Introduction
Breast cancer is the most common malignancy in
Nigeria. The incidence of breast cancer increases with age, with
the majority of women diagnosed >40 years of age (1). Globally, ~25% of women with breast
cancer are pre-menopausal and peri-menopausal, while the remaining
are post-menopausal. A great burden of pre-menopausal women with
breast cancer are found in low-income and middle-income countries
with 55.2% of total breast cancer cases in countries with a low
human development index occurring in pre-menopausal women (2). In Nigeria, the proportion of breast
cancer among pre-menopausal women is close to 60% (3). This group therefore has a higher
proportion of breast cancer cases among individuals of African
ancestry, more than the proportion among Caucasians, where young
women of reproductive age account for the minority of women that
are diagnosed with breast cancer. There is therefore a racial
difference in terms of the incidence of young women with breast
cancer. It has been reported that prior to the age of 35 years, the
percentage of African Americans with breast cancer is more than
twice that of the Caucasians (4).
Although the incidence of breast cancer has
progressively increased recently, there has also been a
simultaneous improvement in the survival rates. This is partly due
to early detection and adjuvant chemotherapy (5). Despite improved survival rates however,
there are certain issues associated with survivorship, such as
fertility concerns, late side-effects of treatment, as well as
psychosocial issues. Pre-menopausal women are those with a regular
menstrual period. Pre-menopausal women pass through different
levels of monthly cyclic changes, which are characterized by
varying hormonal levels that signal each phase of the menstrual
cycle.
Hormonal levels in pre-menopausal women for estrogen
and progesterone, luteinizing hormone (LH), anti-Müllerian hormone
(AMH) and follicle stimulating hormone (FSH) fluctuate during the
menstrual cycle, following the pattern presented in Table I (6).
 | Table IHormonal changes throughout the
menstrual cycle. |
Table I
Hormonal changes throughout the
menstrual cycle.
| Phase of menstrual
cycle | Estrogen (pg/ml) | Progesterone
(ng/ml) | LH (iu/l) | FSH (iu/l) | AMH (ng/ml) |
|---|
| Phase I (menstrual
phase- days 1-5) | 160.00±66.24 | 0.60±0.40 | 7±6.00 | 1.00-9.00 | 0.29-12.70 |
| Phase II (follicular
phase- days 10-13) | 778.00±255.43 | 0.64±0.31 | 20.00±8.00 | 6.00-26.00 | 2.41
(0.15-14.50) |
| Phase Ill (luteal
phase-days 20-23) | 395.00±134.57 | 14.00±5.44 | 7.00±4.00 | 1.00-9.00 | 2.07 (0.08-8.58) |
The peri-menopausal state is regarded as the time
during which a women has irregular menstruation cycles;
menstruation can cease for ≥3 months and then resumes. A woman is
said to be menopausal after having attained the complete cessation
of menstrual periods for at least 12 months (7,8).
Young women with breast cancer are considered to be
high-risk subjects as they are likely to have estrogen
receptor-negative/high-grade tumors; therefore, chemotherapy is
commonly part of their treatment modalities. The majority of
chemotherapeutic regimens for breast cancer have deleterious
effects on the ovaries, thereby affecting fertility. These women
may also attain a state of premature amenorrhea, which can be
temporary or permanent. Women who resume regular menstrual cycles
following the completion of chemotherapy may be less fertile
compared with those who have not received any chemotherapy. They
may also attain menopause earlier than expected (premature
menopause) (9-11).
The rate of amenorrhea varies from 21 to 71% in young women and
49-100% in those >40 years of age treated with chemotherapy
(12).
Fertility concerns are major considerations in women
of childbearing age who are placed on chemotherapy, particularly
among those who still desire to have children. On this account,
concerns for fertility may render treatment decision-making more
complex for young women with breast cancer (13), particularly as young women with
breast cancer experience greater psychosocial distress and anxiety
about infertility compared with their older counterparts.
Primary ovarian insufficiency and premature
menopause are regarded as potential compromises on reproductive
potential. Sometimes a woman may menstruate, but may still suffer
from partial ovarian injury, which may result in subfertility
(14). Due to the increasing
incidence of breast cancer among pre-menopausal women, some
patients are concerned about how they will successfully conceive
following the completion of treatment, which includes the
administration of chemotherapy. Clinicians, reproductive medicine
specialists and gynecologists should be conversant with these
concerns of the patients.
Research has demonstrated that the concentrations of
female sex hormones, such as AMH, inhibin-B, inhibin-A, FSH, LH and
estradiol (E2) exhibit various changes following the
administration of chemotherapy (15-18).
To date, reports on the effects of chemotherapy on female sex
hormones (which are a reflection of ovarian function) among
indigenous populations of African origin are limited, hence the
need for the present study. The aim of the present study was to
determine the effects of chemotherapy on ovarian function/reserve
among the study population which included a high proportion of
pre-menopausal women, most of whom desired child birth following
chemotherapy. The levels of three hormones, namely FSH,
E2 and AMH were measured. AMH is relative stable,
without it being influenced by the menstrual cycle; hence
researchers have selected AMH as a reliable biomarker to assess
ovarian reserve during and after chemotherapy (19) (Table
I).
Patients and methods
The present study was a prospective study conducted
at the Department of Radiation and Clinical Oncology of the Lagos
University Teaching Hospital, Idi-araba, Lagos, Nigeria, between
October, 2020 to November, 2023. Young women within the
reproductive age group (18-42 years) and with a histologically
confirmed diagnosis of breast cancer were counseled appropriately
on the study and their written consent for participation in the
study was obtained. Participants were counselled on fertility
preservation and asked for their preferences.
