Introduction
Malakoplakia is a rare inflammatory disease
characterized by granuloma formation that commonly affects the
genitourinary system (1). However,
the disease can affect various anatomical locations; the bladder,
renal parenchyma, prostate and ureter, are respectively, the most
commonly affected areas (2). Other
documented locations are the gastrointestinal tract, skin,
epididymis, adrenal gland, bone and lungs, as well as multiple
organs in rare conditions (3,4).
Macroscopically, it often appears as soft, yellow-colored plaques
or nodules. Although it usually appears as a single lesion, it can
sometimes manifest as multiple lesions or may even be absent on a
visual inspection (1). Malakoplakia
can affect both sexes and is most common in individuals aged >40
years (2). The most common symptoms
include hematuria, recurrent urinary tract infections and urinary
flow obstruction (3). Although
malakoplakia is an inflammatory condition, it can mimic a tumor,
particularly when it appears as a mass-like lesion (5). The disease typically occurs in
immunocompromised individuals, such as those with diabetes
mellitus, kidney transplants, those with a prolonged use of
systemic corticosteroids, or a history of Escherichia coli
infection (2). However, to the best
of our knowledge, no case of malakoplakia induced by Morganella
morganii and mimicking a bladder tumor has been reported to
date in the literature.
Therefore, the present study describes the case of a
patient with bladder malakoplakia caused by Morganella
morganii mimicking a bladder tumor. In line with the CaReL
guidelines, a brief literature review was also included, and
references were examined to ensure correct citations (6,7). A
search was performed on Google Scholar using the key word
‘allintitle: Bladder Malakoplakia’ for publications dated between
2010 and 2025, yielding 37 results. From these, the 10 most recent
cases were randomly selected for a brief review.
Case report
Patient information
A 78-year-old male patient presented to Smart Health
Tower (Sulaymaniyah, Iraq) on December, 2024 with complaints of
dysuria, associated with urinary frequency, urgency and
incontinence, without hematuria. His past medical history included
cerebrovascular accidents and hypertension. He had undergone a
transurethral resection of the prostate in 2014 and had a femoral
fracture resulting from a road traffic accident in 1990. His
current medications included clopidogrel, rosuvastatin and
amlodipine.
Clinical findings
Upon a general examination, the patient appeared
alert and oriented with no acute distress. He relied on a
wheelchair for mobility assistance. His vital signs were within
normal limits. The abdominal examination revealed a soft,
non-tender abdomen with no palpable masses or suprapubic fullness.
External genitalia appeared normal with no signs of erythema,
discharge, or lesions.
Diagnostic approach
A urinalysis revealed a marked number of pus cells
per high-power field. An abdominal ultrasound demonstrated a
thickened bladder wall, indicative of cystitis. A urine culture
revealed the growth of Morganella morganii. At 20 days
thereafter, the patient returned with similar symptoms despite
antibiotic use [cefditoren pivoxil, 200 mg (1x2) for 3 weeks]. A
repeated urinalysis revealed similar findings. A subsequent
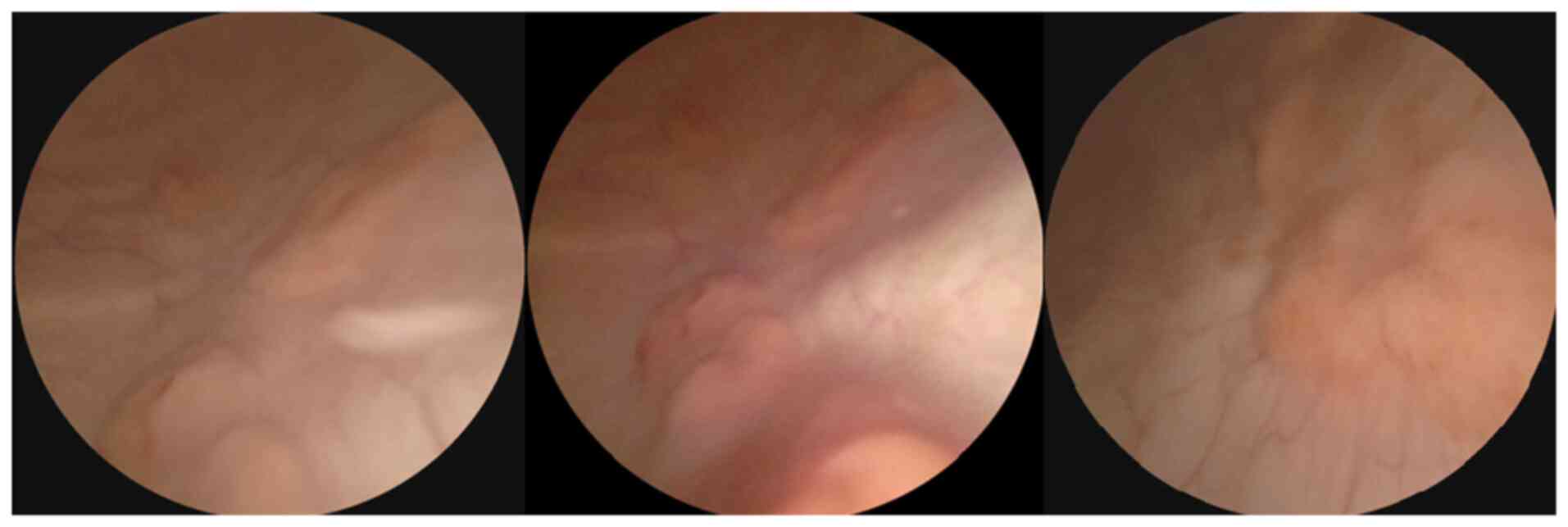
flexible cystoscopy revealed a tight urethra and multiple diffuse
sessile masses throughout the bladder (Fig. 1).
