Introduction
Anterior cruciate ligament (ACL) ruptures are
frequently encountered in clinical practice, and can lead to the
dysfunction of injured joints, increased risk of subsequent injury
of the meniscus and cartilage and even increased risk of early
osteoarthritis (1–4). Due to the lack of blood supply, ACL
ruptures cannot heal independently, and therefore surgical
reconstruction or replacement is necessary (5,6).
Autologous grafts, including quadricep, patellar and hamstring
tendon grafts and iliotibial bands or tracts, are commonly used in
intra-articular replacements of ACL (2,7–9).
However, certain well-recognized drawbacks, including delayed
recovery of the intra-articular donor site, pain in the anterior
part of the knee, reduced strength of the tendon at the harvesting
site, and lengthy surgery required for harvesting the grafts,
restrict the application of autografts (10,11).
Allografts are usually used in lower-demand patients or patients
who are undergoing revision ACL surgery if an ACL reconstruction
fails (12). However, these grafts
are of poor strength and carry a significant risk of disease
transmission (13,14).
In order to overcome such drawbacks, artificial
ligaments have been widely studied over the past 50 years and have
become a potential choice for reconstructing torn ACLs (15–17).
Various materials, including carbon fibers, nylon,
polytetrafluoroethylene (PTFE) and polyethylene terephthalate
(PET), have been employed and have contributed greatly to the
diffusion of artificial ligaments (15,18–20).
Among these materials, PET fibers are considered to be especially
adapted to fabricate artificial ligaments, due to their plasticity,
flexibility and high mechanical resistance to rupturing (21). However, due to the lack of
hydrophilicity, artificial ligaments made of PET fibers appear to
have lower biocompatibility, and therefore cannot integrate into
the surrounding host bone tissue, which can eventually lead to
rupturing of the artificial ligaments and reconstruction failure in
the long-term (21,22).
In this study, in order to improve the
biocompatibility of PET fibers, we modified them by using a
hydrophilic molecule, dimethylolpropionic acid (DMPA), which
contains 2 types of functional groups, 2 primary hydroxyl and 1
tertiary carboxyl group, which make polyesters particularly water
soluble. We postulated that the introduction of DMPA would increase
the biocompatibility of the PET fibers without comprising their
other characteristics, particularly the high mechanical strength.
In order to test this hypothesis, scanning electron microscopy
(SEM), Fourier transform infrared spectroscopy (FTIR), differential
scanning calorimetry (DSC), X-ray diffraction (XRD), mechanical
testing and cell culture were applied to investigate these
fibers.
Materials and methods
Modification of PET by DMPA
Briefly, the original PET fibers (Taiji Co., Ltd.,
Wuxi, China), 5 cm long × 22 μm in diameter, were immersed in a
solution of 2.5 mol/l sodium hydroxide (Sigma-Aldrich, Shanghai,
China) at a ratio of 1:10 (w/w) for 3 h at 50°C. The PET fibers
were then placed into a 75% (w/v) tolylene-2,4-diisocyanate (TDI;
Sigma-Aldrich)/acetone solution, which contained 0.25% (v/v)
dibutyltin dilaurate (DBTDL; Sigma-Aldrich), at a ratio of 1:10
(w/w) for 3 h at 50°C. The PET fibers were then transferred into a
36.5% (w/v) DMPA/acetone solution (Sigma-Aldrich), which contained
0.07% (v/v) fluorite boron (Zibo Shuanglian Petroleum and Chemical
Co., Ltd., Shandong, China) as an activator for 2 h at 20°C.
Finally, the DMPA-modified PET (DMPA-PET) fibers were washed with
distilled water, air-dried and sterilized by γ radiation at 15 kGy
for 6 h. The original PET fibers were also sterilized and used as
the control.
SEM observation
A total of 10 samples from the DMPA-PET and the PET
groups were fixed on stubs with carbon tape and sputter-coated with
gold in an ion coater. The surface characteristics of the samples
were studied under a vacuum using SEM (S-3400N, Hitachi, Osaka,
Japan) to analyze changes in the surface morphology.