Blood samples were collected from eligible
pre-menopausal women after obtaining consent, that were newly
diagnosed with breast cancer and who were planning to receive
chemotherapy as part of their oncological treatment. The samples
were collected prior to treatment with chemotherapy (3-5 days from
the last menstrual period), and then at 3 and 6 months following
the completion of chemotherapy. Patients were also followed-up for
12 months to observe menstrual flow patterns. The patients used a
menstrual diary to track their date and duration of flow. However,
the flow quantity was not measured. The number of sanitary pads
used during each menstrual period was recorded and used to estimate
the quantity of menstrual flow, while the duration of flow was also
recorded. All the patients recruited for the study were on a 3-week
chemotherapeutic regimen. First-line chemotherapy was administered
every 3 weeks amounting to four courses within the first 12 weeks.
This consisted of an anthracycline (doxorubicin 60 m2 or
epirubicin 75 mg/m2) + cyclophosphamide at 600
mg/m2 with or without a 5-fluorouracil regimen at 600
mg/m2 (AC/CAF) or docetaxel at 75 m/m2 +
doxorubicin at 60 mg/m2 + cyclophosphamide at 600
mg/m2 (TAC) regimen. In the second 12 weeks,
participants received four courses administered every 3 weeks of
either a taxane (docetaxel at 75 mg/m2 or paclitaxel at
175 mg/m2) + capecitabine at 2,000 mg twice daily or a
platinum compound (cisplatin at 50 mg/m2 or carboplatin
5 AUC) + a taxane regimen (PT) as a second regimen based on
institutional guidelines. The blood samples obtained from the
participants were stored and analyzed at the Human Reproductive and
Endocrinology Research Laboratory, Department Obstetrics and
Gynecology, College of Medicine, University of Lagos/Lagos
University Teaching Hospital (LUTH), Idi-Araba, Lagos, Nigeria.
Ethical approval for the study was obtained from institutional
Ethics Committee at Lagos University Teaching Hospital Health
Research Ethics Committee with the assigned no.
ADM/DCST/HREC/APP/2736. Written and signed informed consent was
obtained from the participants prior to enrolment in the study.
The participants included in the study were females,
aged 18-42 years, who had histologically confirmed breast cancer at
stages 1-3, who were chemotherapy naïve and with an ECOG
performance status of 0-1. The exclusion criteria were pregnant
patients, those who had been previously treated with chemotherapy,
those who had undergone a hysterectomy, as well as those with an
irregular menstrual cycle, as well as post-menopausal women or with
last menses >3 months prior.
Sample collection and laboratory
analyses
The specimens were blood samples collected by
venipuncture into lithium heparin bottles. Of note, ~5 ml blood
samples were obtained from each participant with minimal pain at
the site of venipuncture. The blood samples collected were
centrifuged at 2,000 x g for 10 min at 4˚C to obtain plasma; these
samples were stored in a freezer at -80˚C and later analyzed.
In total, three different sets of blood samples were
collected for the study: Prior to the commencement of chemotherapy,
at 3 months following the completion of chemotherapy and at 6
months following chemotherapy. The chemotherapy was administered at
3 weekly intervals. The plasma samples were stored at -80˚C and
analyzed in the laboratory for AMH, E2 and FSH. A direct
immunoenzymatic colorimetric method was used for the quantitative
determination of AMH, E2 and FSH concentrations in
plasma using ELISA kits (cat. nos. SEK-0003, SEK-0008 and SEK-0010,
Rapid Labs) as previously described (15).
Statistical analysis
Data analysis was carried out using SPSS version 23
software (IBM Corp.). The descriptive characteristics of the study
participants were summarized using tables and charts. Numerical
data, including the levels of hormonal parameters among the study
participants are expressed as the mean (SD) at pre-chemotherapy, at
3 months post-chemotherapy and at 6 months post-chemotherapy. The
effects of the various chemotherapy combinations on hormone levels
were analyzed using paired t-tests at two specific intervals:
Pre-chemotherapy vs. 3 months post-chemotherapy, and 3 vs. 6 months
post-chemotherapy. Repeated measures analysis of variance (ANOVA)
was used to compare the hormone levels (AMH, E2 and FSH)
measured in the same study participants at three different time
intervals [pre-chemotherapy (baseline), at 3 months
post-chemotherapy and at 6 months post-chemotherapy]. Where
significant differences were observed, pairwise comparisons between
time points were conducted using Bonferroni post hoc tests to
control for multiple comparisons. A Fisher's exact test was
conducted to assess the association between age group and
menstruation patterns at 6 months following chemotherapy. A value
of P<0.05 was considered to indicate a statistically significant
difference.