Therapeutic intervention
A transurethral resection of the masses was
performed and sent for histopathological analysis. Grossly, several
rubbery, gray-tan tissue fragments measuring 2.5 cm in total were
noted. A histopathological examination, was then performed on
5-µm-thick paraffin-embedded sections. The sections were fixed in
10% neutral buffered formalin at room temperature for 24 h and then
stained with hematoxylin and eosin (Bio Optica Co.) for 1-2 min at
room temperature. The sections were then examined under a light
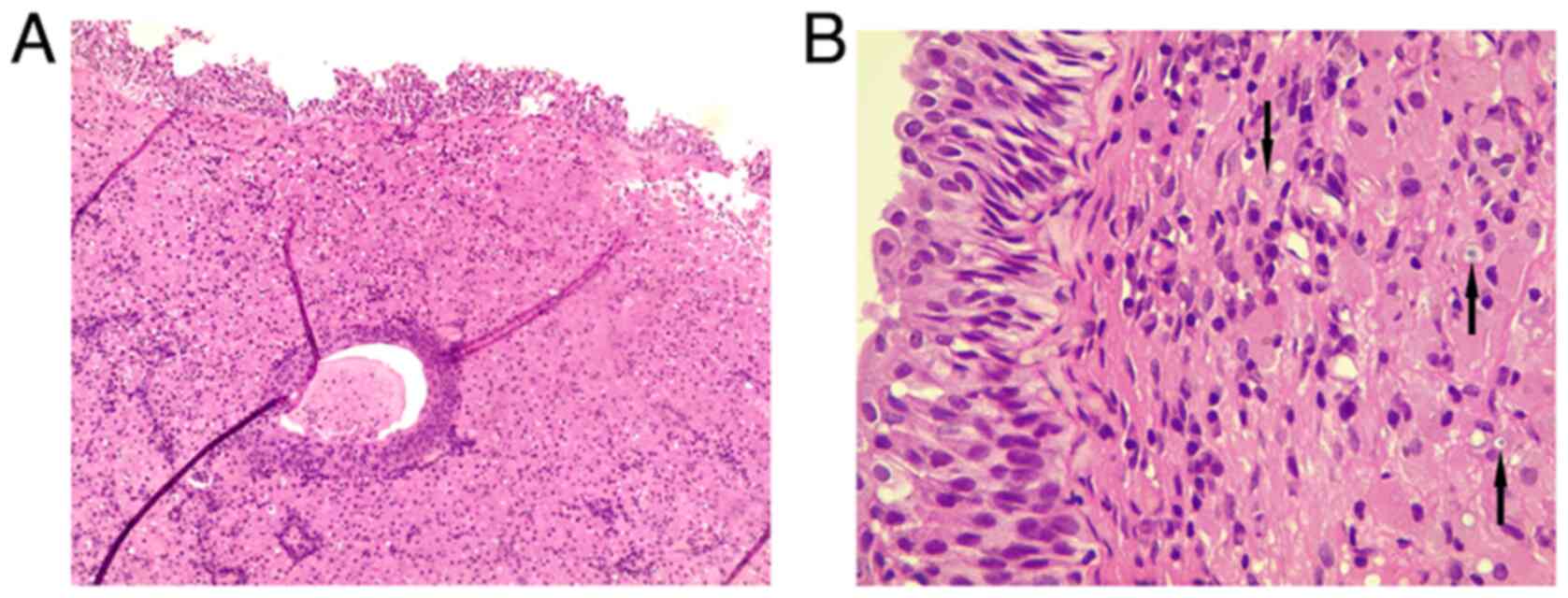
microscope (Leica Microsystems GmbH). Histological analysis
revealed a reactive, flattened urothelial lining with mild
hyperplasia, featuring focal Brunn nests and cystitis glandularis.
