1. Introduction
Invasive candidiasis (IC) is a life-threatening
fungal infection that is caused mainly by Candida albicans
and other non-albicans Candida (NAC) species, including
Candida glabrata, Candida parapsilosis, Candida
tropicalis and Candida krusei. It manifests as
candidemia or disseminated infection involving multiple organs,
such as the liver, spleen, heart and central nervous system.
Diagnosis is complicated due to non-specific symptoms such as
fever, hypotension and multi-organ dysfunction. Blood cultures are
the gold standard for diagnosis, but have limited sensitivity. The
usual treatment involves the use of echinocandins or fluconazole,
while the surgical removal of infected medical devices is also
pivotal for patient management purposes (1,2).
IC is associated with a very high mortality in
intensive care units (ICUs) of 30-50%, which necessitates rapid
identification and treatment with appropriate antifungals. The
emergence of antifungal resistance among NAC species has
complicated management. Thus, infection control strategies,
antifungal stewardship programs, catheter care and the timely
removal of infected central venous lines are required to reduce the
incidence of IC in ICUs. Among the common fungal infections
acquired in ICUs worldwide, Candida species rank 4th among
bloodstream pathogens encountered by hospitalized patients, with
many being NAC species resistant to fluconazole and other
antifungals. The global incidence of IC exhibits a wide variation,
with higher rates in regions with limited healthcare resources and
high antibiotic use. Another significant contributor to mortality
is the delay in diagnosis and inappropriate antifungal therapy in
patients in ICUs whose underlying conditions are already severe
(1-4).
While the recent comprehensive review by Soriano
et al (1) addressed current
clinical challenges and unmet needs in invasive candidiasis across
adult populations, the present review focuses specifically on
critically ill patients in ICUs. The present review highlights
ICU-specific risk factors, diagnostic limitations, therapeutic
complexities due to organ dysfunction and invasive devices, and
unique management strategies. By adopting this focused perspective,
the present review complements prior research and provides
intensivists with practical, ICU-centered insights into the
management of invasive candidiasis (3).
2. Pathogenesis of invasive candidiasis
IC, characterized by the ability to induce
widespread systematic and immune evasion, is a disorder
precipitated by the interaction of colonization, epithelial
invasion, bloodstream dissemination and immune escape (5). Candida species are part of the
normal commensal flora of the skin, gastrointestinal tract and
other mucosal surfaces; these sites function as endogenous
reservoirs rather than sources of external acquisition. Under
normal conditions, host immunity and bacterial microbiota maintain
a balanced state that prevents fungal overgrowth. However, factors
such as immunodepression, broad-spectrum antibiotic use, critical
illness and the insertion of indwelling medical devices disrupt
this equilibrium, enabling Candida to shift from commensal
to pathogen (4-6).
The invasive process begins with opportunistic overgrowth in
response to host defense alterations (as illustrated in Fig. 1), followed by adhesion to epithelial
cells or medical devices through surface adhesins (5,6). A
critical virulence step is the yeast-to-hyphal transition, which
allows penetration of mucosal barriers. Hyphal forms secrete
hydrolytic enzymes, including secreted aspartyl proteases and
phospholipases, leading to tissue damage and deeper invasion
(5). Once the mucosal barrier is
compromised, Candida can translocate into the bloodstream,
resulting in candidemia. Hematogenous dissemination permits
colonization of distant organs such as the liver, spleen, kidneys
and brain (4,5). Biofilm formation, particularly on
central venous catheters, contributes to persistent infection by
enhancing resistance to antifungal therapy and impairing immune
clearance (4,6). Candida employs several immune
evasion strategies, including antigenic variation, secretion of