Results
A total of 96 pre-menopausal women diagnosed with
breast cancer were recruited for the present study. However, 14
dropped out of the study and were exempted from the analysis. The
reasons for dropping out included seeking alternative treatments,
such as spiritual and herbal healing, pregnancy, financial
constraints and a change of residence. Parameters from 82
participants were analyzed. The mean age of all the participants in
the study was 36.6 years (range, 32-41 years). The majority (71%)
had at least 1 child, while 29% were nullipara. Of note, ~51% of
the patients still desired to have more children. The age of the
participants ranged from 32-41 years, with a mean age at diagnosis
of breast cancer of 35.5 years (Table
II). As regards the preference for fertility preservation, 63
(77%) were willing to use methods to preserve fertility, while 19
(23%) declined. Preferred fertility methods were as follows: The
use of drugs 38 (46%); ovum banking 13 (16%) and adoption 25 (31%),
while 6 (7%) patients did not choose any specific method of
fertility preservation. Unfortunately, none of those who preferred
fertility preservation could afford goserelin, which is the least
costly form of fertility preservation currently available in
Nigeria.
 | Table IIBiodata of the study participants. |
Table II
Biodata of the study participants.
| Variable (n=82) | Frequency | Percentage |
|---|
| Age at presentation
(years) | | |
|
≤30 | 10 | 12.2 |
|
31-40 | 56 | 68.3 |
|
41-50 | 16 | 19.5 |
| Mean age,
36.6±4.6 | | |
| Occupation | | |
|
Skilled
professional | 9 | 11.0 |
|
Minimally
skilleda | 58 | 70.7 |
|
Not in
active employmentb | 15 | 18.3 |
| Education
level | | |
|
None | 6 | 7.3 |
|
Primary | 2 | 2.4 |
|
Secondary | 41 | 50.0 |
|
Tertiary | 33 | 40.2 |
| Marital status | | |
|
Married | 55 | 67.1 |
|
Not
marriedc | 27 | 32.9 |
|
Partner's
occupation (n=55) | | |
|
Skilled
professional | 3 | 5.5 |
|
Minimally
skilleda | 50 | 90.9 |
|
Not in
active employmentb | 2 | 3.6 |
| Parity | | |
|
0 | 24 | 29.3 |
|
1 | 10 | 12.2 |
|
2 | 18 | 22.0 |
|
3 | 13 | 15.9 |
|
≥4 | 17 | 20.6 |
| Desire for more
children | | |
|
Yes | 42 | 51.2 |
|
No | 40 | 48.8 |
Most of the participants had AJCC anatomic stage III
disease. Other clinical parameters of participants are presented in
Table III. The first line of
chemotherapy administered within the first 12 weeks consisted of
anthracycline + cyclophosphamide/5-fluorouracil regimen (AC/CAF)
(81 participants), or the taxane + anthracycline + cyclophosphamide
(TAC) regimen (only 1 participant). In the second 12 weeks, 61
participants continued with AC/CAF, no participant continued with
the TAC regimen, while 13 participants had their chemotherapy
changed to taxane + xeloda, and 8 patients received the platinum +
taxane regimen (PT) based on response and toxicity evaluations
following institutional guidelines (Table IV). The plasma levels of the
hormones of interest, namely AMH, E2 and FSH exhibited
variations during the study period, as demonstrated in Table V.
 | Table IIIClinical history of the study
participants. |
Table III
Clinical history of the study
participants.
| Variable
(n=82) | Frequency | Percentage |
|---|
| Disease stage | | |
|
I | 4 | 4.9 |
|
II | 7 | 8.5 |
|
III | 71 | 86.6 |
| ECOG score at
presentation | | |
|
0 | 47 | 57.3 |
|
1 | 35 | 42.7 |
| Nutritional status
(BMI) (kg/m2) | | |
|
Normal | 43 | 52.4 |
|
Overweight
and obese | 39 | 47.6 |
| Primary site of
disease | | |
|
Left | 46 | 58.5 |
|
Right | 36 | 41.5 |
| Presence of
comorbidities | | |
|
Yes | 12 | 13.4 |
|
No | 70 | 86.6 |
| Type of
comorbidities (n=12) | | |
|
Hypertension | 4 | 33.3 |
|
Peptic ulcer
disease | 4 | 33.3 |
|
HIV/AIDS | 4 | 33.3 |
| History of breast
surgery | | |
|
Yes | 29 | 35.4 |
|
No | 53 | 64.6 |
| Surgery type
(n=29) | | |
|
Lumpectomy | 9 | 31.0 |
|
Mastectomy | 20 | 69.0 |
 | Table IVChemotherapeutic regimens among the
study participants. |
Table IV
Chemotherapeutic regimens among the
study participants.
| Regimen (n=82) | Frequency | Percentage |
|---|
| Phase I | | |
|
Anthracycline
+ cyclophosphamide regimen (AC/CAF) | 81 | 98.8 |
|
Taxane +
anthracycline + cyclophosphamide (TAC) | 1 | 1.2 |
| Phase II | | |
|
Anthracycline
+ Cyclophosphamide regimen (AC/CAF) | 61 | 74.4 |
|
Taxane +
xeloda (TX) | 13 | 15.8 |
|
Platinum +
taxane (PT) | 8 | 9.8 |
 | Table VLevels of hormonal parameters among
the study participants. |
Table V
Levels of hormonal parameters among
the study participants.