There was a marked mucosal infiltrate composed of sheets of round
to polygonal histiocyte cells with abundant eosinophilic granular
cytoplasm, resembling von Hansemann cells (Fig. 2). These cells had round nuclei with
variable nucleoli and displayed rounded, concentric basophilic
intracytoplasmic inclusions, Michaelis-Gutmann-like bodies. The
tissue also contained a mixture of acute and chronic inflammation.
The muscularis propria was present and unaffected by the
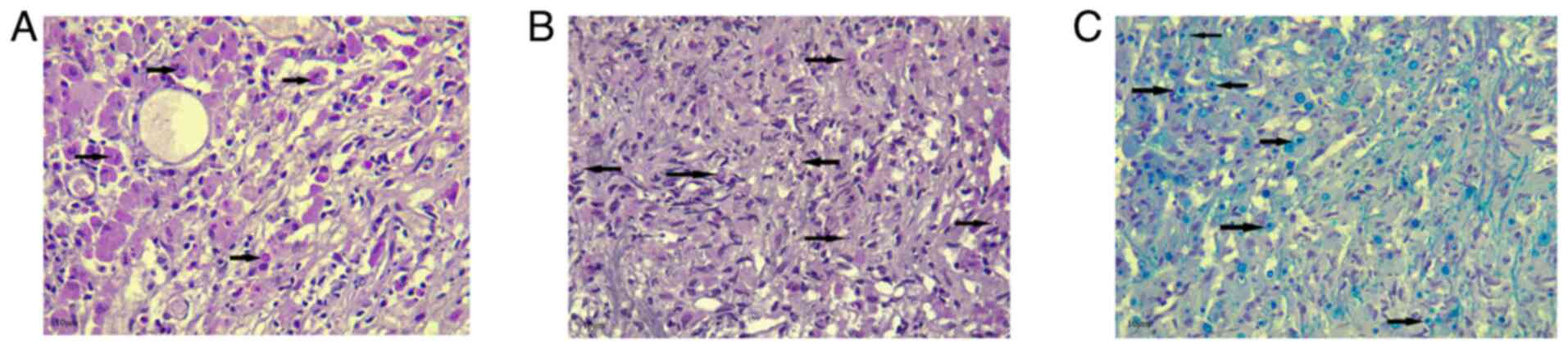
histiocytic infiltrate. Prussian blue, Periodic acid-Schiff (PAS)
and PAS with diastase (D-PAS) staining was performed on
1.5-µm-thick paraffin-embedded sections. The sections were fixed in
10% neutral buffered formalin at room temperature for 24 h.
Prussian blue, PAS and D-PAS staining (MilliporeSigma) were then
applied at room temperature for a duration of 20 min. The sections
were then examined under a light microscope (Leica Microsystems
GmbH). Prussian blue, PAS and D-PAS staining all yielded positive
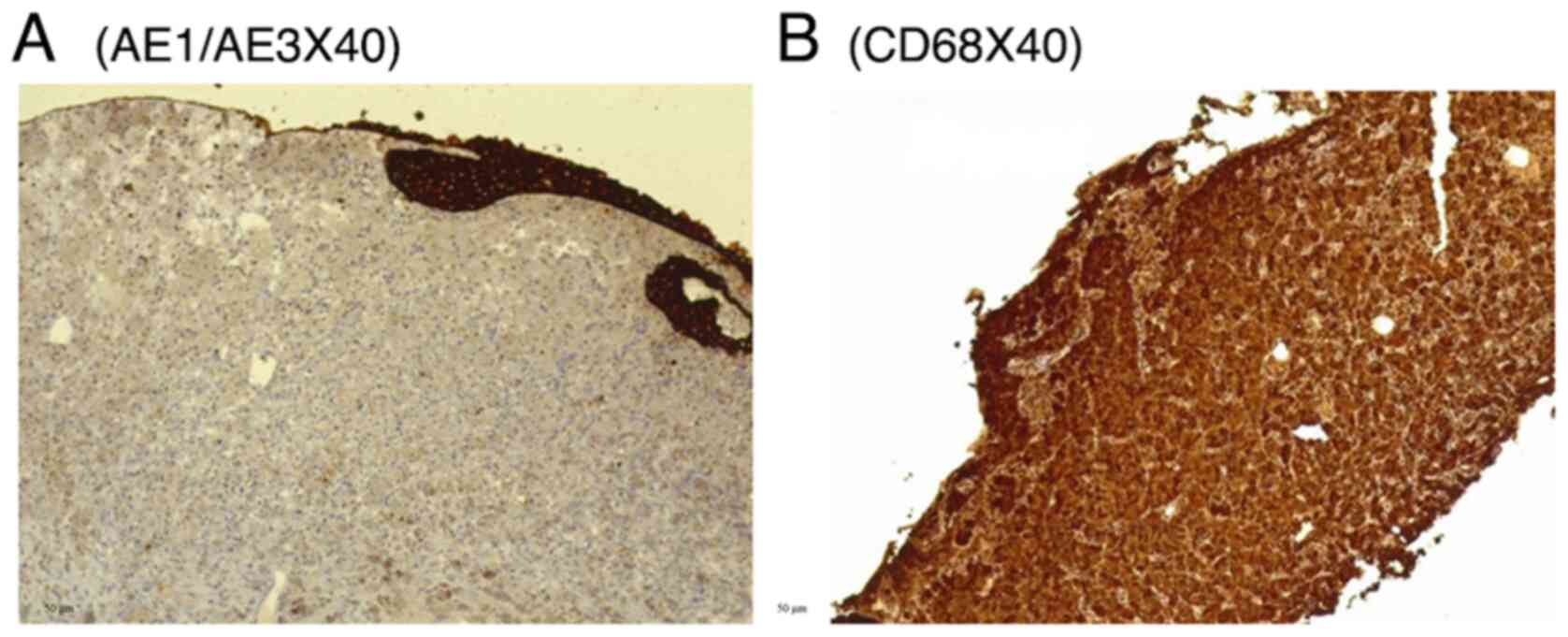
results, highlighting the cytoplasmic inclusion (Fig. 3). Immunohistochemistry (IHC) was then
performed for mucosal infiltrating cells. Serial 5-µm-thick
sections cut from formalin-fixed, paraffin-embedded bladder mass
tissue were mounted on coated slides, deparaffinized in xylene and
rehydrated through graded ethanol to distilled water. Antigen
retrieval was performed by heat-mediated epitope retrieval in 10 mM
citrate buffer (Ph 6.0) at 95-100˚C for 20 min, followed by cooling
for 20 min at room temperature. Endogenous peroxidase activity was
quenched with 3% hydrogen peroxide in PBS for 10 min at room
temperature. Sections were permeabilized with 0.1% Triton X-100 in
PBS for 10 min at room temperature and rinsed with PBS (3x5 min).