FTIR evaluation
A Fourier transform infrared spectrometer (IR-200
Thermo-Nicolet 2.2, Thermo-Nicolet corporation, Madison, WI, USA)
was used to detect the changes in the functional groups of the
DMPA-PET and PET fibers as previously described (23). Briefly, following appropriate
background subtraction, 2 mg of freeze-dried samples of each group
were mixed with dried potassium bromide, and the samples were
scanned from 4,000 to 400 cm−1 by the spectrometer. Each
spectrum reported is the average of at least three spectra measured
in different areas of the samples (n=10).
DSC evaluation
The thermal properties of DMPA-PET and PET were
analyzed by a differential scanning calorimeter (DSC-2041F;
Netzsch, Selb, Germany), which was equipped with an intra-cooler
and a refrigerated cooling system. Indium standard was used to
calibrate the DSC temperature and enthalpy scale. Nitrogen was used
to purge gas through the DSC cell at the flow rate of 50 ml/min
through the cooling unit. A total of 5 μg of samples from the 2
groups were hermetically sealed in an aluminium pan at a heating
rate of 20 K/min (n=10) (24).
Each examination was replicated three times.
XRD evaluation
An X-ray diffractometer (XRD-6000, Shimazu, Japan)
was used to determine the crystalline phases of DMPA-PET and PET.
The generator working parameters were set at 40 kV and 30 mA to
create copper CuKα radiation (λ=1.542 Å). The scanning scope of 2θ
was 100-60° and the scanning rate was 2° per min. To obtain the
maximum diffraction intensity, the DMPA-PET and PET fibers were
prepared in a number of layers to obtain thick samples (total
thickness, 2 mm). This measurement was performed on at least ten
samples (24).
Mechanical testing
The tensile testing was performed by a materials
testing machine (Instron 5565; Instron, Norwood, MA, USA) with a
performance temperature of 20±2°C, a relative humidity of 65±5% and
a humidity controlling time of 4 h. The samples were fixed with a
nominal gauge length of 20 mm and a static pre-tension of 0.15±0.03
cN. The tensile testing was performed at a speed of 20 mm/min until
the testing fibers failed (n=10). The ultimate tensile strength,
tensile stress, elongation percentage at breakage and initial
modulus were determined as previously described (25).
Cytocompatibility evaluation
To determine the cytocompatibility of the 2 PET
fibers, bone marrow stromal cells (BMSCs) transfected with green
fluorescent protein (GFP) (Provided by Dr Lu from the Cell
Laboratory of the Affiliated Dental Hospital at the Fourth Military
Medical University, Xi'an, China) were seeded onto the 2 fibers at
a concentration of 2×10−6 cell/cm2 in
Dulbecco's modified Eagle's medium (DMEM, Sigma, St. Louis, MO,
USA) containing 10% fetal bovine serum (FBS; HyClone, Logan, UT,
USA) at 37°C. The adhesion and growth of the BMSCs on the fibers
were observed under an inverted phase-contrast fluorescence
microscope after 3 and 7 days. A PicoGreen double-stranded DNA
(dsDNA) Quantification kit (Molecular Probes, Eugene, OR, USA) was
used to determine the proliferation of the cells cultured in the
DMEM (10% FBS) with 20% leaching liquor of each type of fiber from
day 1 to 7. The total dsDNA was extracted through enzymatic
digestion and assayed following the manufacturer's instructions.
The proliferation of the cells in the scaffold was interpreted by
changes in the quantity of the dsDNA. The leaching liquors of the
fibers were prepared as previously described (26). Cells cultured in DMEM (10% FBS)
without any leaching liquor were used as the blank control
(n=10).