| Variable
(n=82) |
Pre-chemotherapy | Post-chemotherapy
(3 months) | Post-chemotherapy
(6 months) |
|---|
| AMH (ng/ml) | | | |
|
<1 | 46 (56.1) | 69 (84.1) | 80 (97.6) |
|
1-2 | 16 (19.5) | 6 (7.1) | 2 (2.4) |
|
>2 | 20 (24.4) | 7 (8.5) | 0 (0.0) |
|
Mean
(SD) | 1.66 (0.84) | 0.71 (0.60) | 0.14 (0.16) |
| E2 (IU/ml) | | | |
|
<50 | 57 (69.5) | 61 (74.4) | 62 (75.6) |
|
50-100 | 20 (24.4) | 18 (22.0) | 19 (23.2) |
|
>100 | 5 (6.1) | 5 (6.1) | 1 (1.2) |
|
Mean
(SD) | 57.56 (5.32) | 43.15 (4.66) | 33.82 (3.37) |
| FSH (pg/ml) | | | |
|
<50 | 61 (74.4) | 55 (67.1) | 43 (52.4) |
|
50-100 | 11 (13.4) | 14 (17.1) | 21 (25.6) |
|
>100 | 10 (12.2) | 13 (15.9) | 18 (22.0) |
|
Mean
(SD) | 14.16 (2.39) | 33.70 (4.18) | 47.13 (5.29) |
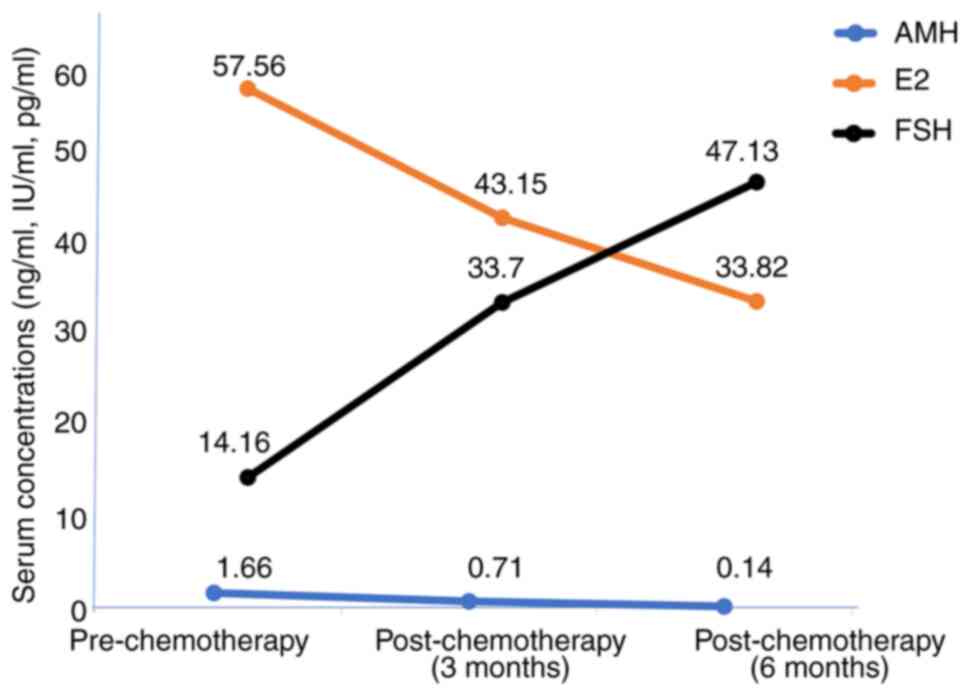
The trend of the levels of the hormones during the
study period is presented in Fig. 1.
To assess changes across the three timepoints pre-chemotherapy
(baseline), at 3 months post-chemotherapy and at 6 months
post-chemotherapy, a repeated measures ANOVA was performed. The
analysis demonstrated a significant time effect for AMH (F=2.56,
P<0.001), E2 (F=0.997, P=0.003) and FSH (F=3.227,
P<0.001). Post-hoc pairwise comparisons with Bonferroni
adjustment revealed a significant decline in AMH levels between
baseline and 3 months with a mean difference of 0.938 ng/ml
(P<0.001) and between baseline and 6 months with a mean
difference of 1.511 ng/ml (P=0.004), as well as between 3 and
6-months post-chemotherapy with a mean difference of 0.573 ng/ml
(P=0.025). By contrast, the E2 levels demonstrated a
significant decrease from baseline to 3 months with a mean
difference of 0.222 IU/ml (P 0.013) and from baseline to 6 months
with a mean difference of 0.300 IU/ml (P=0.004), and with a further
reduction observed between 3 and 6 months, although not
statistically significant [mean difference of 0.121 IU/ml (P=0.177]
). FSH levels on the other hand, exhibited a significant increase
from baseline to 3 months with a mean difference of -19.522 pg/ml
(P<0.001), and from baseline to 6 months with mean difference of
-34.202 pg/ml (P=0.004); however, no further significant increase
was evident between 3- and 6-months post-chemotherapy with mean
difference of -14.680 pg/ml (P=0.076).
The effects of the two chemotherapy combinations on
hormone levels were analyzed using ANOVA and paired t-tests. The
analysis on the AC/CAF chemotherapeutic regimen using repeated
measures ANOVA (pre-chemotherapy vs. 3 months post-chemotherapy,
and 3 vs. 6 months post-chemotherapy) yielded the following
results: AMH (F=1.688, P=0.064), E2 (F=2.111, P=0.073)
and FSH (F=1.711, P=0.172). With the chemotherapeutic regimens of
taxane + xeloda and platinum compounds + taxane, a paired t-test
analysis at two specific intervals of 3 months post-chemotherapy
vs. 6 months-post chemotherapy yielded P-values >0.05 (Table VI, Table VII and Table VIII). The mean values were
recorded, as well as the mean differences in percentages as
presented on the tables. Across all three hormones, there were no
significant differences in hormonal changes attributable to the
chemotherapy combinations (all P-values >0.05). These data
indicated that the type of chemotherapeutic regimen did not
significantly influence ovarian hormone response over time.
 | Table VIEffect of chemotherapy on AMH levels
among the study participants. |
Table VI
Effect of chemotherapy on AMH levels
among the study participants.
