Introduction
Hilar cholangiocarcinoma (hCCA) accounts for 50-60%
of all cholangiocarcinomas (1,2). De
novo hCCA is an aggressive malignancy, with 5-year survival
rates of up to 40% in selected series, particularly when negative
lymph node status and clear resection margins are achieved
(1).
Primary sclerosing cholangitis (PSC) is a
well-recognised risk factor for hCCA, with an annual incidence
estimated between 0.2 and 1.5% following the diagnosis of PSC
(3,4). Outcomes for hCCA arising in the context
of PSC appear to differ markedly from de novo cases. In
selected patients undergoing resection, the 5-year survival rate
for PSC-associated hCCA may reach 60%, compared to 30-40% in the
de novo group (1,3,5).
Further improvements in long-term survival have been
achieved through the implementation of transplant-based protocols,
most notably the Mayo Clinic protocol, which combines neoadjuvant
chemoradiotherapy followed by liver transplantation (4,6). Of
note, 10-year survival rates approaching 70% have been reported in
carefully selected patients (3).
However, challenges remain, namely strict selection criteria,
dropout due to disease progression, false-positive diagnoses with
no tumour in the explant, and increased vascular complications
post-transplant due to prior chemoradiation (4).
Despite these advances, the UK did not have an
approved liver transplant programme for cholangiocarcinoma at the
time of the treatment of the patient described in the present case
report, limiting access to transplant-based pathways. Organ
shortage further restricts curative options in this patient
population, and upfront surgical resection remains the mainstay of
treatment, albeit with key technical challenges. Regardless of