Statistical analysis
Statistical analyses were performed using SPSS
software version 14.0 (SPSS Inc., Chicago, IL, USA). The data are
presented as the means ± SD, and were compared using the
non-parametric Mann-Whitney U test or the Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
SEM observation
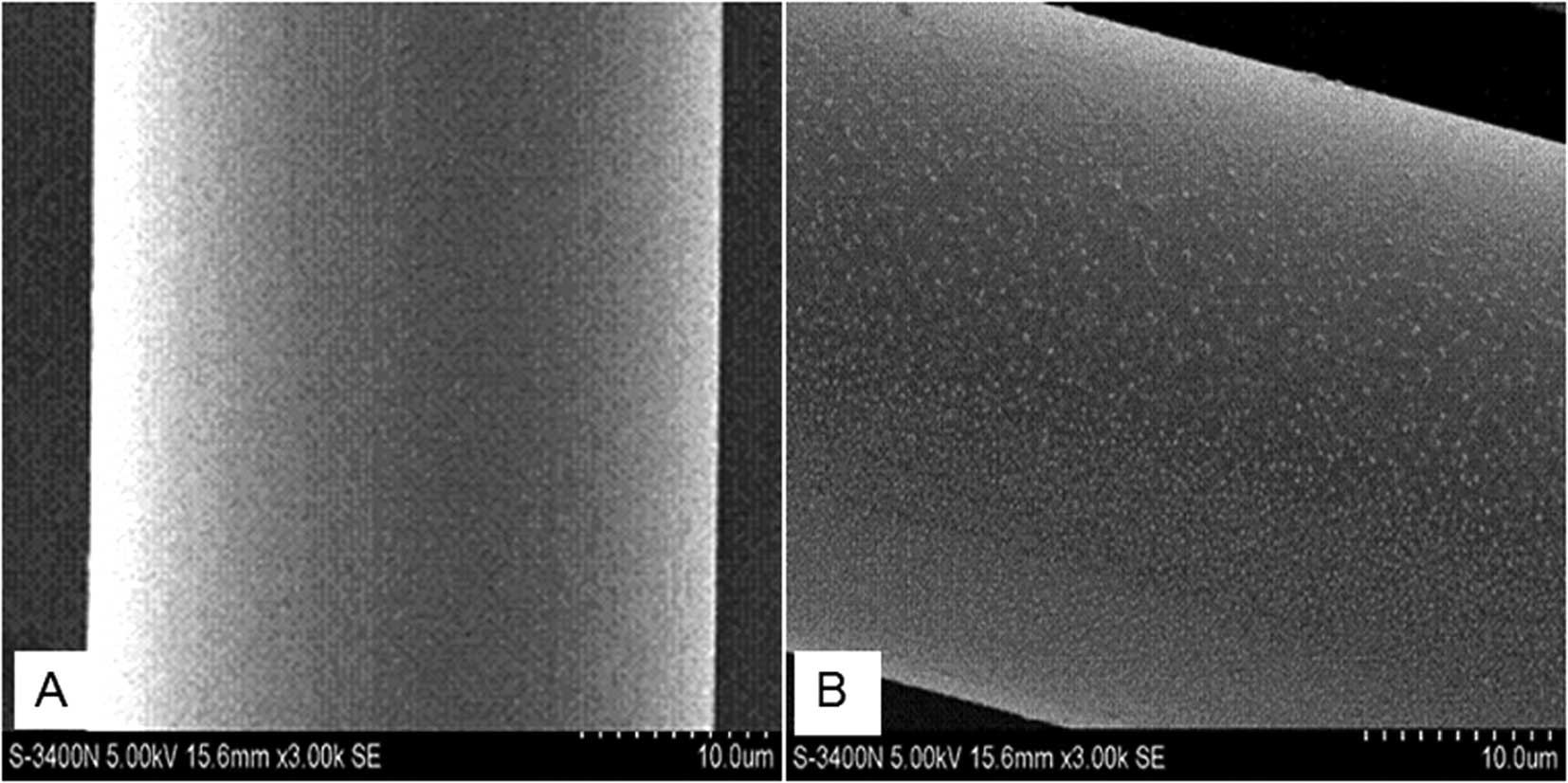
The SEM images demonstrate that the surface of the
original PET fibers was smooth without any deposits (Fig. 1A). By contrast, following
modification the surface of the DMPA-PET fibers became uneven, and
a number of granules, 300 to 500 nm in diameter, were found to be
deposited on the surface (Fig.