| | 1st to 3rd month
(mean values) | 3rd to 6th month
(mean values) |
|---|
| Drugs | No. of
patients |
Pre-chemotherapy | Post-chemotherapy
(3 months) | Mean difference
(%) | No. of
patients | Post-chemotherapy
(3 months) | Post-chemotherapy
(6 months) | Mean difference
(%) |
|---|
| AC/CAF | 81 | 1.67 (ng/ml) | 0.72 (ng/ml) | 0.95 (56.9) | 61 | 0.72 ng/ml | 0.16 ng/ml | 0.56 (77.8) |
| | | | | (F=1.688,
P=0.064) |
| TAC | 1 | 0.82 (ng/ml) | 0.11 (ng/ml) | 0.71 (86.6) | | | | |
| | | P=0.132 | | |
| Taxane +
xeloda | | | | | 13 | 0.31 (ng/ml) | 0.10 (ng/ml) | 0.21 (67.7) |
| | | | | P=0.224 |
| PT | | | | | 8 | 0.52 (ng/ml) | 0.09 (ng/ml) | 0.43 (82.7) |
| | | | | P=0.816 |
 | Table VIIEffect of chemotherapy on
E2 levels among the study participants. |
Table VII
Effect of chemotherapy on
E2 levels among the study participants.
| | 1st to 3rd month
(mean values) | 3rd to 6th month
(mean values) |
|---|
| Drugs | No. of
patients |
Pre-chemotherapy | Post-chemotherapy
(3 months) | Mean difference
(%) | No. of
patients | Post-chemotherapy
(3 months) | Post-chemotherapy
(6 months) | Mean difference
(%) |
|---|
| AC/CAF | 81 | 53.08 (IU/ml) | 38.70 (IU/ml) | 14.38 (27.1) | 61 | 38.70 (IU/ml) | 36.15 (IU/ml) | 2.55 (6.58) |
| | | | | (F=2.111,
P=0.073) |
| TAC | 1 | 57.56 (IU/ml) | 43.15 (IU/ml) | 14.41 (25.0) | | 43.15 (IU/ml) | 33.82 (IU/ml) | 9.35 (21.7) |
| | | P=0.418 | | P=0.079 |
| Taxane +
xeloda | | | | | 13 | 28.81 (IU/ml) | 25.09 (IU/ml) | 3.72 (12.9) |
| | | | | P=0.827 |
| PT | | | | | 8 | 32.90 (IU/ml) | 21.09 (IU/ml) | 11.81 (35.9) |
| | | | | P=0.308 |
 | Table VIIIEffect of chemotherapy on FSH levels
among the study participants. |
Table VIII
Effect of chemotherapy on FSH levels
among the study participants.
| | 1st to 3rd month
(mean values) | 3rd to 6th month
(mean values) |
|---|
| Drugs | No. of
patients |
Pre-chemotherapy | Post-chemotherapy
(3 months) | Mean difference
(%) | No. of
patients | Post-chemotherapy
(3 months) | Post-chemotherapy
(6 months) | Mean difference
(%) |
|---|
| AC/CAF | 81 | 14.22 (pg/ml) | 34.03 (pg/ml) | 19.81 (139.3) | 61 | 34.03 (pg/ml) | 42.77 (pg/ml) | 8.74 (25.68) |
| | | | | (F=1.711,
P=0.172) |
| TAC | 1 | 8.87 (pg/ml) | 7.25 (pg/ml) | -1.62 (-18.3) | | | | |
| | | P=0.938 | | |
| Taxane +
xeloda | | | | | 13 | 41.46 (pg/ml) | 49.51 (pg/ml) | 8.05 (19.4) |
| | | | | P=0.177 |
| PT | | | | | 8 | 46.12 (pg/ml) | 83.21 (pg/ml) | 37.09 (80.4) |
| | | | | P=0.853 |
The effects of chemotherapy on the menstrual cycle
of the participants differed slightly according to age. A Fisher's
exact test was conducted to assess the association between age
group and menstruation patterns at 6 months following chemotherapy.
The association was not statistically significant (P=0.146)
(Table IX). Some participants
experienced changes in their menstrual patterns at 6 months
following chemotherapy. At the 12-month follow-up, the menstrual
pattern was as follows: Regular, 38 (46%); irregular, 19 (23%);
while 25 (31%) exhibited the complete cessation of
menstruation.
 | Table IXAssociation between age and
menstruation patterns (at 6 months following chemotherapy). |
Table IX
Association between age and
menstruation patterns (at 6 months following chemotherapy).
| | Menstruation
pattern |
|---|
| | Regular (%) | Irregular (%) | Absent (%) | Total | P-value |
|---|
| Age (years) | | | | | 0.146 |
|
≤30 | 6(7) | 4(5) | 0 (0.0) | 10 | |
|
31-40 | 37(45) | 11(14) | 8(10) | 56 | |
|
41-50 | 6(7) | 6(7) | 4(5) | 16 | |
Discussion
The diagnosis of breast cancer is increasing and is
likely to occur prior to the completion of creating a family in
numerous females. The use of chemotherapy is an integral part of
the treatment of breast cancer among most pre-menopausal women.