treatment modality, recurrence rates remain high, affecting ~50% of
cases (3,4).
The present study describes a rare case of long-term
survival in a patient with PSC-associated hCCA initially treated
with extended liver resection. Liver transplantation was performed
2 years thereafter, an unorthodox point in the disease course.
Despite multiple episodes of metastatic recurrence under
immunosuppression, each was successfully managed. The case
described herein highlights the potential importance of tumour
biology in determining long-term outcomes in PSC-associated
hCCA.
Case report
A 52-year-old male patient was diagnosed with PSC in
2015 at King s College Hospital (London, UK), confirmed by
liver biopsy. He remained under regular surveillance by the
hepatology team until April, 2017, when he presented with new-onset
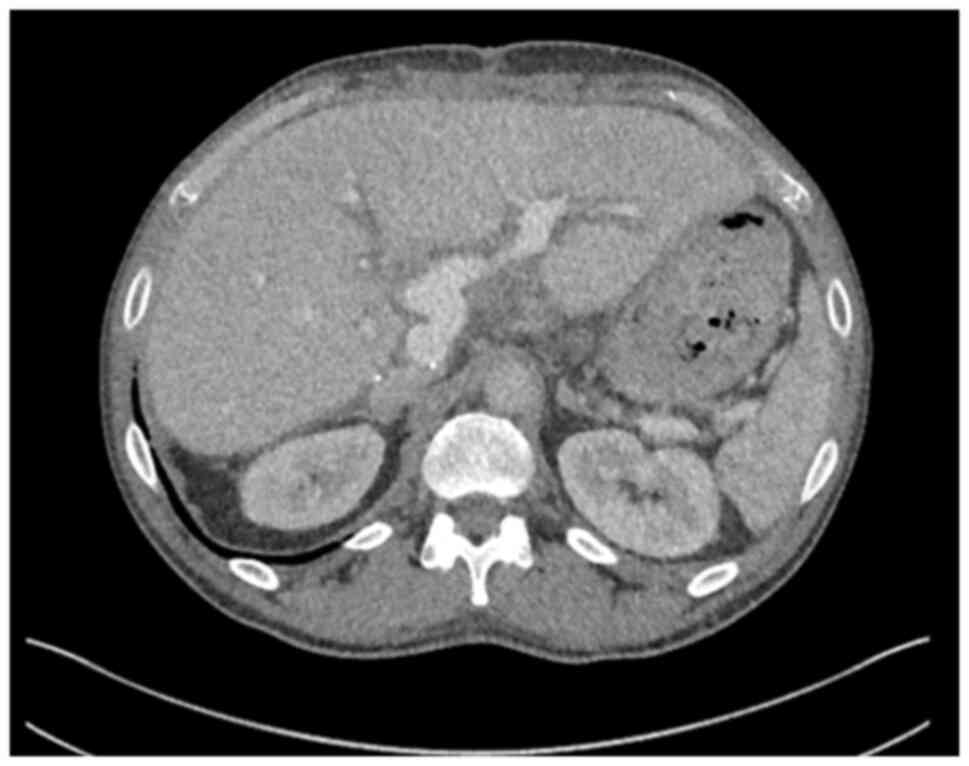
obstructive jaundice. Contrast-enhanced computed tomography (CT)
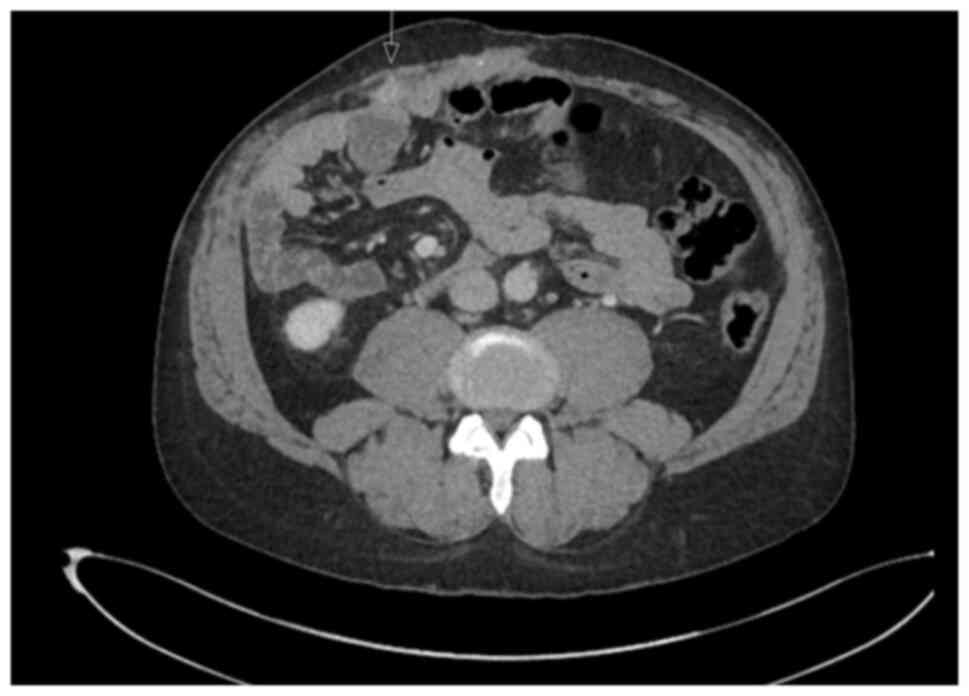
revealed a hilar mass causing bilateral biliary dilation, and the
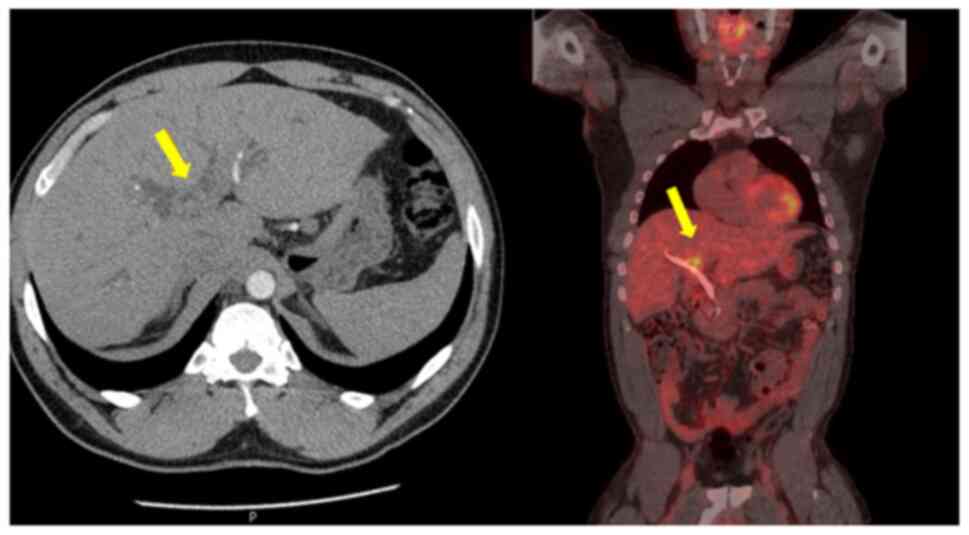
working diagnosis was that of hCCA. After staging with
fluorodeoxyglucose positron emission tomography (FDG-PET) and
diagnostic laparoscopy, no evidence of metastatic disease was
identified (Fig. 1). Portal vein
embolization was performed to induce hypertrophy of the future
liver remnant (FLR) in preparation for an extended right
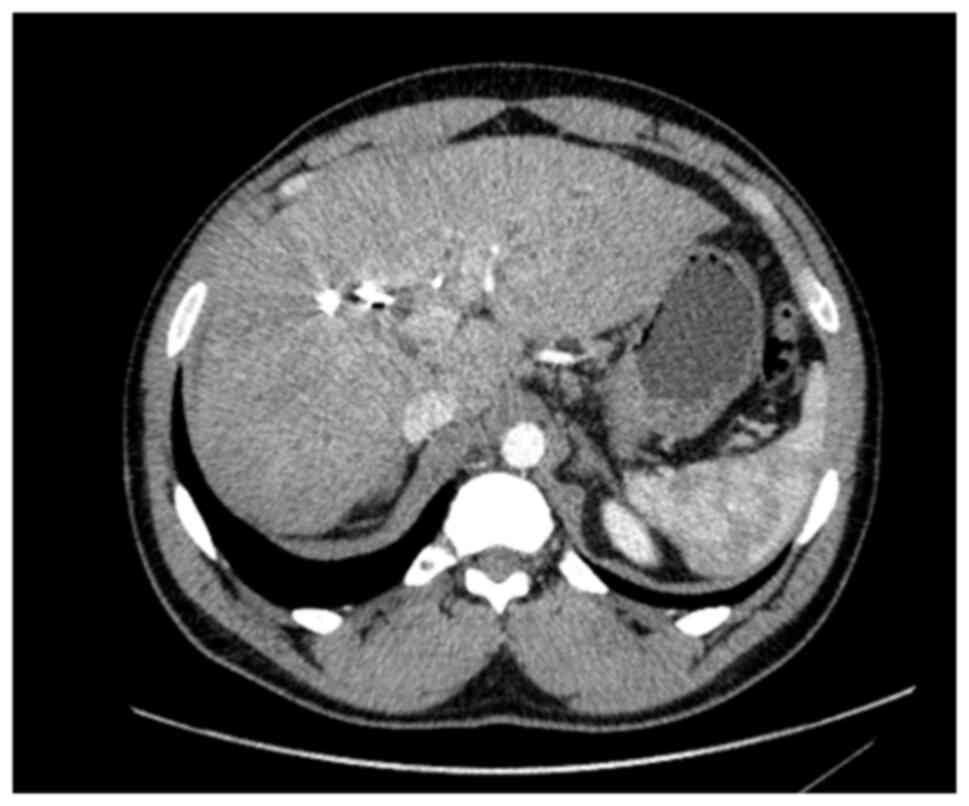
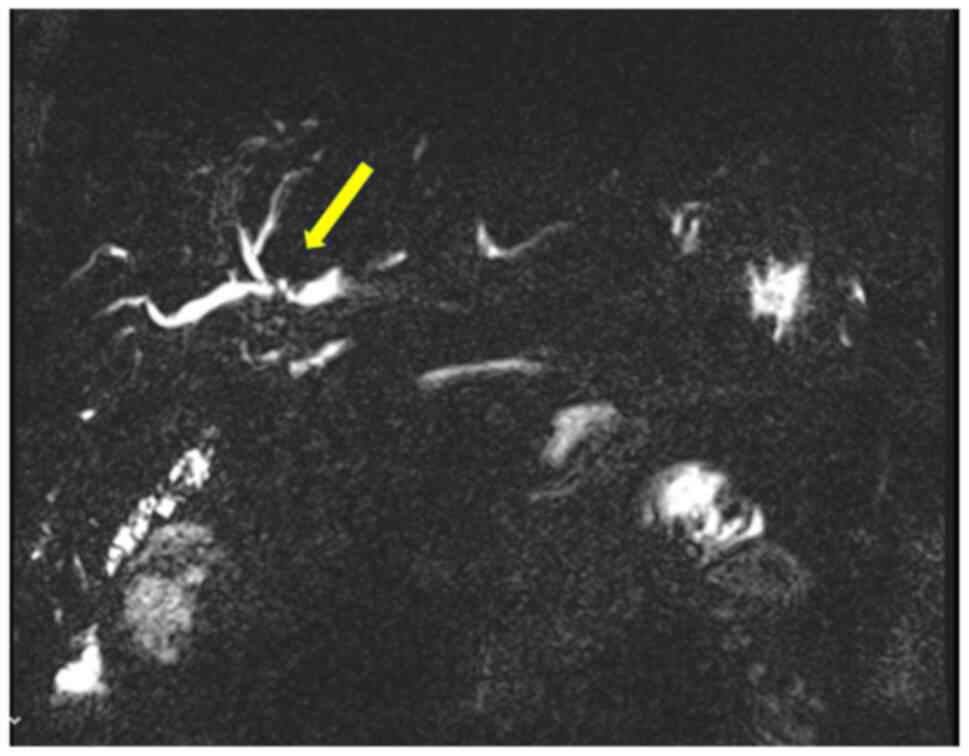
hepatectomy (ERH). Given the background of PSC, a 10-week interval
was observed before repeat imaging, which demonstrated marginal FLR
growth. The future liver remnant-to-body weight ratio (FLRBWR) was
0.42 at the time of surgery, corresponding to ~20% of total liver
volume (Fig. 2).