1B).
FTIR evaluation
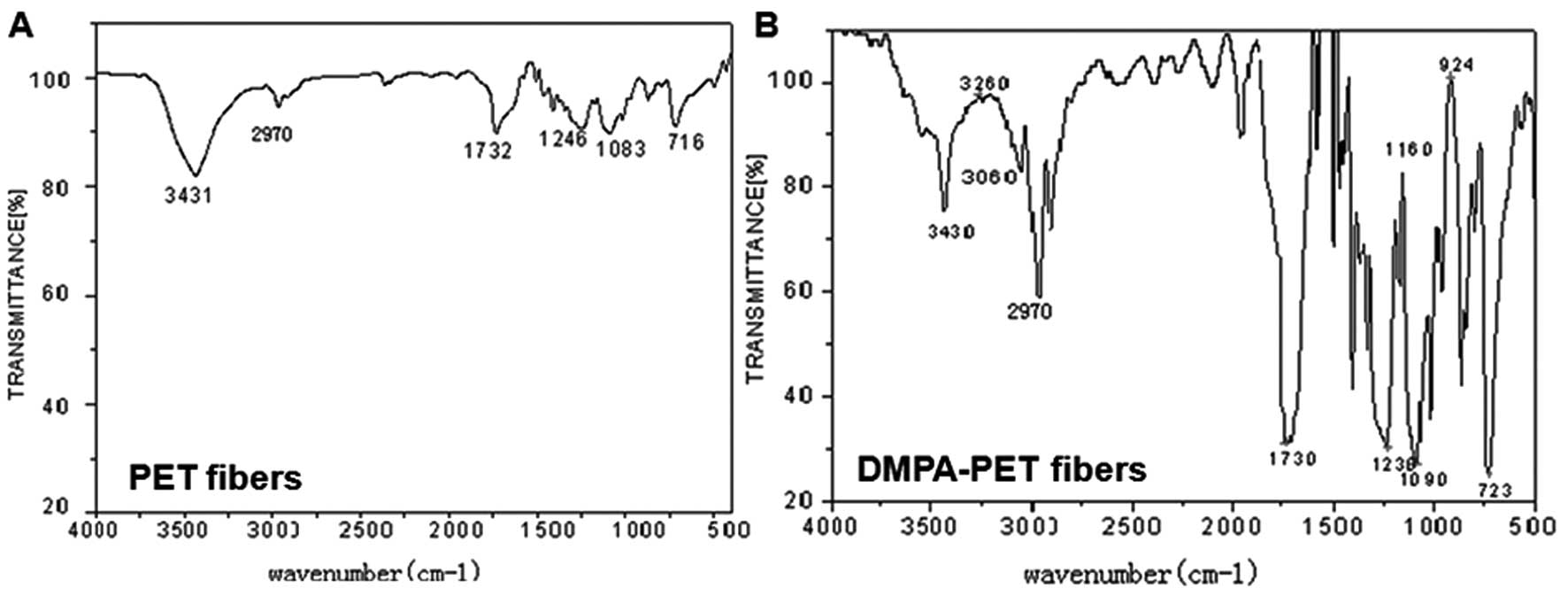
The different infrared spectra of the PET and
DMPA-PET fibers were demonstrated by FTIR evaluation. The
characteristic peaks of PET appeared at 3,431 and 2,970, 1,732,
1,246, 1,083 and 716 cm−1, corresponding to the hydroxyl
(-OH), methyl (-CH3), carbonyl (C=O), and methanediyl
(-CH2-) groups, and ethers (C-O-C), respectively. By
contrast, a number of newly-formed absorption bands were found in
the spectrum of DMPA-PET at 3,400–3,500, 2,852–3,000 and
1,700–1,730 cm−1, which represented the newly-formed
peptide bonds (N-H), -OH and carboxy (-COOH) groups, respectively.
Furthermore, the greater absorption band at the 3,200
cm−1 area in the DMPA-PET group also confirmed the
increase in the hydrophilic -OH and -COOH groups (Fig. 2B).