immunomodulatory molecules and resistance to oxidative stress
(5,6). Host factors, such as neutropenia,
corticosteroid therapy and impaired T-cell responses significantly
increase the risk of systemic dissemination, culminating in sepsis,
multiorgan failure and high mortality in critically ill patients
(4-7).
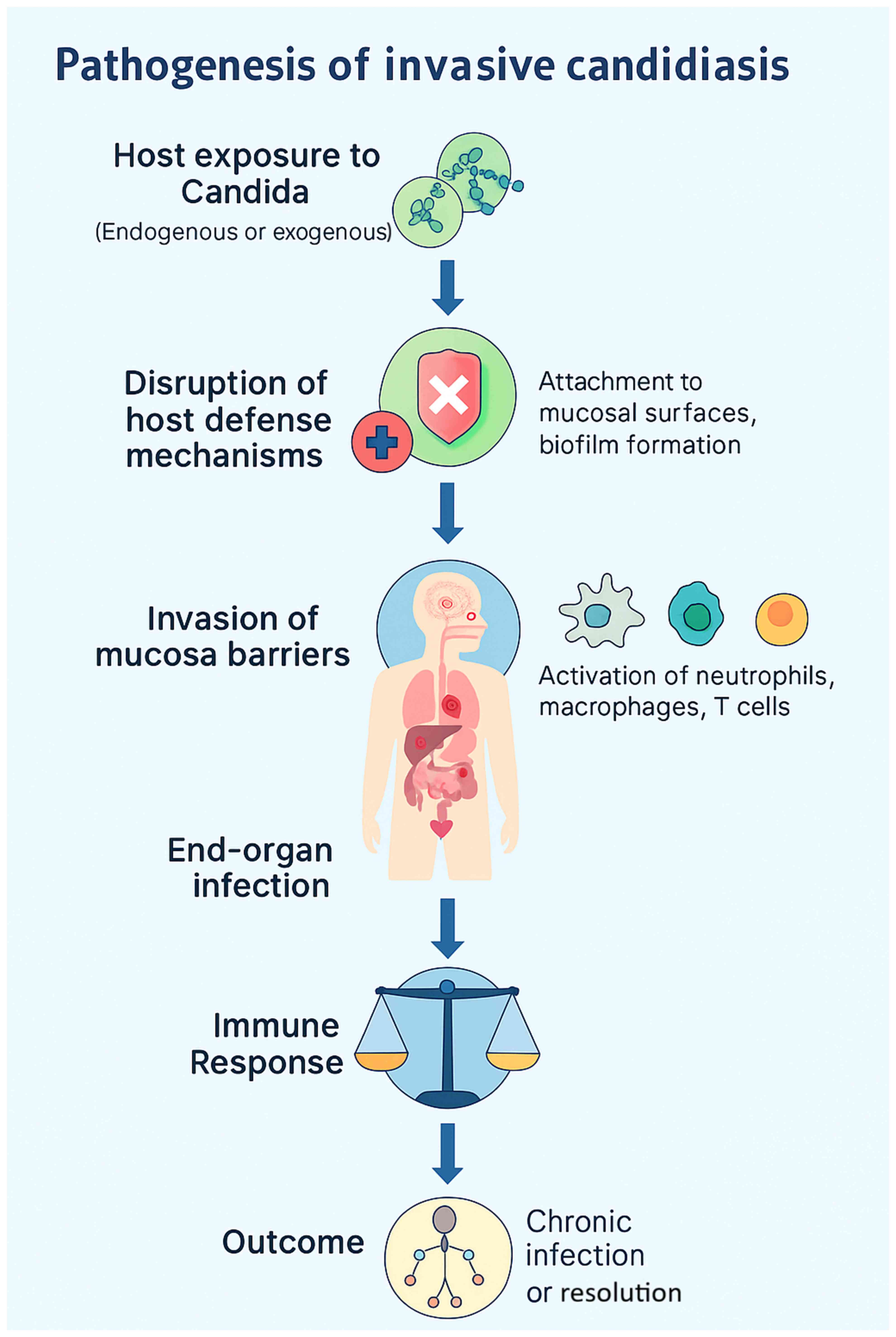 | Figure 1Pathogenesis of invasive candidiasis.
Schematic flowchart illustrating the sequence from the initial
colonization of mucosal surfaces and skin, through the disruption
of host defenses (e.g., broad-spectrum antibiotics,
immunosuppression, total parenteral nutrition), adhesion and
biofilm formation on indwelling devices (central venous catheters),
yeast-to-hyphae morphogenesis with the secretion of hydrolytic
enzymes (secreted aspartyl proteases and phospholipases), mucosal
invasion and translocation into the bloodstream (candidemia),
hematogenous dissemination to distant organs (liver, spleen,
kidneys and brain), immune evasion mechanisms (antigenic variation,
oxidative stress resistance, modulation of host response), and the
resulting clinical outcomes including sepsis, multi-organ failure,
and high mortality rates in intensive care units. |
Risk factors
IC in patients in ICUs is strongly associated with
multiple predisposing factors, including the prolonged use of
broad-spectrum antibiotics, central venous catheterization,
mechanical ventilation, parenteral nutrition, renal replacement
therapy, corticosteroid therapy and underlying immunosuppression.
Recent systematic reviews confirm that these risk factors
significantly increase susceptibility to candidemia and invasive
fungal disease in critically ill patients (6-8). A
summary of the key ICU-specific risk factors for IC is presented in
Table I.
 | Table IRisk factors: Key ICU-specific risk
factors for invasive candidiasis, including device-related,
pharmacological and host-related contributors. |
Table I
Risk factors: Key ICU-specific risk
factors for invasive candidiasis, including device-related,
pharmacological and host-related contributors.
| Risk factor | Description |
|---|
| Immunocompromised
state | Conditions
including HIV/AIDS, cancer, and other disorders involving
immunosuppressive therapies (e.g., corticosteroids, chemotherapy)
reduce the ability of the body to oppose infections. |
| Diabetes
mellitus | High blood sugar
and insulin resistance may enhance Candida growth,
particularly in patients with uncontrolled glucose levels. |
| Antibiotic use | Broad-spectrum
antibiotics disrupt the normal microbiota, promoting Candida
overgrowth. |
| Indwelling medical
devices | Catheters,
prosthetics, and central venous lines provide surfaces for
Candida adherence, increasing the risk of infection. |
| Recent surgery or
trauma | Surgical
interventions or mucosal injuries may create entry points for
Candida invasion into deeper tissues. |
| Prolonged periods
of hospitalization | Longer
hospitalization periods increase exposure to hospital-acquired
infections and invasive devices. |
| Corticosteroid
therapy | Long-term steroid
use alters immune function, favoring Candida growth. |
| Pregnancy | Hormonal changes
during pregnancy alter vaginal pH, predisposing women to vaginal
candidiasis. |
| High estrogen
levels | Estrogen (e.g.,
oral contraceptives) may displace normal vaginal flora,
facilitating Candida overgrowth. |
| Obesity | Excess body weight,
particularly abdominal fat, creates moist environments favorable
for fungal growth. |
| Poor oral
hygiene | Inadequate oral
care predisposes to oral Candida overgrowth (oral thrush). |
| Malnutrition/poor
diet | Micronutrient
deficiencies impair immune function, increasing susceptibility to
infection. |
| Age | Infants, elderly
persons, and immunocompromised patients are more susceptible. |
|
Chemotherapy/radiation | Cancer therapies
weaken immune defenses, predisposing to infection. |
| Intravenous drug
use (IVDU) | Contaminated
needles or unsterile practices may introduce Candida into
the bloodstream, causing systemic infection. |
Symptoms
The clinical presentation of IC in patients in the
ICU is usually non-specific, often mimicking bacterial sepsis.