In September 2017, the patient underwent ERH with
Roux-en-Y hepaticojejunostomy and regional lymphadenectomy.
Intraoperative portal pressure measurements exceeded 17 mmHg,
prompting portal flow modulation with splenic artery ligation to
mitigate the risk of post-hepatectomy liver failure (PHLF). The
gastroduodenal artery was also ligated. The patient developed PHLF
grade B, according to ISGLS criteria (7), although he did not meet the 50:50
criteria (8), and recovered well
post-operatively. Pre-operative CA 19-9 was 43 U/ml (Fig. 3).
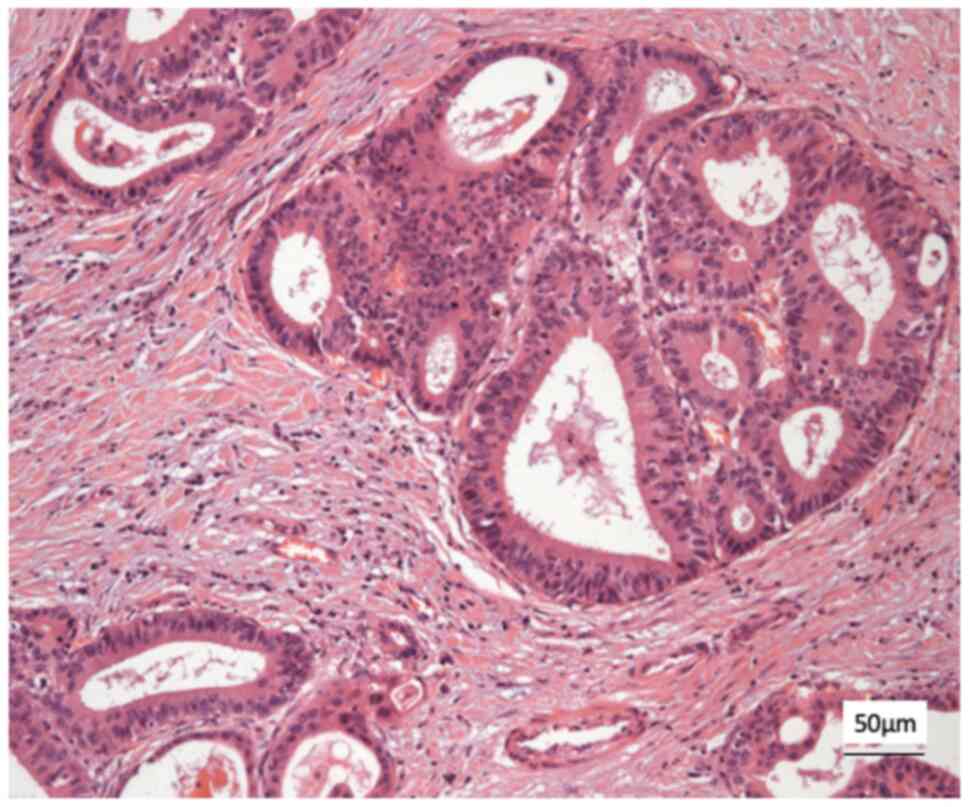
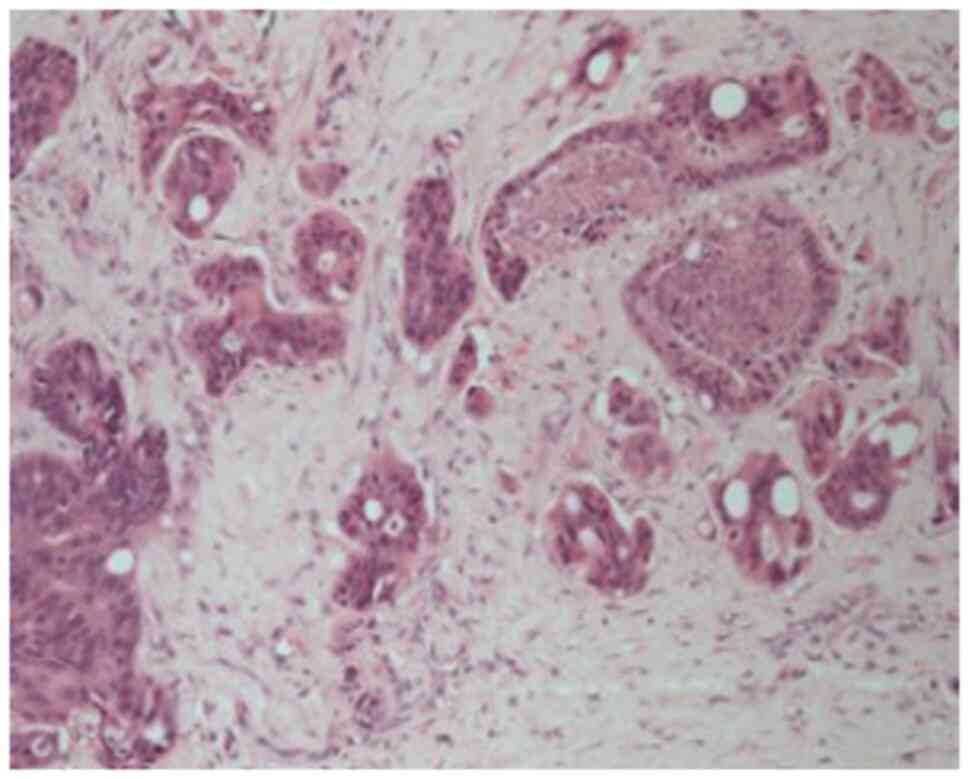
Histological analysis revealed a moderately
differentiated perihilar cholangiocarcinoma measuring 21 mm,
arising in the setting of biliary intraductal papillary neoplasm of
the extrahepatic ducts (30 mm) on a background of severe PSC.