Non-specific binding was blocked with 5% normal goat serum (Thermo
Fisher Scientific, Inc.) in PBS for 20 min at room temperature.
Primary antibodies [AE1/AE3 (1:100, clone AE1/AE3, cat. no. M3515),
S100 (1:200, polyclonal rabbit, cat. no. Z0311) and CD68 (1:100,
clone KP1, cat. no. M0814) (all from Dako; Agilent Technologies,
Inc.)] were applied and incubated with the sections for 30-60 min
at room temperature (alternatively overnight at 4˚C if required for
optimization). Following washes with distilled water, the sections
were incubated with HRP-conjugated goat anti-mouse IgG (1:200, cat.
no. P0447) for AE1/AE3 and CD68, and HRP-conjugated goat
anti-rabbit IgG (1:200, cat. no. P0448) for S100 (both from Dako;
Agilent Technologies, Inc.), for 30 min at room temperature. HRP
activity was visualized using DAB chromogen according to
manufacturer's instructions (monitoring 3-10 min), followed by a
water rinse. The sections were counterstained with Gill's
hematoxylin II (Thermo Fisher Scientific, Inc.) for ~30-60 sec,
rinsed, blued in running tap water, dehydrated, cleared and
coverslipped. Images were captured on a Leica light microscope
(Leica Microsystems GmbH); scale bars (50 µm) were added following
objective calibration. IHC revealed negativity for AE1/AE3 and S100
(data not shown), and strong positivity for CD68 (Fig. 4). Negativity for AE1/AE3 argued
against an epithelial cell origin, while negativity for S100 argued
against a granular cell tumor. Positivity for CD68 supported the
presence of histiocytic-type cells. The patient was administered
oral sulfamethoxazole-trimethoprim (800/160 mg) twice daily for 5
days. Following the urine culture results, treatment was switched
to intravenous meropenem (1 g) twice daily for 4 days. No
side-effects from the treatment were observed.