Common features include persistent fever despite broad-spectrum
antibiotics, hypotension, signs of septic shock, multi-organ
dysfunction, and occasionally, the focal involvement of organs such
as the liver, spleen, or kidneys (4-9).
These manifestations are summarized in Table II for clarity.
 | Table IISymptoms: Common clinical features of
invasive candidiasis in critically ill patients, highlighting
non-specific symptoms that often mimic bacterial sepsis. |
Table II
Symptoms: Common clinical features of
invasive candidiasis in critically ill patients, highlighting
non-specific symptoms that often mimic bacterial sepsis.
| Symptom
type/site | Symptoms |
|---|
| Systemic
symptoms | Persistent fever,
chills, hypotension, tachycardia, fatigue, sepsis-like symptoms
(severe cases). |
| Bloodstream
Infection (candidemia) | Persistent fever,
positive blood cultures for Candida, sepsis (fever, chills,
low blood pressure). |
| Endocarditis
(heart) | Fever, heart
murmurs, embolic phenomena (e.g., stroke, skin lesions). |
| Ophthalmic
(endophthalmitis) | Vision changes, eye
pain, redness. |
| Renal
(kidneys) | Fever, flank pain,
dysuria, hematuria. |
| Hepatosplenic
candidiasis | Abdominal pain,
jaundice, hepatomegaly (enlarged liver). |
| Peritonitis
(abdomen) | Abdominal
tenderness, distension, fever. |
| Pulmonary
(lungs) | Respiratory
distress if lungs are involved. |
| Gastrointestinal
symptoms | Nausea, vomiting
(if digestive tract is affected). |
Diagnosis
Diagnosis remains challenging due to the
non-specific clinical features and the limited sensitivity of blood
cultures. Culture-based methods remain the gold standard but
require up to 72 h for results. Non-culture-based assays, including
(1→3)-β-D-glucan (BDG), mannan antigen/anti-mannan antibody and
PCR-based tests, provide a more rapid detection, while advanced
platforms, such as the T2Candida panel allow for rapid species
identification directly from blood samples (9-11).
The strengths and limitations of available diagnostic modalities
are summarized in Table III.
 | Table IIIDiagnostic modalities for invasive
candidiasis: Performance characteristics, turnaround time and
limitations in intensive care unit settings. |
Table III
Diagnostic modalities for invasive
candidiasis: Performance characteristics, turnaround time and
limitations in intensive care unit settings.
| Diagnostic
method | Description |
|---|
| Clinical
Evaluation | Detailed history
and physical examination, particularly in immunocompromised
patients (cancer, diabetes, transplant). |
| Blood cultures | Gold standard for
candidemia; samples collected before antifungal initiation. |
| Urine culture | Used in suspected
renal involvement (candiduria). |
| Tissue biopsy and
culture | Obtained from
affected organs (liver, kidney, heart) for definitive
diagnosis. |
| PCR testing | Detects
Candida DNA in blood or body fluids. |
| Serological
tests | Mannan/anti-mannan
antibodies and β-D-glucan test (fungal cell wall component). |
| Imaging
analyses | Computed tomography
scan or chest X-ray for pulmonary or abdominal involvement. |
| Endoscopy | Used for
gastrointestinal candidiasis to visualize mucosal lesions. |
| Microscopic
examination | Direct microscopy
of tissue/fluid (e.g., abscess or peritoneal fluid) for
yeasts/pseudohyphe. |
| Antigen
detection | Detection of
Candida antigens in serum or body fluids, adjunctive
diagnostic tool. |
3. Management of invasive candidiasis
The management of IC in the ICU is built upon three
essential principles: The timely initiation of empiric antifungal
therapy, targeted treatment tailored to pathogen identification and
susceptibility results, and effective source control. These
pillars, supported by careful monitoring and supportive care,
remain the cornerstone for improving the survival of critically ill
patients. The early initiation of empiric therapy is crucial, as
delays are consistently associated with increased morbidity and
mortality rates. Echinocandins (caspofungin, micafungin and
anidulafungin) are generally recommended as first-line agents in
this setting, owing to their broad antifungal spectrum, fungicidal
activity against the majority of Candida species and
favorable safety profile. In hemodynamically stable patients
without prior azole exposure and with a high likelihood of
Candida albicans infection, fluconazole may be considered as
an alternative. Amphotericin B, while highly effective, is
generally reserved for patients with resistant species infection or
intolerance to other agents, given its nephrotoxicity and
infusion-related side-effects (12-14).
Once the infecting species and susceptibility
profile are available, de-escalation to targeted therapy is
essential. Candida albicans is usually susceptible to
fluconazole, which remains an effective step-down option, whereas
non-albicans species such as Candida glabrata and Candida
krusei often necessitate continued echinocandin or amphotericin
B therapy due to resistance patterns. This highlights the
importance of routine susceptibility testing, which allows
clinicians to optimize therapy and reduce the risk of treatment
failure or resistance emergence (12-15).
The recommended duration of antifungal therapy is
typically at least 14 days following the documented clearance of
Candida from the bloodstream and the resolution of clinical
signs and symptoms. However, deeper foci of infection, such as
endocarditis, osteomyelitis, or intra-abdominal abscesses may
require extended courses of therapy lasting several weeks. The
removal of central venous catheters or other indwelling devices
implicated as the source of infection is critical, as antifungal
therapy alone is often insufficient in the presence of
biofilm-associated colonization. Similarly, surgical drainage or
debridement may be required for abscesses or localized invasive
disease (12,15-17).
Supportive care plays an equally critical role in
management. This includes hemodynamic stabilization, glycemic
control (particularly in diabetic patients receiving
corticosteroids), nutritional optimization and organ support
measures, as needed. Regular monitoring with repeat blood cultures,
imaging analyses to evaluate persistent infection, and the
laboratory surveillance of renal and hepatic function is essential
to assess the treatment response and mitigate drug-related
toxicities (14-17).