DSC evaluation
DSC was used to characterize the thermophysical
properties of the fibers. In the PET group, the first sharp
endothermic peak was formed at 259.8±1.1°C, with a fusion enthalpy
of 39.5±0.5 J/g and a crystallinity of 28.2±1.5%. The second peak
formed at 254.1±1.2°C, with a fusion enthalpy of 28.0±0.5 J/g and a
crystallinity of 20.0±1.7%. Similarly, the first endothermic peak
of the DMPA-PET group was demonstrated at 258.1±1.2°C, with a
fusion enthalpy of 40.8±0.7 J/g and a crystallinity of 29.2±1.6%.
The second peak of DMPA-PET was formed at 253.0±1.4°C, with a
fusion enthalpy of 28.8±0.6 J/g and a crystallinity of 20.6±1.9%.
There were no significant differences in the endothermic peaks,
fusion enthalpies and crystallinity indices between the 2 groups
(P>0.05, n=10; Table I).
 | Table IThermophysical properties of the PET
and DMPA-PET fibers (n=10). |
Table I
Thermophysical properties of the PET
and DMPA-PET fibers (n=10).
| Endothermic peak
(°C) | Fusion enthalpy
(J/g) | Crystallinity
(%) |
|---|
|
|
|
|
|---|
| Groups | 1st heating | 2nd heating | 1st heating | 2nd heating | 1st heating | 2nd heating |
|---|
| PET | 259.8±1.1 | 254.1±1.2 | 39.5±0.5 | 28.0±0.5 | 28.2±1.5 | 20.0±1.7 |
| DMPA-PET | 258.1±1.2 | 253.0±1.4 | 40.8±0.7 | 28.8±0.6 | 29.2±1.6 | 20.6±1.9 |