Follow-up and outcome
Following antibiotic therapy, the symptoms of the
patient partially improved at 1-month follow-up. The patient was
subsequently lost to follow-up.
Discussion
When involving the genitourinary system,
malakoplakia is more commonly observed in women (8). However, malakoplakia outside the
urinary tract is more common among males and is uncommon in
children (3). The precise cause of
malakoplakia is not yet fully understood. It is hypothesized that
malakoplakia results from impaired phagocytosis, linked to reduced
levels of intracellular cyclic guanosine monophosphate. A low
cyclic guanosine monophosphate to cyclic adenosine monophosphate
ratio disrupts the redox balance of the cell, compromising its
ability to kill bacteria effectively. This leads to a granulomatous
response, where bacterial remnants accumulate. These partially
digested bacteria calcify and cluster inside macrophages, forming
the characteristic Michaelis-Gutmann bodies (5). While a direct cause-and-effect
connection between coliform bacteria and malakoplakia has not yet
been firmly established, it has been reported that ~90% of patients
with malakoplakia also have coliform infections (8).
Morganella morganii, a bacterium from the
Enterobacteriaceae family, is a rod-shaped, Gram-negative,
facultatively anaerobic bacillus that includes two subspecies:
Morganii and sibonii. Formerly known as Proteus
morganii, it is typically part of the normal human gut
microbiota. However, in certain cases, particularly in hospital
settings, following surgery, or in individuals with weakened immune
systems and young children, it can lead to severe and potentially
life-threatening systemic infections, and naturally exhibits
resistance to several antibiotic classes (9). Herein, following a detailed literature
review, no cases of malakoplakia caused by Morganella
morganii were identified. In addition, Polisini et al
(2) reviewed the literature on
bladder malakoplakia and, across 35 articles reporting 36 cases,
did not identify any case of bladder malakoplakia associated with
Morganella morganii. Hence, the case presented herein may be
the first reported case of its kind.
Polisini et al (2) reported that approximately half of the
cases had recurrent urinary tract infections, particularly caused
by Escherichia coli, and that ~20% had immune system
disorders. Antibiotic therapy was administered in ~70% of cases,
and surgery was performed in 67% of cases. Among the surgical
procedures, approximately three-quarters involved transurethral
resection of the bladder. The recurrence rate was lower in patients
managed with both antibiotics and surgery than in those treated
with antibiotics alone (2).
Malakoplakia can also occur in unusual sites, such as the brain and
skeleton, which may indicate atypical Escherichia coli
strains or immune system abnormalities (3). A total of 10 cases of bladder
malakoplakia were reviewed herein (1,3,4,8,10-15).
All cases involved older individuals or adults, apart from 1
patient who was <2 years of ag. Michaelis-Gutmann bodies were
identified in all histopathological examinations, with 2 cases also
reporting von Hansemann cells. Among the cases that performed urine
cultures, only 2 cases were negative, while the others were
infected with Escherichia coli; 1 case also had a positive
blood culture for Pseudomonas aeruginosa. Surgical
intervention was performed in 6 cases, while 4 cases were managed
conservatively, one of which experienced recurrent bladder
malakoplakia after 6 months (Table
I).
 | Table IReview of 10 cases of malakoplakia in
the urinary bladder identified in the literature. |
Table I
Review of 10 cases of malakoplakia in
the urinary bladder identified in the literature.
| First author, year of
publication | Sex | Age (years) | Chief
complaint/symptoms | Imaging tests | Histopathological and
immunohistochemical examination | Culture tests | Other
examinations | Treatment | Follow-up | (Refs.) |
|---|
| Ye, 2025 | F | N/A | Recurrent urinary
frequency for >1 year | CT: Intravesical mass
and upper ureteral calculi Cystoscopy: A mass at bladder neck and
urethral opening | HPE:
Michaelis-Gutmann bodies | N/A | N/A | Transurethral bladder
tumor resection | Not mentioned. | (11) |
| Tinguria, 2024 | F | 86 | Nausea, diarrhea,
foul-smelling urine, dysuria, hematuria, and low blood
pressure | CT: Atrophy of the
left kidney, & diffuse bladder wall thickening. | HPE: Foamy
epithelioid histiocytes, PAS-positive eosinophilic, calcified
Michaelis-Gutmann bodies. IHC: +ve vimentin and CD-68, -ve
AE1/AE3. | Urine: +ve E.
coli, and P. aeruginosa, blood: +ve P.