In summary, the optimal management of invasive
candidiasis in the ICU requires an integrated approach that
combines the rapid initiation of empiric antifungal therapy,
adjustment based on pathogen-directed susceptibility, effective
source control and comprehensive supportive care. Despite these
well-established principles, clinicians continue to face
substantial barriers that compromise timely diagnosis, appropriate
therapy and patient outcomes.
4. Challenges in the management of invasive
candidiasis in the ICU
The management of IC in critically ill patients
remains particularly challenging due to the interplay between host
factors, diagnostic limitations, therapeutic complexity and
healthcare system constraints. Patients in ICUs frequently present
with multiple organ dysfunction (renal, hepatic, or
cardiovascular), that not only complicates the course of illness,
but also alters antifungal pharmacokinetics, necessitating dose
adjustments and vigilant monitoring for toxicity. Immunocompromised
states, whether due to hematologic malignancies, organ
transplantation, corticosteroid therapy, or HIV infection, further
diminish host defenses and predispose patients to persistent or
recurrent infections. Delayed diagnosis continues to be one of the
most crucial obstacles. Clinical manifestations are non-specific
fever, hypotension, or an unexplained clinical deterioration and
are easily confounded with bacterial sepsis or other ICU-related
infections. While blood culture remains the gold standard for
candidemia, it is a lengthy process, requiring 48-72 h for results.
This diagnostic delay, compounded by the limited availability of
advanced molecular or antigen-based tests in numerous ICUs, often
postpones the initiation of effective antifungal therapy, thereby
worsening outcomes (17,18).
Antifungal resistance represents another growing
concern, particularly among NAC species, such as Candida
glabrata and Candida krusei, which exhibit a reduced
susceptibility to azoles. Although echinocandins have emerged as
effective alternatives, resistance, albeit uncommon, has been
reported. The absence of oral echinocandin formulations also
complicates step-down therapy and prolongs outpatient management.
Drug-drug interactions and polypharmacy further complicate the care
of patients in ICUs, as antifungal agents may potentiate toxicities
or alter the efficacy of other commonly administered drugs,
including immunosuppressants, vasopressors and nephrotoxic
antimicrobials (19-22).
The presence of invasive medical devices, including
central venous catheters, endotracheal tubes and urinary catheters,
also amplifies the risk of biofilm-associated infections that are
poorly responsive to antifungal therapy alone. While device removal
is critical for achieving a cure, this may be difficult or
impossible in critically ill patients who depend on these
interventions for survival. Such challenges, compounded by the lack
of ICU-specific clinical guidelines for antifungal dosing, the
duration of therapy and source control strategies, often leave
clinicians to rely on individualized decision-making rather than
standardized evidence-based protocols (22-24).
Beyond clinical complexities, IC imposes a
significant economic burden, primarily due to prolonged periods of
hospitalization in the ICU, repeated interventions and the high
cost of antifungal agents (17,18,20).
Prolonged periods of hospitalization, device-related procedures and
the need for intensive supportive care substantially increase
direct and indirect healthcare expenditures. Furthermore, the
healthcare-associated nature of IC underscores the importance, but
also the difficulty, of prevention in ICUs, where factors such as
overcrowding, frequent use of invasive devices, and extended
hospitalization create an environment conducive to nosocomial
transmission (17,18,21).
The psychological and emotional toll on patients is
another underrecognized dimension. Extended critical illness,
invasive procedures, uncertain prognoses and the high risk of
complications contribute to anxiety, depression and psychological
distress in patients and their families, further compounding the
burden of IC in ICU settings (20).
Collectively, these clinical, economic and
psychosocial challenges highlight the urgent need for earlier
diagnosis, individualized therapy, improved infection prevention,
and ICU-specific clinical guidance to improve outcomes in this
vulnerable population (17,18,21).
Notably, the growing recognition of these
limitations has catalyzed significant innovations across
diagnostics, therapeutics and preventive strategies. Novel
diagnostic tools include rapid non-culture-based assays such as
(1→3)-β-D-glucan, mannan antigen and anti-mannan antibody tests,
PCR-based platforms, and the T2Candida panel. These modalities
significantly reduce the time to detection compared with blood
cultures, enabling earlier initiation of targeted therapy (19-24).
The T2Candida panel, in particular, can detect Candida DNA
directly from whole blood within 3-5 h with high sensitivity and
specificity, representing a major advance in early diagnosis
(23,24).
On the therapeutic front, newer antifungal agents
and treatment strategies are reshaping management. Echinocandins
remain first-line agents; however, the recent development of new
triazoles, such as isavuconazole and investigational agents such as
rezafungin and ibrexafungerp provides expanded options, including
improved pharmacokinetics and oral formulations for step-down
therapy (25-29).
Combination antifungal therapy is being explored to overcome
resistance among non-albicans Candida species (28,29).
Therapeutic drug monitoring (TDM) for agents, such as voriconazole
and posaconazole further enables individualized dosing in
critically ill patients with altered pharmacokinetics (30-32).
In parallel, infection prevention and stewardship
programs have become central components of management. These
include strict catheter care protocols, antifungal prophylaxis in
selected high-risk populations, and antifungal stewardship
initiatives that promote judicious drug use and de-escalation based
on local epidemiology and biomarker data (30,31,33-41).
Collectively, these innovations in diagnostics, therapeutics and
prevention represent a paradigm shift toward more rapid, tailored
and multidisciplinary approaches to IC in the ICU (17-24,28-31).