Proximal, distal, and circumferential margins were negative for
malignancy. Perineural (PN) and lymphovascular (LV) invasion were
both present. Final pathological staging was pT2aN0 (0/14) PN1LV1R0
(Fig. 4). Fig. 4 depicts routine haematoxylin and
eosin (H&E) staining performed by the Department of
Histopathology at King s College Hospital. Sections were
paraffin-embedded and cut at a standard diagnostic thickness of 3-4
µm. Fixation was performed in 10% neutral-buffered formalin at room
temperature for a minimum of 24 h. A standard H&E stain was
used, procured from established hospital suppliers under
ISO-accredited protocols. Staining was carried out using automated
staining systems under standard diagnostic laboratory conditions.
Microscopic evaluation was performed using a Leica DM1000
microscope. These procedures are in accordance with institutional
diagnostic protocols.
Subsequently, the patient received two cycles of
adjuvant capecitabine at 1,250 mg/m2 twice daily,
administered on days 1-14 of a 21-day cycle. However, systemic
therapy was discontinued in January, 2018 due to liver function
deterioration. The patient remained disease-free on surveillance
imaging for 18 months. He later developed progressive liver
dysfunction and weight loss. Cross-sectional imaging revealed signs
of progressive cholangiopathy and portal hypertension in addition
to synthetic dysfunction. Given this, the patient underwent a
successful appeal for listing and evaluation for liver
transplantation.
In November, 2019, 26 months later, he underwent
orthotopic liver transplantation using a whole liver graft from a
brain-dead donor (cold ischemia time, 19 h) (Fig. 5). Intraoperatively, extensive
adhesions involving the right colon resulted in a serosal tear
requiring right hemicolectomy and temporary ileostomy.
A histopathological examination of the explanted
liver revealed no evidence of residual hCCA. However, a 9-mm nodule
on the serosal surface of the resected colon demonstrated
histological features identical to the primary hCCA, confirming it
as a metastatic deposit. This assessment was performed by the
Department of Histopathology, King s College Hospital, using
routine diagnostic protocols on formalin-fixed, paraffin-embedded
tissue with standard haematoxylin and eosin (H&E) staining.
Archived digital images of these slides are not available.
Following a prolonged recovery complicated by acute
rejection, the patient was discharged in a stable condition. In
February, 2020, following conversion to an mTOR-based
immunosuppression regimen (9), he
began treatment with adjuvant capecitabine at the standard BILCAP
dose (1,250 mg/m2 twice daily, days 1-14 of a 21-day
cycle) according to routine oncology protocols used for biliary
tract cancers. However, treatment was interrupted after two cycles
due to the COVID-19 pandemic. Surveillance imaging continued every
3 months.
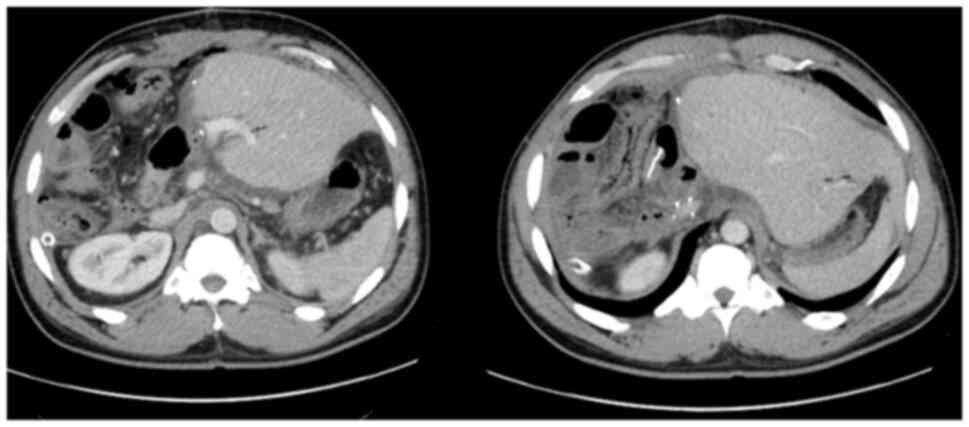
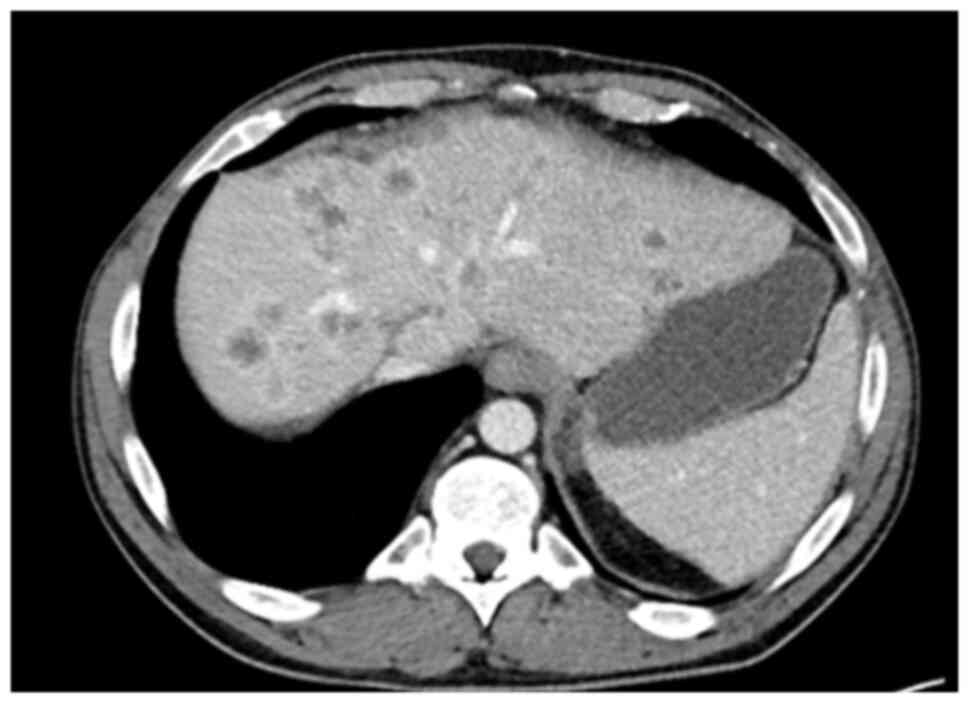
In March 2021, a new hilar stricture was identified
in the liver graft. Magnetic resonance cholangiopancreatography and
biopsy revealed features of ischemic cholangiopathy, possibly from
prior rejection, although recurrent PSC could not be excluded
(Fig. 6). FDG-PET revealed no
evidence of disease recurrence. A suspected anastomotic stricture
at the hepaticojejunostomy was treated with balloon dilation via
percutaneous transhepatic cholangiography, leading to the
resolution of liver function abnormalities. The ileostomy was
reversed in March, 2022.