However, its administration is not without significant reproductive
side-effects in those who are still within their reproductive age
group. Understanding the impact of chemotherapy on future fertility
is of utmost importance. The present study demonstrated that
chemotherapy significantly affected key hormones that regulate the
reproductive cycle of pre-menopausal women being treated with
chemotherapy for breast cancer. In the present study, three
hormones (AMH, E2 and FSH) were evaluated for the
effects of chemotherapy on the plasma concentrations of these
hormones. The mean values of both AMH and E2
progressively decreased within the 6 months of their evaluation,
while the mean value of FSH increased (Table V). These derangements suggest that
the fertility of these pre-menopausal women had been negatively
affected. These findings are in agreement with those of previous
studies reporting a significant decrease in E2 and AMH
levels following chemotherapy for breast cancer (17,20). On
the other hand, the plasma level of FSH increased following
chemotherapy. This is consistent with the findings of previous
reports demonstrating reduced ovarian function (21,22).
These findings thus support the need for ovarian protection during
chemotherapy. There are studies demonstrating that the
administration of hormonal therapy (aromatase inhibitor/tamoxifen),
particularly in hormone receptor-positive women, or
gonadotropin-releasing hormone (GnRH) agonist e.g., goserelin,
administered during chemotherapy exerts some degree of protection
on the ovaries (23,24).
The present study also indicated changes in the
monthly menstrual pattern of some of the participants at 6 months
following treatment. Although the quantity of monthly menstrual
flow was not measured, the quantity of flow was estimated with fair
accuracy, particularly by younger patients, since individuals are
already conversant with their menstrual flow pattern (25). In addition, using the records of the
number of sanitary pads used during each menstrual period and the
duration of flow as recorded by the participants, the authors deem
that the quantity of monthly flow was fairly estimated. Almost 15%
of the participants became amenorrhoeic following chemotherapy,
while 26% developed irregular menstrual patterns (Table IX). This is less than the previously
reported proportion of 21 to 71% in young women treated with
chemotherapy, although these data are from other populations
(26). The data may also reflect the
higher number of young patients in the present study. It is also
worthwhile to note that those who were ≤30 years of age did not
experience the absence of menstruation even though the number was
low. A previous study in Nigeria indicated that ~33% of the
participants experienced the cessation of the menstrual cycle
(27). In that study, participants
with various malignancies with different chemotherapeutic regimens
were included, whereas the present study only included patients
with breast cancer patients with a fairly uniform chemotherapeutic
regimens. However, this result is indicative of the higher ovarian
reserve in younger patients than older ones following
chemotherapy.
In the present study, the effects of the different
chemotherapeutic regimens on the expression of the FSH,
E2 and AMH hormone levels during the study period were
also assessed, with no significant differences observed (Table VI, Table VII and Table VIII). The chemotherapeutic
regimens used were standard combination regimens used for breast
cancer administered every 3 weeks sequentially. All patients
received either of the two combinations (anthracyclines +
cyclophosphamide) first and then shifted to the second combination
(taxanes and platinum compounds) or vice versa. At the end, they
were exposed to similar chemotherapeutic agents based on their body
surface areas. Thus, the authors deem that the results reflected
the cumulative effects of chemotherapy. The analyses did not reveal
significant effects of the three regimens on the hormonal levels.
This demonstrates that in the present study, the effects of
chemotherapy on ovarian function were not dependent on the breast
cancer chemotherapeutic regimen administered. This is similar to
the previous study by Goldfarb et al (28) who reported no difference in the
decline of AMH levels among pre-menopausal women receiving
cyclophosphamide methotrexate and the 5 fluorouracil (CMF)
combination; adriamycin, cyclophosphamide and taxane (AC-T)
combination, and then taxane and herceptin (TH) for breast cancer.
In addition, Goldfarb et al (28) also observed that age was an important
predictor of AMH levels as the older age of the participants was
associated with lower AMH values. The present study also yielded
similar findings.
Almost one third of the participants had
hormone-positive breast cancer and were treated with tamoxifen.
This may affect the hormonal levels; however, a previous study
reported no significant difference in the levels of E2
post-chemotherapy and post-hormonal therapy as the patients in each
group had decreased E2 levels following treatment
(17).
A notable number of the participants (77%) preferred
fertility preservation; however, they could not afford goserelin,
which is the only form of fertility preservation currently
available in Nigeria. Some patients had comorbidities, namely
hypertension, diabetes and HIV infection, and all were well
controlled. A previous study demonstrated that HIV infection does
not have significant effect on estrogen levels in pre-menopausal
HIV-positive women with an unsuppressed CD4 count (29). The observed changes may mainly be due
to the chemotherapy received by the participants. It was also
observed that at 6 months post-chemotherapy, none of the hormonal
levels indicated recovery from the effects of chemotherapy,
indicating that the effects last far beyond 6 months
post-treatment. This was supported by the observation that at 12
months, those with regular menses were reduced from 49 (58%) to 38
(46%), while 25 (31%) of the participants experienced cessation of
menstrual flow compared with 12 (16%) at 6 months. A longer period
of follow-up is required to identify the time and pattern of
recovery for those whose ovarian function recovered from the
effects of chemotherapy. From the findings of the present study, it
is highly recommended that fertility preservation services, such as
the use of oocyte/embryo cryopreservation, ovarian suppression and
GnRH agonists with counselling be provided to patients scheduled to
undergo cancer chemotherapy if they still desire to have children
folowing treatment.
Limitations of the study
The lack of data comparing study completers and
non-completers is a limitation to the present study. This could not
allow the assessment of the characteristics of those who completed
the study against those who did not. Nevertheless, the results
obtained still provide useful information about the population
under study. However, multivariate analysis adjusting for potential
confounders (e.g., age, parity and baseline AMH) was not performed
on the study data. A simple analysis was used, since this was
within a specific age group of <42 years, and we looked for
characteristics of 30 years and >30 years based on previous
reports on the literature (30,31).