5. Advances in the management of invasive
candidiasis in the ICU
In recent years, notable advances have been made to
address the challenges encountered in the management of IC,
spanning from earlier diagnostic approaches to novel therapeutic
strategies and multidisciplinary care models. Early recognition
remains the cornerstone of improved outcomes, and several
non-culture diagnostic tools have markedly shortened the diagnostic
window compared with conventional blood cultures. Blood cultures,
although considered the reference standard, have limited
sensitivity (~50%) and require 24-72 h to yield positive results,
with performance declining in deep-seated infections without
candidemia (17,18). By contrast, non-culture assays enable
earlier the initiation of antifungal therapy, often before overt
clinical manifestations, thereby reducing diagnostic delays and
potentially improving survival.
Among these, BDG testing demonstrates a pooled
sensitivity of ~70-80% and a specificity of ~80-85%, with results
available within hours; however, its specificity is reduced in the
ICU due to false positives associated with hemodialysis,
intravenous immunoglobulins, albumin and certain antibiotics,
rendering it most useful as a rule-out test (19,20).
Mannan antigen and anti-mannan antibody assays, when used in
combination, provide improved sensitivity (~83%) and specificity
(~86%), often yielding positive results several days before blood
cultures, though their availability remains variable (21). PCR-based assays yield higher pooled
sensitivity (~95%) and specificity (~92%) and provide rapid
turnaround, but lack standardization and are constrained by costs
and accessibility (22). Recently,
the T2Candida panel, which uses magnetic resonance to directly
detect Candida DNA from blood within 3-5 h, has demonstrated
a sensitivity of ~89-100% and a specificity of ~96-99% for the most
common species; however, it is limited by high costs, instrument
availability and a restricted species panel (24). The practical utility of these assays
lies in combining them with culture-based methods to accelerate
rule-in or rule-out decisions, while ensuring species
identification and susceptibility testing for definitive
management.
Therapeutically, echinocandins have become the
standard of care for empiric treatment due to their potent activity
and favorable safety profile. The development of newer triazoles,
such as isavuconazole, provides additional options, including oral
formulations for step-down therapy. Combination regimens and novel
antifungal agents under investigation are being explored to counter
emerging resistance and broaden the therapeutic armamentarium
(28,29). In parallel, pharmacokinetic and
pharmacodynamic optimization has gained importance in critically
ill patients, whose altered physiology often disrupts drug
distribution and clearance. Therapeutic drug monitoring (TDM),
particularly for agents, such as voriconazole, enables
individualized dosing and enhances treatment precision, while
minimizing toxicity (37-41).
Addressing biofilm-associated infections has also
emerged as a key focus area. Biofilm formation on indwelling
medical devices contributes to persistent infection and therapeutic
failure; current research is investigating antifungal agents with
biofilm-disruptive properties and innovative device technologies to
reduce colonization and infection risk (4,6,8,12,13,28,29).
Complementary to pharmacologic advances, infection
prevention and control measures play a pivotal role. These include
antifungal stewardship programs, stricter catheter care protocols,
and bundled prevention strategies, all of which have been shown to
reduce ICU-acquired Candida infections (17,18,30,31).
Stewardship initiatives emphasize judicious antifungal use, early
de-escalation based on clinical and microbiological data, and the
integration of local epidemiology and biomarkers into therapeutic
decision-making, thereby reducing antifungal resistance and
improving patient outcomes (8,28,30,31).
Emerging therapies extend beyond antifungal drugs themselves.
Immunomodulatory approaches, such as granulocyte colony-stimulating
factor and experimental vaccines against Candida species are
under investigation to augment host defenses, particularly in
immunocompromised patients in the ICU (30,31).
Furthermore, the adoption of multidisciplinary teams, including
infectious disease specialists, intensivists, pharmacists and
microbiologists has improved diagnostic accuracy, treatment
optimization and stewardship.
Recent developments in predictive scoring systems,
such as the Candida Score and APACHE II-based models, now allow
clinicians to stratify patients and prioritize early therapy for
those at greatest risk. Simultaneously, artificial intelligence and
machine learning are being applied for predictive modeling, early
detection and risk assessment of invasive candidiasis, providing a
personalized and data-driven approach to ICU care (32,33).
Finally, host factors and comorbidities have become
integral to management. Optimizing glycemic control, managing renal
dysfunction, and minimizing unnecessary immunosuppression are
essential strategies to reduce susceptibility and enhance response
to therapy. Prophylactic antifungal use in select high-risk ICU
populations, such as patients undergoing abdominal surgery, those
with hematologic malignancies, or those undergoing transplantation
remains an area of active investigation, although concerns
regarding resistance, costs and toxicity necessitate cautious
application. Collectively, these advances signal a paradigm shift
toward earlier, more individualized and multidisciplinary care,
integrating diagnostics, therapeutics, infection prevention and
host optimization to improve the outcomes of patients with ICU
(34-36).
6. Diagnostic modalities in the ICU
Blood culture remains the gold standard for the
diagnosis of candidemia; however, its performance in critically ill
patients is suboptimal. Sensitivity is estimated at only ~50%, and
the time to positivity can range from 24-72 h, which delays
targeted therapy and limits its value in deep-seated candidiasis
without bloodstream involvement (17,18).
Despite these drawbacks, cultures provide species identification
and susceptibility testing, which remain indispensable for
tailoring antifungal therapy.
To overcome the limitations of culture, several
non-culture-based assays have been introduced. The BDG assay is
among the most widely used biomarkers, with a pooled sensitivity of
~70-80% and specificity of ~80-85%, and a turnaround time of hours,
once samples are processed. In the ICU, however, false positives
are common due to exposure to hemodialysis membranes, intravenous
immunoglobulin, albumin infusions and certain broad-spectrum
antibiotics. As such, BDG is particularly valuable as a rule-out
test in patients with low-to-intermediate pre-test probability
rather than as a confirmatory tool (19,20).