The patient remained in a good condition clinically,
although mild biochemical evidence of recurrent PSC was noted. In
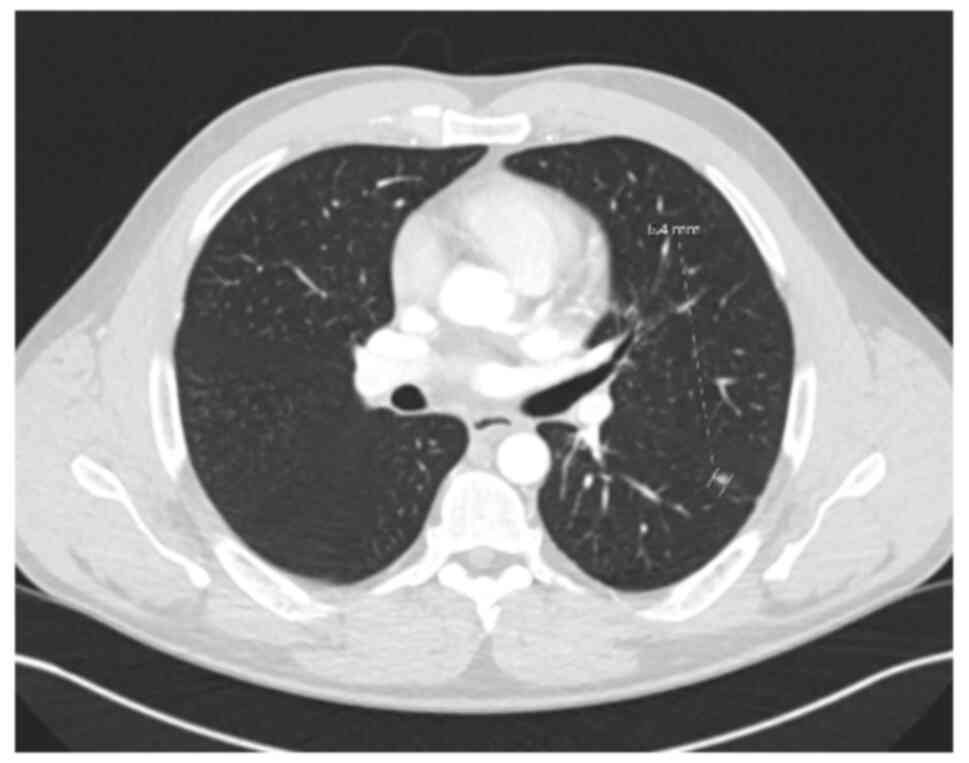
June, 2022, a solitary left lower lobe lung nodule was detected on
surveillance imaging (Fig. 7). Wedge
resection confirmed a 5-mm metastatic lesion from the original hCCA
(pTisN0R0). The patient was commenced on capecitabine in November,
2022, typically administered at a dose of 1,250 mg/m2
twice daily on days 1-14 of a 21-day cycle. He completed seven
cycles by April, 2023 with no observed toxicities.
In July 2023, PET imaging revealed a new FDG-avid
lesion in the anterior rectus sheath. Diagnostic biopsy was
performed with histology again confirmed metastatic hCCA. The
patient began systemic palliative chemotherapy. The patient began
systemic palliative chemotherapy with gemcitabine (1,000
mg/m2 on days 1 and 8) and cisplatin (25
mg/m2 on days 1 and 8 of each 21-day cycle). By January,
2024, following eight cycles, surveillance imaging demonstrated
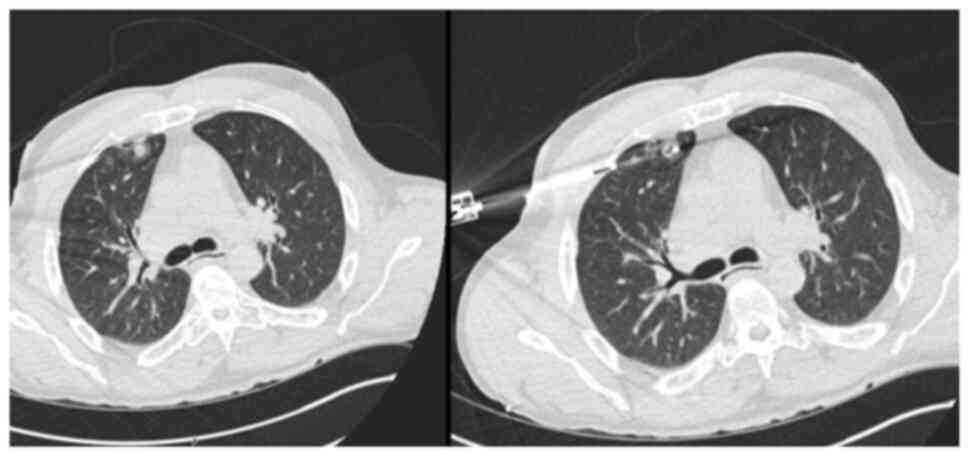
increased uptake in a previously stable lung nodule. The rectus
sheath metastasis and lung lesion were treated with surgical
excision and microwave ablation, respectively. A new solitary lung
recurrence was treated with ablation in October, 2024 (Figs. 8 and 9).