Furthermore, since individual hormonal levels were measured at
baseline and then serially, each patient stood as a self-control.
Additionally, hormone level variability exists across menstrual
phases, and this may have confounded the E2 and FSH
results. The patients were on 3 weekly chemotherapy treatments,
with some participants residing at a distance from the hospital.
Measuring the hormone levels based on the menstrual cycle phase
would have necessitated more visits to the clinic, which would have
been too stressful for the patients with breast cancer receiving
chemotherapy. The exact volume of menstrual flow per cycle could
not be measured for the purpose of determining reduction in
menstrual flow following chemotherapy. However, using the number of
sanitary pads used during each menstrual cycle and recording the
duration in a diary was deemed to have provided a fair estimate of
the quantity of menstrual blood loss by an individual during the
study.
The present study was conducted in southwest
Nigeria, which represents a fraction of the Nigerian population.
The results cannot therefore be generalized to the entire
population of Nigeria. Nevertheless, the results provide useful
information that can be used to guide the design of larger studies
that can give information about the Nigerian population.
In conclusion, 82 Nigerian participants with a
pre-menopausal onset of breast cancer treated with chemotherapy
were analyzed in the present study. The findings revealed that
chemotherapy led to a significant reduction in ovarian function, as
reflected by the negative changes in the plasma concentrations of
E2, FSH and AMH. The effects were more pronounced in
participants >30 years of age, as some developed an irregular or
cessation of menstrual flow. The different chemotherapeutic
regimens for breast cancer did not significantly affect ovarian
function. The hormonal levels did not exhibit a recovery at 6
months post-chemotherapy, while the induced reduction in menstrual
patterns persisted for 12 months following chemotherapy. These
findings suggest that the effects of chemotherapy last >6
months. Clinicians should thus be aware of the deleterious effects
of chemotherapy on ovarian function and counsel such patients who
desire childbearing appropriately on fertility issues to ensure
good survivorship.
Acknowledgements
The authors acknowledge the contributions of Ms.
Oluwawemimo Akinlabi of the Department of Radiation Oncology,
College of Medicine, University of Ibadan, Ibadan, Nigeria for her
assistance with data analysis.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
OP and MH conceptualized the study and provided
resources and funding for the project. AA, OAl and GO took part in
the investigations and data acquisition. UO and OAd contributed to
the study methodology and formal analysis. AN was involved in the
conception and design of the study, and also supervised the study
and was involved in the drafting of the manuscript. OP and AN
confirm the authenticity of all the raw data. All authors edited
the manuscript, and read and approved the submission and final
version of the manuscript. All authors have access to the study
data.
Ethics approval and consent to
participate
The present study was approved by the Lagos
University Teaching Hospital Health Research Ethics Committee
(LUTHHREC) with the approval no. ADM/DCST/HREC/APP/2736. Written
and signed informed consent to participate in the study was
obtained from all participants prior to enrolment into the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Azubuike SO, Muirhead C, Hayes L and
McNally R: Rising global burden of breast cancer: The case of
sub-Saharan Africa (with emphasis on Nigeria) and implications for
regional development: A review. World J Surg Oncol.
16(63)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Heer E, Harper A, Escandor N, Sung H,
McCormack V and Fidler-Benaoudia MM: Global burden and trends in
premenopausal and postmenopausal breast cancer: A population-based
study. Lancet Glob Health. 8:e1027–e1037. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ali-Gombe M, Mustaph MI, Folasire A,
Ntekim A and Campbell OB: Pattern of survival of breast cancer
patients in a tertiary hospital in south west Nigeria.
Ecancermedicalscience. 15(1192)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hung MC, Ekwueme DU, Rim SH and White A:
Racial/ethnicity disparities in invasive breast cancer among
younger and older women: An analysis using multiple measures of
population health. Cancer Epidemiol. 45:112–118. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
El Saghir NS, Khalil LE, El Dick J, Atwani
RW, Safi N, Charafeddine M, Al-Masri A, El Saghir BN, Chaccour M,
Tfayli A, et al: Improved survival of young patients with breast
cancer 40 years and younger at diagnosis. JCO Glob Oncol.
9(e2200354)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Reed BG and Carr BR: The normal menstrual
cycle and the control of ovulation. In: Feingold KR, ASF AB (eds)
et al. Endotext. 2nd edition. South Dartmouth (MA):
MDText.com, Inc.; 2018.
|
|
7
|
Delamater L and Santoro N: Management of
the perimenopause. Clin Obstet Gynecol. 61:419–432. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Davis SR, Pinkerton J, Santoro N and
Simoncini T: Menopause-Biology, consequences, supportive care, and
therapeutic options. Cell. 186:4038–4058. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Partridge AH, Gelber S, Peppercorn J,
Sampson E, Knudsen K, Laufer M, Rosenberg R, Przypyszny M, Rein A
and Winer EP: Web-based survey of fertility issues in young women
with breast cancer. J Clin Oncol. 22:4174–4183. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hong YH, Park C, Paik H, Lee KH, Lee JR,
Han W, Park S, Chung S and Kim HJ: Fertility preservation in young
women with breast cancer: A review. J Breast Cancer.