Mannan antigen and anti-mannan antibody assays
provide another non-culture diagnostic approach, with diagnostic
yield improving significantly when both are combined. Multiple
studies have reported a sensitivity of ~83-89% and a specificity of
86-90%, with serological positivity often preceding blood culture
results by several days (21,42,43).
However, test performance varies across Candida species, and
availability remains inconsistent across ICU settings (21). Molecular methods, particularly
PCR-based assays, have demonstrated a high pooled sensitivity
(~95%) and specificity (~92%) for IC, with results available within
24 h. These assays allow for the much earlier initiation of
antifungal therapy, although the lack of assay standardization,
variable performance across platforms and higher costs limit
widespread adoption (22).
A notable advancement in this field is the T2Candida
panel, which detects Candida DNA directly from whole blood using
magnetic resonance technology. The assay provides results within
3-5 h and covers the five most common Candida species, with
a reported sensitivity of ~89-100% and specificity of ~96-99%.
Despite these advantages, its clinical utility is tempered by high
costs, limited species coverage and restricted availability in
numerous ICUs (23). Taken together,
culture- and non-culture-based diagnostics are best viewed as
complementary rather than competitive. While blood cultures remain
essential for definitive identification and susceptibility testing,
non-culture assays, such as BDG, mannan antigen and anti-mannan
antibody assays, PCR and T2Candida can greatly accelerate
diagnosis, facilitate the earlier initiation or de-escalation of
antifungal therapy, and improve the outcomes of critically ill
patients. The optimal diagnostic approach in the ICU likely
involves a multimodal strategy, integrating rapid biomarkers with
traditional cultures and guided by clinical risk stratification to
balance speed, accuracy and cost-effectiveness.
7. Dosing and therapeutic drug monitoring in
the ICU
TDM has become an increasingly valuable tool in
optimizing antifungal therapy in critically ill patients, in whom
altered pharmacokinetics can result in either subtherapeutic
exposure or toxicity (23,40). Although not all antifungals require
TDM, it is particularly indicated for agents with narrow
therapeutic windows, a high interpatient variability, or
concentration-dependent toxicities (26,29).
Voriconazole is the most well-established candidate, as trough
concentrations are closely associated with both efficacy and
toxicity. Subtherapeutic levels (<1-2 µg/ml) are associated with
treatment failure, while supratherapeutic levels (>5-6 µg/ml)
increase the risk of hepatotoxicity, neurotoxicity and visual
disturbances; the generally recommended target trough range is 2-5
µg/ml (37,40,41).
Posaconazole, particularly the oral suspension, demonstrates
variable absorption in critically ill patients; TDM is therefore
recommended, with prophylaxis targets of >0.7 µg/ml and
treatment targets of >1.0-1.25 µg/ml (37,38,40).
Itraconazole is another azole for which TDM is advised, with
suggested trough levels >1 µg/ml to ensure efficacy (23,26). For
5-flucytosine, TDM is essential due to its narrow therapeutic
index, and the risk of bone marrow suppression and hepatotoxicity
at higher exposures. Target peak plasma concentrations are
typically 30-80 µg/ml, with the avoidance of sustained levels
>100 µg/ml (39,40). By candida.
In the ICU, where factors such as renal replacement
therapy, extracorporeal membrane oxygenation and drug-drug
interactions can alter antifungal disposition (23,40), TDM
provides a practical means of tailoring therapy to achieve optimal
exposure while minimizing toxicity. Its incorporation into routine
practice for selected agents represents a critical advance in
improving antifungal stewardship and clinical outcomes in
critically ill patients with IC (8,28,40).
8. Prevention and infection control
strategies
Prevention and control strategies for IC are
particularly important for reducing incidence and improving patient
outcomes in ICU settings. The routine screening and monitoring of
at-risk patients, including those with central venous catheters,
those on mechanical ventilation, or those treated with
broad-spectrum antibiotics in recent days, may aid in identifying
potential infections at an early stage. Accordingly, antifungal
prophylaxis is frequently considered in patients who are considered
high-risk, particularly when undergoing invasive surgery or whether
they are in an immunocompromised state. Although antifungals for
prophylaxis may be beneficial, the careful selection of patients
needs to be undertaken so as not to expose any others unnecessarily
and risk furthering resistance development. Strict infection
control measures, apart from pharmacological interventions, need to
be maintained. The standard precautions, such as hand washing, the
sterilization of medical products, and the use of standard sterile
techniques during invasive procedures, greatly limit the
transmission of Candida species. The timely removal of
central venous catheters when not medically necessary is another
preventive strategy to reduce the risk of catheter-related
bloodstream infection. Personalized antifungal stewardship programs
in hospitals will limit antifungal use to situations in which it is
truly required, while at the same time preventing the development
of resistance and aiding in the implementation of proper empirical
therapy on the basis of local resistance patterns. With these
surveillance, prophylactical and infection prevention strategies,
the incidence of developing IC in the ICU can be greatly reduced
(37-40).
9. Conclusion and future perspectives
Future aspects and research gaps in the management
of IC in ICU settings concern improving early diagnosis, optimizing
treatment strategies and resolving antifungal resistance; one of
the major areas of future studies could be the development of
rapid, non-culture-based, advanced molecular and serological assays
for the earlier detection of Candida infections, leading to
timelier and more targeted treatment. Furthermore, personalized
antifungal therapy could then be further improved, using genetic
and pharmacokinetic profiling, as patients in the ICU may also have
altered drug metabolism and clearance patterns. Research into novel
classes of antifungal agents and into combination therapies could
also pave the way, particularly in light of increasing resistance
to azoles and echinocandins. Furthermore, the identification of
biomarkers to predict resistance and outcomes in patients would
enable more directed treatment strategies. Research on prevention
strategies in high-risk ICU populations, particularly antifungal
prophylaxis, de-escalation protocols and the management of
indwelling devices, appears to be warranted. Furthermore,
understanding host-pathogen interactions and the role of the
microbiome in the development of IC may provide useful insights for
prevention and treatment. Research into these issues would
ultimately help to reduce the high morbidity and mortality rates
associated with IC and benefit the management of patients in ICUs
(36-37,40-42)
(https://dig.pharmacy.uic.edu/faqs/2024-2/nov-2024-faqs/what-information-is-available-to-guide-the-practice-of-therapeutic-drug-monitoring-for-itraconazole-posaconazole-voriconazole-and-isavuconazonium-sulfate/).