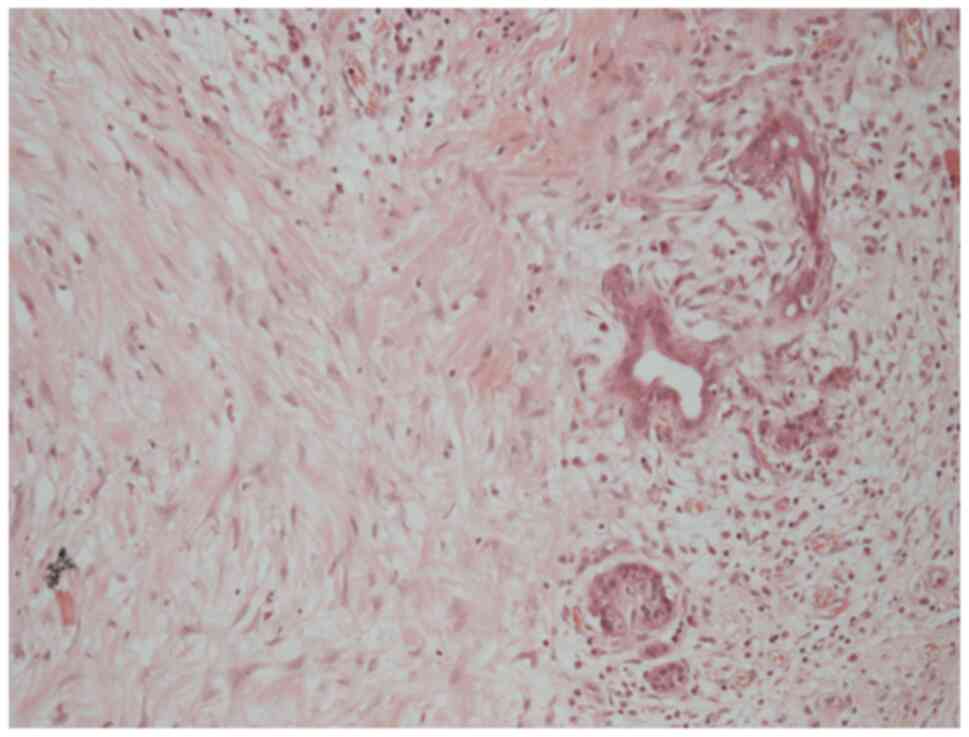
A histological examination of the rectus sheath
deposit confirmed metastatic moderately differentiated
adenocarcinoma, morphologically consistent with the patient s
original diagnosis of hilar cholangiocarcinoma (Fig. 10). Histology was performed by the
Department of Histopathology at King s College Hospital using
routine paraffin-embedded sections, formalin fixation and standard
diagnostic laboratory protocols.
In January, 2025, the patient developed multiple
intrahepatic and peritoneal metastases (Fig. 11). Notably, HER2 overexpression was
confirmed in the most recent metastatic specimen, prompting
initiation of palliative systemic therapy with zanidatamab, a
bispecific HER2-targeted antibody. HER2 immunohistochemistry was
performed on 4-µm paraffin-embedded sections using the BOND ORACLE
HER2 IHC System (cat. no. DS9800 Leica Biosystems), an automated,
standardised assay in which all steps were executed according to
the manufacturer s validated protocol.
The primary hilar cholangiocarcinoma specimen
resected in 2017 was retrospectively retrieved and subjected to the
same HER2 IHC protocol, confirming HER2 overexpression in the
original tumour (Fig. 12). Although
HER2 testing was not routinely performed in 2017, the concordant
HER2-positive status of both primary and metastatic sites supports
the rationale for HER2-directed therapy in this patient.
Despite initial disease progression, he maintained a
good performance status and received palliative chemotherapy, which
was administered at a different institution. As such, detailed
information regarding the specific agents and dosing schedule is
unavailable. The patient ultimately passed away in November 2025,
103 months following his initial diagnosis of PSC-associated hilar
cholangiocarcinoma in April, 2017. A summary of the disease course
of the patient and key clinical events is provided in Table I.
 | Table ITimeline summary of the course of the
disease. |
Table I
Timeline summary of the course of the
disease.
| 1. | 2015: Diagnosis of
PSC |
| 2. | April 2017: Diagnosis
of hCCA |
| 3. | September 2017: ERH
with Roux enY hepaticojejunostomy |
| | Histology
pT2N0PN1LV1R0 |
| | SACT two cycles
discontinued due to liver dysfunction |
| 4. | November 2019: Liver
transplantation and right hemicolectomy, terminal ileostomy |
| | Incidental finding of
serosa deposit on right colon |
| | DBD whole graft CIT
19 h |
| 5. | March 2022: GI
restoration |
| 6. | August 2022: Lung
wedge resection for pTisN0R0 lung deposit |
| | SACT seven cycles of
adjuvant capecitabine completed |
| 7. | July 2023: New
deposit on rectus sheath and new Lung lesion |
| | SACT eight cycles Aug
23-Jan 24 of palliative gemcitabine/cisplatin completed |
| | April 2024: Resection
of rectus sheath deposit and ablation of lung metastasis |
| 8. | October 2024: Another
lung deposit treated with ablation |
| 9. | January 2025: CT
multiple liver metastases with peritoneal spread |
| | SACT commenced FOLFOX
palliative chemotherapy (cycle 5 received April, 2025) |
| 10. | August 2025: Disease
progression on FOLFOX; commenced compassionate-use Zanidatamab for
HER2-positive cholangiocarcinoma |
| 11. | November 2025:
Patient succumbed to the disease |
Discussion
hCCA is an aggressive malignancy often requiring
extended liver resection to achieve oncological clearance and
improve survival outcomes (1,10). The
5-year overall survival ranges between 30-40% (3,5), while
the 5-year disease-free survival is reported to be ~20%,
particularly when favourable features, such as well-differentiated
tumours, negative lymph nodes and R0 margins are present (4,5,11).
PSC is a known predisposing factor, with an
estimated lifetime risk of cholangiocarcinoma approaching 20%, and
reported prevalence ranging from 6.5 to 13% (12). Accumulating evidence suggests that
hCCA arising in the context of PSC has a more favourable prognosis
compared to de novo hCCA. Patients with PSC-associated hCCA
undergoing resection can achieve 5-year survival rates up to 60%,
while the survival rates of those with de novo hCCA rarely
exceed 30-40% (3,5).