XRD evaluation
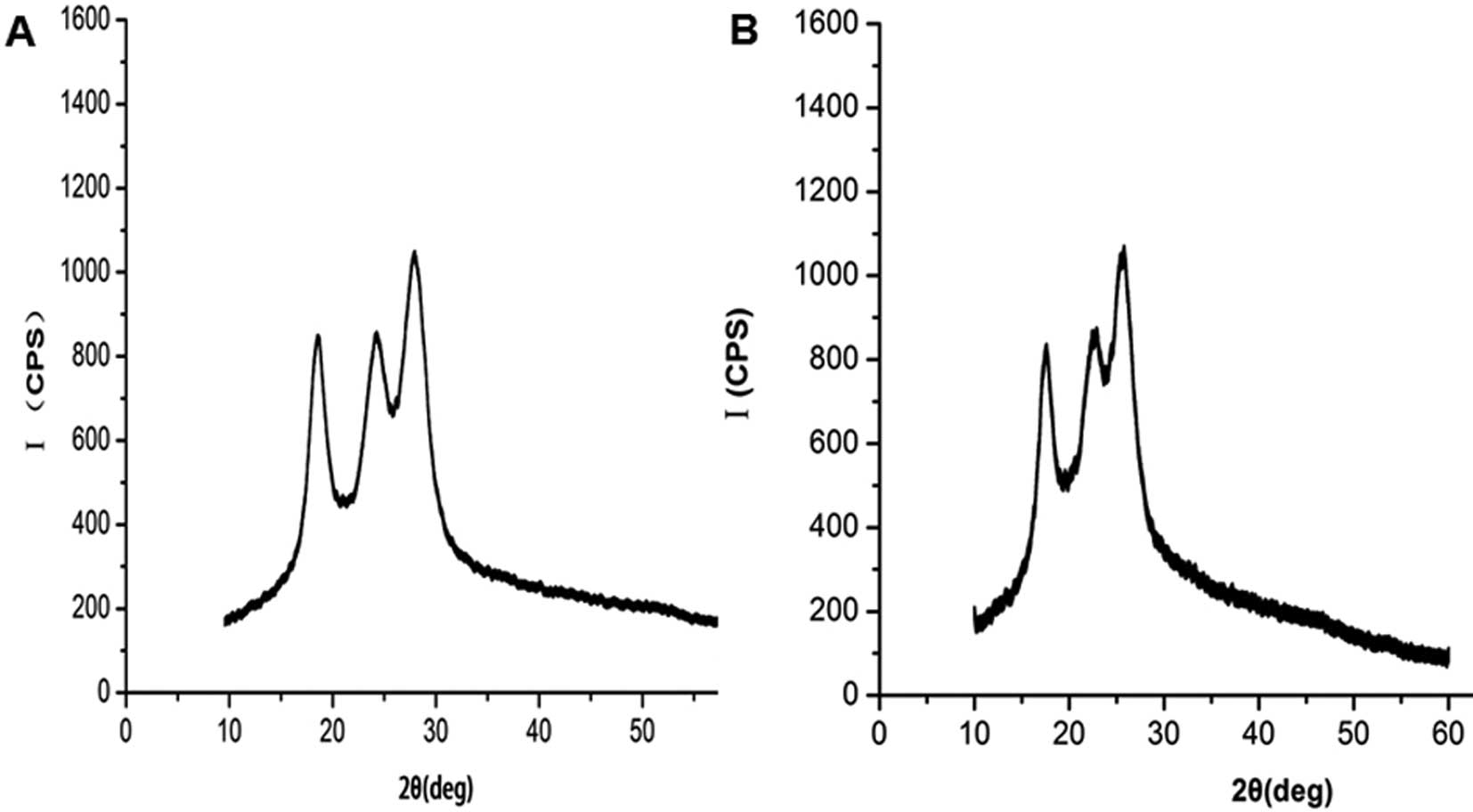
The XRD spectra recorded for the PET and the
DMPA-PET groups are shown in Fig.
3. The XRD pattern of the PET fibers demonstrated that the
original PET was mostly amorphous in nature with less
crystallinity. The tip of the amorphous halo of the original PET
was observed at 2θ=27.42±0.02° in the diffraction pattern as
demonstrated in the diffractogram (Fig. 3A). Only a small change was observed
in the the XRD pattern of the DMPA-PET samples compared with the
original one. The amorphous peak tip of the DMPA-PET samples was
found to be shifted slightly towards a lower angle (26.23±0.01°),
but the change was not significant (n=10, P<0.05; Fig. 3B).
Mechanical testing
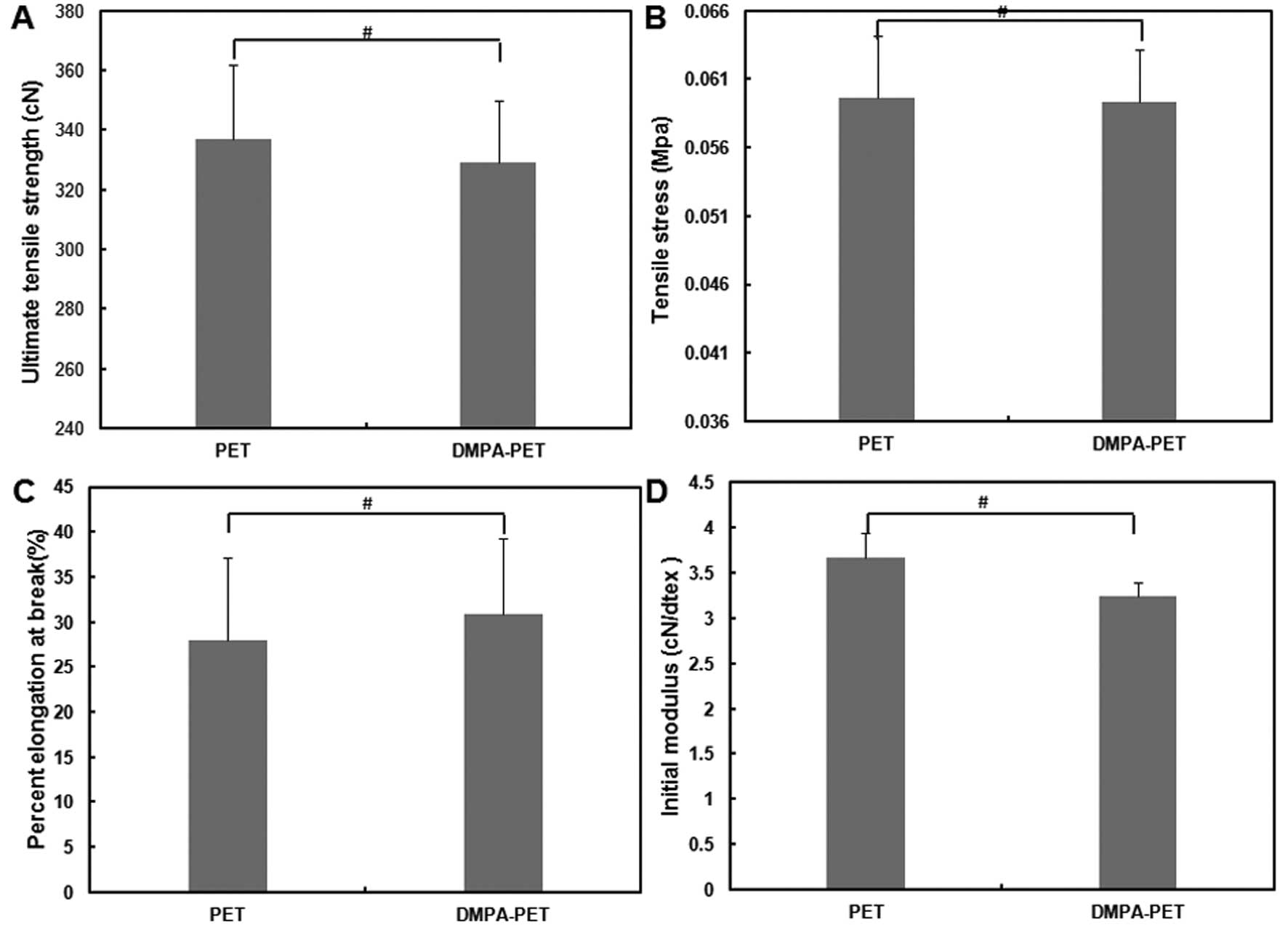
As shown in Fig. 4,
the ultimate tensile strength and tensile stress of the samples
from the DMPA-PET group were 329.2±20.62 cN and 0.05934±0.0038 MPa,
respectively, which were similar to those from the PET group
(336.8±25.15 cN and 0.05966±0.00445 MPa, respectively; n=10,
P>0.05; Fig. 4A and B).
Although the elongation percentage at breakage of the samples from
the DMPA-PET group (30.9±8.4%) was slightly higher than that from
the PET group (27.9±9.2%), there was no significant difference
between them (n=10, P>0.05; Fig.