In conclusion, the management of IC in patients in
the ICU remains a complex challenge characterized by delayed
diagnoses, difficulties in risk stratification, antifungal
resistance and the requirement for pharmacokinetic adjustments. The
persistently high mortality rates among patients in ICUs, despite
advances in antifungal therapy and diagnostics, reflects the
non-specific nature of clinical presentation and the critical
baseline status of these patients. However, with the advent of
rapid diagnostic techniques, novel antifungal agents and improved
infection prevention strategies, there are promising avenues toward
improving patient outcomes. Optimal results will be achieved
through antifungal stewardship programs and multidisciplinary
collaboration. Notably, unlike prior reviews that have addressed IC
in broader adult populations (1-5,15,16),
the present review provides an ICU-specific synthesis that
integrates advances in diagnostics, antifungal pharmacology,
therapeutic drug monitoring, infection control and predictive risk
models. By tailoring insights to the critical care environment, the
present review aimed to bridge the gap between general antifungal
guidance and the practical realities faced in ICUs. Moving forward,
research on personalized treatment protocols, novel antifungal
therapies and prevention strategies will be of utmost importance to
overcoming current challenges and improving the survival and
quality of life of patients in ICUs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Author's contributions
All authors (WGE, AMA and AHAA) contributed to the
conception and design of the study, supervised the work, and
provided resources including access to institutional libraries,
clinical guidelines, and scientific databases (PubMed, Scopus, and
Embase), as well as reference management tools. They participated
in literature collection, screening, and processing, data analysis
and interpretation, and drafting and critical revision of the
manuscript. Data authentication is not applicable. All authors have
read and approved the final manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Soriano A, Honore PM, Puerta-Alcalde P,
Garcia-Vidal C, Pagotto A, Gonçalves-Bradley DC and Verweij PE:
Invasive candidiasis: Current clinical challenges and unmet needs
in adult populations. J Antimicrob Chemother. 78:1569–1585.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kullberg BJ and Arendrup MC: Invasive
candidiasis. N Engl J Med. 373:1445–1456. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Paramythiotou E, Frantzeskaki F, Flevari
A, Armaganidis A and Dimopoulos G: Invasive fungal infections in
the ICU: How to approach, how to treat. Molecules. 19:1085–1119.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gonzalez-Lara MF and Ostrosky-Zeichner L:
Invasive Candidiasis. Semin Respir Crit Care Med. 41:3–12.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Van de Veerdonk FL, Kullberg BJ and Netea
MG: Pathogenesis of invasive candidiasis. Curr Opin Crit Care.
16:453–459. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Thomas-Rüddel DO, Schlattmann P, Pletz M,
Kurzai O and Bloos F: Risk factors for invasive candida infection
in critically Ill patients: A systematic review and meta-analysis.
Chest. 161:345–355. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Barantsevich N and Barantsevich E:
Diagnosis and treatment of invasive candidiasis. Antibiotics
(Basel). 11(718)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Oliva A, De Rosa FG, Mikulska M, Pea F,
Sanguinetti M, Tascini C and Venditti M: Invasive Candida
infection: Epidemiology, clinical and therapeutic aspects of an
evolving disease and the role of rezafungin. Expert Rev Anti Infect
Ther. 21:957–975. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Clancy CJ and Nguyen MH: Diagnosing
invasive candidiasis. J Clin Microbiol. 56:e01909–17.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ellepola AN and Morrison CJ: Laboratory
diagnosis of invasive candidiasis. J Microbiol. 43:65–84.
2005.PubMed/NCBI
|
|
11
|
Ylipalosaari P, Ala-Kokko TI, Koskenkari
J, Laurila JJ, Ämmälä S and Syrjälä H: Use and outcome of empiric
echinocandins in critically ill patients. Acta Anaesthesiol Scand.
65:944–951. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ben-Ami R: Treatment of invasive
candidiasis: A narrative review. J Fungi (Basel).
4(97)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De Oliveira Santos GC, Vasconcelos CC,
Lopes AJO, de Sousa Cartágenes MDS, Filho AKDB, do Nascimento FRF,
Ramos RM, Pires ERRB, de Andrade MS, Rocha FMG and de Andrade
Monteiro C: Candida infections and therapeutic strategies:
Mechanisms of action for traditional and alternative agents. Front
Microbiol. 9(1351)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mermel LA, Allon M, Bouza E, Craven DE,
Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ and Warren
DK: Clinical practice guidelines for the diagnosis and management
of intravascular catheter-related infection: 2009 Update by the
Infectious Diseases Society of America. Clin Infect Dis. 49:1–45.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Peçanha-Pietrobom PM and Colombo AL: Mind
the gaps: Challenges in the clinical management of invasive
candidiasis in critically ill patients. Curr Opin Infect Dis.