This difference in outcomes is most notably
demonstrated in transplant-based approaches pioneered by the Mayo
Clinic, which developed the first structured protocol combining
neoadjuvant chemoradiotherapy with liver transplantation for
PSC-associated hCCA. In carefully selected patients, this strategy
has achieved 10-year survival rates approaching 67% (3,4),
significantly outperforming outcomes observed in de novo
hCCA. However, its success is contingent upon stringent selection
criteria, with dropout rates reaching 15% and up to 5% of explanted
livers found to be tumour-free on final histology (4). Despite these limitations, the Mayo
Protocol remains the gold standard and the most effective
therapeutic framework for long-term survival in PSC-associated
hCCA.
Recurrence remains a key concern. Even with liver
transplantation, recurrence has been reported in up to 50% of cases
(3,4), although this decreased to as low as 22%
in carefully selected PSC hCCA cases treated as per the Mayo
protocol (4).
The prospective study by Groot Koerkamp et al
(13) in 2015 evaluated time to
recurrence and disease-free survival in 306 patients with resected
hCCA. The overall recurrence rate was 58%, with local recurrence in
26% and combined local and distant recurrence in 40% of cases
(13). Isolated distant recurrence
was not clearly documented, and only 2 patients experienced
recurrence >8 years following resection. The median overall
survival following recurrence was only 8 months (13). Notably, PSC-associated hCCA cases
were not separately analysed (13).
The present study described the case of a patient
with an isolated distant recurrence of PSC-associated hCCA
occurring 5 years following initial resection and 2 years after
liver transplantation, under immunosuppression. The serosal
metastatic deposit discovered incidentally at the time of
transplantation may represent the earliest sign of recurrence.
However, there was no evidence of widespread progression during the
subsequent 3 years of immunosuppression. Later recurrences in the
lung and anterior abdominal wall remained solitary and were
amenable to resection or ablation, with histology in some cases
confirming only in situ disease. These features suggest a
less aggressive biological phenotype, raising the possibility that
tumour biology, rather than treatment modality alone, may play a
key role in long-term survival.
Late-onset, isolated distant metastases are
extremely rare. A similar case in the literature describes a
solitary rib metastasis developing 10 years following the resection
of de novo hCCA, treated with surgical excision (14). However, to the best of our knowledge,
the present case report is the first to describe multiple late
metastatic recurrences following an unorthodox treatment sequence,
including delayed liver transplantation, with long-term survival
>8 years.
A key point of discussion is the paradox between
immunosuppression and tumour behaviour following transplantation.
While chronic immunosuppression is generally associated with an
increased oncological risk, emerging evidence suggests that
immunosuppressive regimens containing mTOR inhibitors may have
antitumour properties by inhibiting angiogenesis and tumour
proliferation. In the patient in the present study, conversion to
an mTOR-based immunosuppressive regimen shortly following liver
transplantation may have contributed to delayed metastatic
recurrence, synergising with the underlying favourable tumour
biology (9).
Advances in biomarker discovery for PSC-related hCCA
may support earlier diagnosis (15),
while molecular profiling of cholangiocarcinoma is likely to
enhance histological subtyping and facilitate the development of
personalised treatment algorithms. In the case described herein,
the tumour on the latest resected recurrence site was found to be
HER2-positive, allowing the palliative initiation of zanidatamab, a
bispecific HER2-targeted antibody recently shown to be effective in
HER2-amplified biliary tract cancers (16).
Notably, the retrospective assessment of the primary
tumour specimen resected in 2017 confirmed HER2 overexpression,
corroborating the findings from the metastatic site and further
supporting the rationale for HER2-directed therapy at this final
stage.
HER2 overexpression has been identified in ~10-15%
of biliary tract cancers (BTCs), including extrahepatic
cholangiocarcinoma. Several studies have explored the prognostic
relevance of HER2 overexpression in BTC, though findings have
varied. In 2020, Vivaldi et al (17) reported HER2 overexpression and
associated it with a significantly shorter disease-free survival,
suggesting a more aggressive biological behaviour. In 2021, the
multicentre study by Hori et al (18) found no significant impact of HER2
expression on survival outcomes across intrahepatic and
extrahepatic cholangiocarcinomas, thus challenging its utility as a
prognostic marker. Recently, in 2024, Kim et al (19) demonstrated that HER2 amplification
was significantly associated with a reduced overall survival,
reinforcing its potential role as a negative prognostic factor in
BTC and a rationale for HER2-targeted therapies.
Notably, routine HER2 testing in cholangiocarcinoma
was not widely implemented in clinical practice until ~2020,
coinciding with early-phase trials exploring HER2 blockade in
biliary tract cancers. Retrospective HER2 assessment in older
cases, such as the patient in the present study (diagnosed in
2017), was not standard practice at the time.
Biological factors may have contributed to the
relatively indolent clinical course observed in the patient
described herein. PSC-associated cholangiocarcinoma often arises in
a chronically inflamed and fibrotic biliary microenvironment, which
promotes stepwise neoplastic transformation, but may also limit
angiogenesis and metastatic dissemination (2,15).
Compared with de novo cases, PSC-related tumours have been
shown to exhibit lower proliferative indices, dense lymphocytic
infiltration and a dominant inflammatory stroma, features that may
contribute to a more immunologically active and less aggressive
phenotype (4,15,20).
Additionally, mismatch repair deficiency and microsatellite
instability, observed in a subset of PSC-related
cholangiocarcinomas, may underlie reduced tumour growth kinetics
and more favourable treatment responses s(21).
In conclusion, organ shortage and limited transplant
programme availability, as was the case in the UK at the time of
the treatment of the patient described herien, continue to limit
access to potentially curative strategies for patients with
PSC-associated hCCA. In such settings, upfront resection remains
the mainstay of treatment despite its technical demands and
uncertain long-term efficacy.
The superior survival outcomes observed in
PSC-associated hCCA, both with resection and transplant-based
protocols, may reflect an underlying difference in tumour biology
compared to de novo cases. The case described herein underscores
the potential role of tumour biology as a key prognostic driver,
particularly when durable survival is achieved despite recurrence
and long-term immunosuppression.