aeruginosa. | CR: 252 µmol/l,
troponin: 2,695 ng/l, WBC count: 15.4x109/l, cystoscopy:
Plaque-like whitened lesions |
Piperacillin/tazobactam, | Recurrent bladder
malakoplakia after 6 months. | (3) |
| Jdiaa, 2023 | F | 70 | Fatigue, weakness,
decreased oral intake | CT: Bilateral
ureteral fullness suspicious for urothelial malignancy, bilateral
hydronephrosis | HPE and IHC: Sheets
of epithelioid histiocytes (CD68 positive), numerous neutrophils,
numerous CD38-positive plasma cells and occasional
Michaelis-Gutmann bodies | Blood and urine: +ve
E. coli | CR: 5.0 mg/dl
Hemoglobin: 6.9 g/dl WBC: 13.9x109/l | Transurethral bladder
tumor resection, ceftriaxone | Recovery with return
of kidney function to baseline | (12) |
| Gao, 2021 | M | 48 | Frequent and urgent
urination, right lumbago swelling and pain, as well as lower
abdominal discomfort | CT: A mass on the
right lateral bladder wall invading the right ureteral orifice | HPE: large numbers of
eosinophils and foam cells containing Michaelis-Gutmann bodies | Urine: +ve E.
coli | ESR: 98 mm/h CR: 123
µmol/l | Transurethral bladder
mass resection, tazobactam | No recurrence after 4
years | (13) |
| Pham, 2022 | F | 81 | Frequent and urgent
urination, Urge incontinence | CT: Severe bilateral
hydrou-reteronephrosis and bladder wall thickening | HPE:
Michaelis-Gutmann bodies | N/A | CR: 221 µmol/l | Cephalexin,
methenamine hippurate, supplemental vitamin C, ntravesical
gentamicin wash | Not mentioned | (14) |
| Parkin, 2020 | F | 82 | History of T1D,
rigors, lethargy, back pain, irritative voiding and urgency | CT: Nilateral
hydroureterone-phrosis and marked thickening of the bladder
base | HPE:
Michaelis-Gutmann bodies & von Hansemann cells. No evidence of
dysplasia or malignancy was seen. | Urine: recurrent
pan-sensitive E. coli. | WBC count:
5.4×10^9/l, hemoglobin: 96 g/l & CR: 234 µmol/l. | Surgical removal of
the bladder mass, amoxicillin-clavulanic acid | No recurrence at 2
months. | (10) |
| Rabani, 2019 | F | 1.7 | voiding dysfunction,
pyuria & repeated UTI. | CT: right lateral
bladder wall mass. | HPE:
Michaelis-Gutmann bodies. | Urine: +ve E.
coli. | Normal blood profile,
renal, and liver function tests. Cystoscopy: bladder mass. |
Trimethoprim-sulfamethoxazole | No recurrence after 9
years. | (8) |
| Sirithanaphol,
2018 | F | 66 | Gross hematuria,
dysuria | Cystoscopy:
white-yellowish plaque in bladder | HPE: sheets of large
macrophages with granular eosinophilic cytoplasm, mixed
inflammatory infiltrate, and Michaelis-Gutmann bodies | Urine: -ve | Urinalysis: Pyuria
and hematuria | Ciprofloxacin,
prednisolone, methotrexate, trimethoprim-sulfamethoxazole | Recovery of
symptoms | (15) |
| Lee, 2015 | F | 74 | Chills,
unremarkable physical examination. | CT: urinary bladder
postero-lateral wall mass. | HPE: papillary
urothelial carcinoma, & macrophages with Michaelis-Gutmann
bodies. IHC: +ve CD163 & CD68 & -ve Cam 5.2. | Urine: -ve. | WBC count:
12.9x10^3/µl, CRP: 77.4 mg/l, PCT: 64.28 µg/l CR: 101 µmol/l,
Urinalysis: hemopyuria. | IV ceftriaxone for
possible sepsis, transu-rethral resection | No recurrence at
12-month follow-up. | (1) |
| Ristić-Petrović,
2013 | F | 53 | General weakness,
low-grade fever and urinary hesitancy. | Not mentioned | HPE: von Hansemann
cells, Michaelis-Gutmann bodies, infiltrated by dense collections
of lymphocytes. IHC: -ve cytokeratin, and Ki-67, +ve CD68 | Persistent E.
coli infections for 2 years. | Urine analysis:
Albuminuria, macrohematuria, pyuria and bacteriuria. Cystoscopy:
Thickened mucosa of the bladder similar to a neoplastic mass. | Surgical removal of
yellowish polypoidal lesions of the bladder | Not mentioned | (4) |
There may be instances in which malakoplakia occurs
without clinical or laboratory evidence of infection, but rather in
association with underlying neoplastic conditions. Darvishian et
al (16) reported the case of a
74-year-old female patient with papillary urothelial carcinoma of
the urinary bladder, with malakoplakia identified incidentally in a
bladder biopsy. Urinalysis revealed only a marked presence of
numerous red blood cells, with no evidence of pyuria or
bacteriuria. The urine culture did not reveal bacterial growth. A
tumor on the lateral wall of the urinary bladder was noted in
cystoscopy following initial examinations, and a biopsy was
subsequently done. Histological analysis and immunohistochemistry
were performed, exhibiting CD68 positivity. She underwent
conservative treatment without additional invasive intervention
(16).