33:441–448. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mehta Y: Challenges in the diagnosis and
treatment of invasive candidiasis in India. Int J Crit Care Emerg
Med. 10(165)2024.
|
|
17
|
Logan C, Martin-Loeches I and Bicanic T:
Invasive candidiasis in critical care: Challenges and future
directions. Intensive Care Med. 46:2001–2014. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hankovszky P, Társy D, Öveges N and Molnár
Z: Invasive candida infections in the ICU: Diagnosis and therapy. J
Crit Care Med (Targu Mures). 1:129–139. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Aldardeer NF, Albar H, Al-Attas M, Eldali
A, Qutub M, Hassanien A and Alraddadi B: Antifungal resistance in
patients with Candidaemia: A retrospective cohort study. BMC Infect
Dis. 20(55)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mei-Sheng Riley M: Invasive fungal
infections among immunocompromised patients in critical care
settings: Infection prevention risk mitigation. Crit Care Nurs Clin
North Am. 33:395–405. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Delaloye J and Calandra T: Invasive
candidiasis as a cause of sepsis in the critically ill patient.
Virulence. 5:161–169. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Almirante B, Rodríguez D, Park BJ,
Cuenca-Estrella M, Planes AM, Almela M, Mensa J, Sanchez F, Ayats
J, Gimenez M, et al: Epidemiology and predictors of mortality in
cases of Candida bloodstream infection: Results from
population-based surveillance, barcelona, Spain, from 2002 to 2003.
J Clin Microbiol. 43:1829–1835. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bellmann R and Smuszkiewicz P:
Pharmacokinetics of antifungal drugs: Practical implications for
optimized treatment of patients. Infection. 45:737–779.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pfaller MA: Application of
Culture-independent rapid diagnostic tests in the management of
invasive candidiasis and cryptococcosis. J Fungi (Basel).
1:217–251. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bassetti M, Mikulska M and Viscoli C:
Bench-to-bedside review: Therapeutic management of invasive
candidiasis in the intensive care unit. Crit Care.
14(244)2010.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Lepak AJ and Andes DR: Antifungal
pharmacokinetics and pharmacodynamics. Cold Spring Harb Perspect
Med. 5(a019653)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ademe M: Immunomodulation for the
treatment of fungal infections: Opportunities and challenges. Front
Cell Infect Microbiol. 10(469)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Alhamad H, Abu-Farha R, Albahar F, Jaber
D, Abu Assab M, Edaily SM and Donyai P: The impact of antifungal
stewardship on clinical and performance measures: A global
systematic review. Trop Med Infect Dis. 9(8)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pappas PG, Kauffman CA, Andes DR, Clancy
CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez
JA, Walsh TJ, et al: Clinical practice guideline for the management
of candidiasis: 2016 update by the infectious diseases society of
America. Clin Infect Dis. 62:e1–e50. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kumar NR, Balraj TA, Kempegowda SN and
Prashant A: Multidrug-resistant sepsis: A critical healthcare
challenge. Antibiotics (Basel). 13(46)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hurley JC: Trends in ICU mortality and
underlying risk over three decades among mechanically ventilated
patients. A group level analysis of cohorts from infection
prevention studies. Ann Intensive Care. 13(62)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mayer LM, Strich JR, Kadri SS, Lionakis
MS, Evans NG, Prevots DR and Ricotta EE: Machine learning in
infectious disease for risk factor identification and hypothesis
generation: Proof of concept using invasive candidiasis. Open Forum
Infect. 9(ofac401)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Noppè E, Eloff JRP, Keane S and
Martin-Loeches I: A Narrative review of invasive candidiasis in the
intensive care unit. Ther Adv Pulm Crit Care Med.
19(29768675241304684)2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Baltogianni M, Giapros V and Dermitzaki N:
Recent challenges in diagnosis and treatment of invasive
candidiasis in neonates. Children (Basel). 11(1207)2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pham D, Sivalingam V, Tang HM, Montgomery
JM, Chen S and Halliday CL: Molecular diagnostics for invasive
fungal diseases: Current and future approaches. J Fungi (Basel).
10(447)2024.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Pappas PG, Lionakis MS, Arendrup MC,
Ostrosky-Zeichner L and Kullberg BJ: Invasive candidiasis. Nat Rev
Dis Primers. 4(18026)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yi WM, Schoeppler KE, Jaeger J, Mueller
SW, MacLaren R, Fish DN and Kiser TH: Voriconazole and posaconazole
therapeutic drug monitoring: A retrospective study. Ann Clin
Microbiol Antimicrob. 16(60)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
König C, Göpfert M, Kluge S and Wichmann
D: Posaconazole exposure in critically ill ICU patients: A need for
action. Infection. 51:1767–1772. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sigera LSM and Denning DW: Flucytosine and
its clinical usage. Ther Adv Infect Dis.
10(20499361231161387)2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Baracaldo-Santamaría D, Cala-Garcia JD,
Medina-Rincón GJ, Rojas-Rodriguez LC and Calderon-Ospina CA:
Therapeutic drug monitoring of antifungal agents in critically Ill
patients: Is there a need for dose optimisation? Antibiotics
(Basel). 11(645)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jiang L and Lin Z: Voriconazole: A review
of adjustment programs guided by therapeutic drug monitoring. Front
Pharmacol. 15(1439586)2024.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sendid B, Poirot JL, Tabouret M, Bonnin A,
Caillot D, Camus D and Poulain D: Combined detection of mannanaemia
and antimannan antibodies as a strategy for the diagnosis of
systemic infection caused by pathogenic Candida species. J Med
Microbiol. 51:433–442. 2002.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Prella M, Bille J, Pugnale M, Duvoisin B,
Cavassini M, Calandra T and Marchetti O: Early diagnosis of
invasive candidiasis with mannan antigenemia and antimannan
antibodies. Diagn Microbiol Infect Dis. 51:95–101. 2005.PubMed/NCBI View Article : Google Scholar
|