Late isolated distant metastasis is exceptionally
rare in hCCA. The present case report demonstrates that long-term
survival and the control of metastatic disease may be achievable in
highly selected patients through multimodal treatment and
biologically favourable tumour behaviour.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors contributions
EF was responsible for the conception, design and
drafting of the study, the retrieval of the patient s clinical
and pathological data, and final revision. PS, DS and MH were
involved in the interpretation of the imaging and histopathological
findings, contributed to the design and critical review of the case
presentation, and provided specialist input on oncological and
hepatology management. AP performed the surgeries and liver
transplantation and subsequent surgical follow-up, contributed to
the study design, and critically revised the manuscript for
important intellectual content.
Ethics approval and consent to
participate
The present case report was conducted in accordance
with institutional requirements. Written informed consent was
obtained from the patient for his participation in the present
study.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this report and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Soares KC and Jarnagin WR: The landmark
series: Hilar cholangiocarcinoma. Ann Surg Oncol. 28:4158–4170.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Banales JM, Marin JJG, Lamarca A,
Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen
JB, Braconi C, et al: Cholangiocarcinoma 2020: The next horizon in
mechanisms and management. Nat Rev Gastroenterol Hepatol.
17:557–588. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tan EK, Taner T, Heimbach JK, Gores GJ and
Rosen CB: Liver transplantation for peri-hilar cholangiocarcinoma.
J Gastrointest Surg. 24:2679–2685. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Villard C, Jorns C and Bergquist A:
Treatment of cholangiocarcinoma in patients with primary sclerosing
cholangitis: A comprehensive review. eGastroenterology.
2(e100045)2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kang MJ, Jang JY, Chang J, Shin YC, Lee D,
Kim HB and Kim SW: Actual long-term survival outcome of 403
consecutive patients with hilar cholangiocarcinoma. World J Surg.
40:2451–2459. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Heimbach JK, Gores GJ, Haddock MG, Alberts
SR, Nyberg SL, Ishitani MB and Rosen CB: Liver transplantation for
unresectable perihilar cholangiocarcinoma. Semin Liver Dis.
24:201–207. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rahbari NN, Garden OJ, Padbury R,
Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo
RP, Christophi C, et al: Posthepatectomy liver failure: A
definition and grading by the international study group of liver
surgery (ISGLS). Surgery. 149:713–724. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Balzan S, Belghiti J, Farges O, Ogata S,
Sauvanet A, Delefosse D and Durand F: The ‘50-50 criteria’ on
postoperative day 5: An accurate predictor of liver failure and
death after hepatectomy. Ann Surg. 242:824–829. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Semaan S, Connor AA, Saharia A, Kodali S,
Elaileh A, Patel K, Soliman N, Basra T, Victor DW III, Simon CJ, et
al: Transplantation for peri-hilar and intrahepatic
cholangiocarcinoma with mTOR immunosuppression. Transplant Proc.
57:255–263. 2025.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gilbert TM, Hackett J, Holt L, Bird N,
Quinn M, Gordon-Weeks A, Diaz-Nieto R, Fenwick SW, Malik HZ and
Jones RP: Long-term morbidity after surgery for perihilar
cholangiocarcinoma: A cohort study. Surg Oncol.
45(101875)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nooijen LE, Banales JM, de Boer MT,
Braconi C, Folseraas T, Forner A, Holowko W, Hoogwater FJH, Klümpen
HJ, Groot Koerkamp B, et al: Impact of positive lymph nodes and
resection margin status on the overall survival of patients with
resected perihilar cholangiocarcinoma: The ENSCCA registry. Cancers
(Basel). 14(2389)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Safarpour AR, Askari H, Ejtehadi F,
Azarnezhad A, Raeis-Abdollahi E, Tajbakhsh A, Abazari MF, Tarkesh
F, Shamsaeefar A, Niknam R, et al: Cholangiocarcinoma and liver
transplantation: What we know so far? World J Gastrointest
Pathophysiol. 12:84–105. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Groot Koerkamp B, Wiggers JK, Allen PJ,
Besselink MG, Blumgart LH, Busch OR, Coelen RJ, D Angelica MI,
DeMatteo RP, Gouma DJ, et al: Recurrence rate and pattern of
perihilar cholangiocarcinoma after curative intent resection. J Am
Coll Surg. 221:1041–1049. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ota Y, Matsuyama R, Taniguchi K, Ueda M,
Takeda K, Tanaka K, Nakayama T and Endo I: Solitary rib recurrence
of hilar cholangiocarcinoma 10 years after resection: Report of a
case. Clin J Gastroenterol. 6:485–489. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Catanzaro E, Gringeri E, Burra P and
Gambato M: Primary sclerosing cholangitis-associated
cholangiocarcinoma: From pathogenesis to diagnostic and
surveillance strategies. Cancers (Basel). 15(4947)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Harding JJ, Fan J, Oh DY, Choi HJ, Kim JW,
Chang HM, Bao L, Sun HC, Macarulla T, Xie F, et al: Zanidatamab for
HER2-amplified, unresectable, locally advanced or metastatic
biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm,
phase 2b study. Lancet Oncol. 24:772–782. 2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vivaldi C, Fornaro L, Ugolini C, Niccoli
C, Musettini G, Pecora I, Cacciato Insilla A, Salani F, Pasquini G,
Catanese S, et al: HER2 overexpression as a poor prognostic
determinant in resected biliary tract cancer. Oncologist.
25:886–893. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hori Y, Yoh T, Seo S, Minamiguchi S, Haga
H and Taura K: Limited Impact of HER2 Expression on Survival
Outcomes in Patients with Intrahepatic Cholangiocarcinoma After
Surgical Resection. Oncologist. 26:e1893–e1894. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim Y, Jee S, Kim H, Paik SS, Choi D, Yoo
SH and Shin SJ: EGFR, HER2, and MET gene amplification and protein
expression profiles in biliary tract cancer and their prognostic
significance. Oncologist. 29:e1051–e1060. 2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oura K, Morishita A, Nakahara M, Tadokoro
T, Fujita K, Tani J, Masaki T and Kobara H: Chronic Liver Disease
Associated Cholangiocarcinoma: Genomic Insights and Precision
Therapeutic Strategies. Cancers (Basel). 17(3052)2025.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Goeppert B, Roessler S, Renner M, Singer
S, Mehrabi A, Vogel MN, Pathil A, Czink E, Köhler B, Springfeld C,
et al: Mismatch repair deficiency is a rare but putative
therapeutically relevant finding in non-liver fluke associated
cholangiocarcinoma. Br J Cancer. 120:109–114. 2019.PubMed/NCBI View Article : Google Scholar
|