There is currently no universally accepted standard
for the diagnosis and treatment of malakoplakia (5). Since the majority of studies on bladder
malakoplakia are case reports, with a notable absence of
comprehensive reviews (2),
diagnosing malakoplakia clinically can be challenging due to its
non-specific features. However, its appearance may mimic carcinoma,
which often leads to invasive or minimally invasive treatment, as
in the present case report, since malakoplakia generally requires
conservative treatment (17).
Its histological morphology changes over three
stages. In the first stage, a few cells with lymphocyte and plasma
cell infiltration represent glucolipid aggregation in
Michaelis-Gutmann bodies. The second stage is marked by increased
histiocytes and von Hansemann cells, along with the formation of
Michaelis-Gutmann bodies, which is the most distinctive feature. In
the third stage, histiocytes and von Hansemann cells decrease, and
fibrosis and collagen hyperplasia occur. Stains such as PAS,
Prussian blue, Alcian Blue and von Kossa effectively highlight
Michaelis-Gutmann bodies (18).
Increased levels of immunoreactive α1-antitrypsin are observed in
macrophages in malakoplakia, potentially aiding in the differential
diagnosis. Malakoplakia also presents with non-specific imaging
findings, and the affected areas may develop tumor-like nodules
(5). Still, imaging techniques, such
as computed tomography, ultrasonography and intravenous pyelogram
can help detect associated hydroureteronephrosis and identify upper
urinary tract filling defects, which are suggestive of its presence
(2). Immunohistochemistry also aids
in diagnosing malakoplakia by exhibiting negativity for AE1/AE3 and
S100, and positivity for CD68(3).
Immunohistochemistry staining in the present case report revealed
negativity for AE1/AE3 and S100, and positivity for CD68, similar
to has been previously reported (3).
The treatment of malakoplakia depends on the
severity of the condition and the underlying health conditions of
the patient. In the majority of cases, patients with bilateral or
multifocal involvement recover following antibiotic therapy. A
cholinergic agonist such as bethanechol chloride may be used
alongside antibiotics to help correct lysosomal dysfunction
(17). There are no established
guidelines regarding selection or duration of antibiotic therapy to
prevent malakoplakia recurrence. However, the use of antibiotics
that are effective against Gram-negative bacteria and those that
accumulate within macrophages has been recommended, as they may
help compensate for impaired phagocytic function. These antibiotics
include rifampicin, ciprofloxacin and trimethoprim. Due to the
potential adverse effects associated with quinolones, trimethoprim
is often the preferred option (7).
Vitamin C supplementation is recommended to help reduce the
inflammatory response. In patients with malakoplakia-related
complications, the regular monitoring of renal function and ongoing
cystoscopic surveillance are essential (10). Surgical removal has also been
recommended as an alternative treatment option for large lesions,
especially when complete elimination through medical therapy alone
may not be possible (8). Unifocal
disease may require surgical excision, and transurethral resection
may be needed for large lesions blocking the ureter. Based on the
risk-benefit ratio, the termination of immunosuppressive drugs is
often necessary (3). The patient in
the present case report was managed using a combination of
transurethral resection of the mass-like lesions and antibiotic
therapy. The patient demonstrated a favorable initial response with
a partial improvement of symptoms; however, the case was lost to
follow-up, preventing the further assessment of disease
progression. Consequently, the major limitation of the present case
report is the short follow-up period, which restricts the ability
to accurately evaluate the long-term outcome of the patient. In
addition, there is a lack of a representative image for S100
reactivity. The present study presents only a single-center case;
thus, further research is warranted to elucidate the potential role
and underlying mechanisms of Morganella morganii infection
in the pathogenesis of malakoplakia and to provide deeper insights
into this association.
