid Redox Signal.
30:1369–1386. 2019.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Xiao H, Jedrychowski MP, Schweppe DK,
Huttlin EL, Yu Q, Heppner DE, Li J, Long J, Mills EL, Szpyt J, et
al: A quantitative tissue-specific landscape of protein redox
regulation during aging. Cell. 180:968–983.e24. 2020.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Subramani J, Kundumani-Sridharan V and Das
KC: Chaperone-mediated autophagy of eNOS in myocardial
ischemia-reperfusion injury. Circ Res. 129:930–945. 2021.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Wu X, Liu L, Zheng Q, Ye H, Yang H, Hao H
and Li P: Dihydrotanshinone I preconditions myocardium against
ischemic injury via PKM2 glutathionylation sensitive to ROS. Acta
Pharm Sin B. 13:113–127. 2023.PubMed/NCBI View Article : Google Scholar
|