26(221)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mannion S, Higgins A, Larson N, Stewart
EA, Khan Z, Shenoy C, Nichols HB, Su HI, Partridge AH, Loprinzi CL,
et al: Prevalence and impact of fertility concerns in young women
with breast cancer. Sci Rep. 14(4418)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Turnbull AK, Patel S, Martinez-Perez C,
Rigg A and Oikonomidou O: Risk of chemotherapy-related amenorrhoea
(CRA) in premenopausal women undergoing chemotherapy for early
stage breast cancer. Breast Cancer Res Treat. 186:237–245.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun M, Liu C, Zhang P, Song Y, Bian Y, Ke
S, Lu Y and Lu Q: Perspectives and needs for fertility preservation
decision-making in childbearing-age patients with breast cancer: A
qualitative study. Asia Pac J Oncol Nurs. 11(100548)2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Letourneau JM, Ebbel EE, Katz PP, Oktay
KH, McCulloch CE, Ai WZ, Chien AJ, Melisko ME, Cedars MI and Rosen
MP: Acute ovarian failure underestimates age-specific reproductive
impairment for young women undergoing chemotherapy for cancer.
Cancer. 118:1933–1939. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lambert-Messerlian G, Plante B, Eklund EE,
Raker C and Moore RG: Levels of antimüllerian hormone in serum
during the normal menstrual cycle. Fertil Steril. 105:208–213.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Furlanetto J, Marmé F, Seiler S, Thode C,
Untch M, Schmatloch S, Schneeweiss A, Bassy M, Fasching PA, Strik
D, et al: Chemotherapy-induced ovarian failure in young women with
early breast cancer: Prospective analysis of four randomised
neoadjuvant/adjuvant breast cancer trials. Eur J Cancer.
152:193–203. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Soewoto W and Agustriani N: Estradiol
levels and chemotherapy in breast cancer patients: A prospective
clinical study. World J Oncol. 14:60–66. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bala J, Seth S, Dhankhar R and Ghalaut VS:
Chemotherapy: Impact on Anti-Müllerian Hormone Levels in Breast
Carcinoma. J Clin Diagn Res. 10:BC19–BC21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kevenaar ME, Meerasahib MF, Kramer P, van
de Lang-Born BMN, de Jong FH, Groome NP, Themmen AP and Visser JA:
Serum anti-mullerian hormone levels reflect the size of the
primordial follicle pool in mice. Endocrinology. 147:3228–3234.
2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zong X, Yu Y, Chen W, Zong W, Yang H and
Chen X: Ovarian reserve in premenopausal women with breast cancer.
Breast. 64:143–150. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Malisic E, Susnjar S, Milovanovic J,
Todorovic-Rakovic N and Kesic V: Assessment of ovarian function
after chemotherapy in women with early and locally advanced breast
cancer from Serbia. Arch Gynecol Obstet. 297:495–503.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yu B, Lobo R, Crew K, Ferin M, Douglas N,
Nakhuda G and Hershman D: Predictive markers of
chemotherapy-induced menopause in premenopausal women under the age
of 40 with breast cancer. Cancer Res. 69 (2_Suppl)(S1118)2009.
|
|
23
|
Moore HCF, Unger JM, Phillips KA, Boyle F,
Hitre E, Porter D, Francis PA, Goldstein LJ, Gomez HL, Vallejos CS,
et al: Goserelin for ovarian protection during breast-cancer
adjuvant chemotherapy. N Engl J Med. 372:923–932. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wong M, O'Neill S, Walsh G and Smith IE:
Goserelin with chemotherapy to preserve ovarian function in
pre-menopausal women with early breast cancer: Menstruation and
pregnancy outcomes. Ann Oncol. 24:133–138. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cerminaro RM, Gardner HM, Shultz SJ,
Dollar JM, Wideman L and Duffy DM: The accuracy of early
reproductive history and physical activity participation recall
across multiple age ranges. J Womens Health (Larchmt). 32:715–722.
2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pourali L, Taghizadeh Kermani A,
Ghavamnasiri MR, Khoshroo F, Hosseini S, Asadi M and Anvari K:
Incidence of chemotherapy-induced amenorrhea after adjuvant
chemotherapy with taxane and anthracyclines in young patients with
breast cancer. Iran J Cancer Prev. 6:147–150. 2013.PubMed/NCBI
|
|
27
|
Obadipe J, Samuel T, Akinlalu A, Ajisafe
A, Olajide E and Albdulmumin L: Chemotherapy-induced ovarian
toxicity in female cancer patients from selected nigerian tertiary
health care. Niger J Exp Clin Biosci. 9(89)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Goldfarb SB, Bedoschi G, Turan V, Crown A,
Abdo N, Dickler MN and Oktay K: Impact of breast cancer
chemotherapy on ovarian damage and recovery. J Clin Oncol. 38
(15_suppl)(e24059)2020.
|
|
29
|
Coburn SB, Dionne-Odom J, Alcaide ML,
Moran CA, Rahangdale L, Golub ET, Massad LS, Seidman D, Michel KG,
Minkoff H, et al: The association between HIV status, estradiol,
and sex hormone binding globulin among premenopausal women in the
women's interagency HIV study. J Womens Health (Larchmt).
31:183–193. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mauri D, Gazouli I, Zarkavelis G, Papadaki
A, Mavroeidis L, Gkoura S, Ntellas P, Amylidi AL, Tsali L and
Kampletsas E: Chemotherapy associated ovarian failure. Front
Endocrinol (Lausanne). 11(572388)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ben-Aharon I, Granot T, Meizner I, Rizel
S, Yerushalmi R, Sulkes A and Stemmer SM: Chemotherapy-induced
ovarian failure in young breast cancer patients: The role of
vascular toxicity. J Clin Oncol. 32(15_suppl)(S9595)2014.
|