In conclusion, bladder malakoplakia can also develop
due to infection with Morganella morganii and mimic a tumor;
thus, it should be considered in the differential diagnosis of
bladder inflammation or masses.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RB and IA were major contributors to the conception
of the study, as well as to the literature search for related
studies. TOS, PWB and FHK were involved in the design of the study,
literature review and in the writing of the manuscript. FMF, BOM
and MNH were involved in the literature review, the design of the
study, the critical revision of the manuscript, and in the
processing of the table. AHA was the radiologist who performed the
assessment of the case. HAY was the pathologist who performed the
diagnosis of the case. FHK and RB confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient for participation in the present study.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the present case report and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee SL, Teo JK, Lim SK, Salkade HP and
Mancer K: Coexistence of malakoplakia and papillary urothelial
carcinoma of the urinary bladder. Int J Surg Pathol. 23:575–578.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Polisini G, Delle Fave RF, Capretti C,
Marronaro A, Costa AM, Quaresima L, Mazzaferro D and Galosi AB:
Malakoplakia of the urinary bladder: A review of the literature.
Arch Ital Urol Androl. 94:350–354. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tinguria M: Recurrent bladder
malakoplakia: A rare bladder lesion mimicking malignancy. Bladder
(San Franc). 11(e21200018)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ristić-Petrović A, Stojnev S,
Janković-Veličković L and Marjanović G: Malakoplakia mimics urinary
bladder cancer: A case report. Vojnosanit Pregl. 70:606–608.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boualaoui I, El Aboudi A, Mikou MA, El
Abidi H, Ouskri S, Mesbahi S, Ibrahimi A, Hashem ES and Yassine N:
Malignant masquerade: A case report of urachal malakoplakia
disguised as urachal tumor. Urol Case Rep.
60(103012)2025.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Abdullah HO, Abdalla BA, Kakamad FH, Ahmed
JO, Baba HO, Hassan MN, Bapir R, Bapir HM, Bapir DA, Bapir SH, et
al: Predatory publishing lists: A review on the ongoing battle
against fraudulent actions. Barw Med J. 2:26–30. 2024.
|
|
7
|
Prasad S, Nassar M, Azzam AY,
García-Muro-San José F, Jamee M, Sliman RK, Evola G, Mustafa AM,
Abdullah HQ, Abdalla B, et al: CaReL guidelines: A consensus-based
guideline on case reports and literature review (CaReL). Barw Med
J: 2, 2024 doi: 10.58742/bmj.v2i2.89.
|
|
8
|
Rabani SM and Rabani SH: Bladder
malakoplakia simulating neoplasm in a young girl: Report of a case
and review of literature. Urol J. 16:614–615. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zaric RZ, Jankovic S, Zaric M,
Milosavljevic M, Stojadinovic M and Pejcic A: Antimicrobial
treatment of Morganella morganii invasive infections:
Systematic review. Indian J Med Microbiol. 39:404–412.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Parkin CJ, Acland G, Sulaiman B, Johnsun
ML and Latif E: Malakoplakia, a malignant mimic. Bladder (San
Franc). 7(e44)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ye H, Yu L and Chen Y: Ureteral calculus
complicated by bladder malakoplakia: A case report. Medicine
(Baltimore). 104(e42926)2025.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jdiaa SS, Degheili JA, Matar CF, Mocadie
MF, Khaled CS and El Zakhem AM: Acute kidney injury secondary to
obstructive bladder malakoplakia: A case report. African J Urol.
29(40)2023.
|
|
13
|
Gao P, Hu Z and Du D: Malakoplakia of the
bladder near the ureteral orifice: A case report. J Int Med Res.
49(03000605211050799)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pham CT, Edwards M, Chung AS and Chalasani
V: Malakoplakia causing poor bladder compliance and bilateral
hydroureteronephrosis. Société Int d'Urologie J. 3:281–282.
2022.
|
|
15
|
Sirithanaphol W, Sangkhamanon S,
Netwijitpan S and Foocharoen C: Bladder malakoplakia in systemic
sclerosis patient: A case report and review literature. J Endourol
Case Rep. 4:91–93. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Darvishian F, Teichberg S, Meyersfield S
and Urmacher CD: Concurrent malakoplakia and papillary urothelial
carcinoma of the urinary bladder. Ann Clin Lab Sci. 31:147–150.
2001.PubMed/NCBI
|
|
17
|
Purnell SD, Davis B, Burch-Smith R and
Coleman P: Renal malakoplakia mimicking a malignant renal
carcinoma: A patient case with literature review. Case Reports.
2015(bcr2014208652)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang XY, Li J, Chen SL, Li Y, Wang H and
He JH: Malakoplakia with aberrant ALK expression by
immunohistochemistry: A case report. Diagn Pathol.
18(97)2023.PubMed/NCBI View Article : Google Scholar
|