4C). The initial modulus of the DMPA-PET group (3.234±0.1530
cN/dtex) was a little lower than that of the PET group
(3.661±0.2734 cN/dtex), but the difference was not significant
(n=10, P>0.05; Fig. 4D).
Cytocompatibility evaluation
Using inverted phase-contrast fluorescence
microscopy, we found that the BMSCs seeded onto the DMPA-PET fibers
appeared flattened, polygonal, spindle-shaped and evenly
distributed after 3 days of culture (Fig. 5A). On the 7th day, a number of
cells were spread and colonized patches appeared on the surface of
DMPA-PET (Fig. 5B). By contrast,
the number of cells that had adhered to the surface of the PET
fibers on the 3rd day had decreased (Fig. 5C). On the 7th day, only a few cells
were found on the surface of PET (Fig.
5D). The dsDNA content of the DMPA-PET group gradually
increased from days 1 to 5, with a slight decline from days 5 to 7
after confluence. There were no differences in the dsDNA content
between the DMPA-PET group and the blank control group (n=10,
P>0.05; Fig. 5E). On the
contrary, the dsDNA content of the PET group increased very slowly,
and began to decrease from the 4th day. The dsDNA content of the
PET group was much lower than that of the DMPA-PET group and the
blank control group after day 4 (n=10, P<0.05; Fig. 5E).
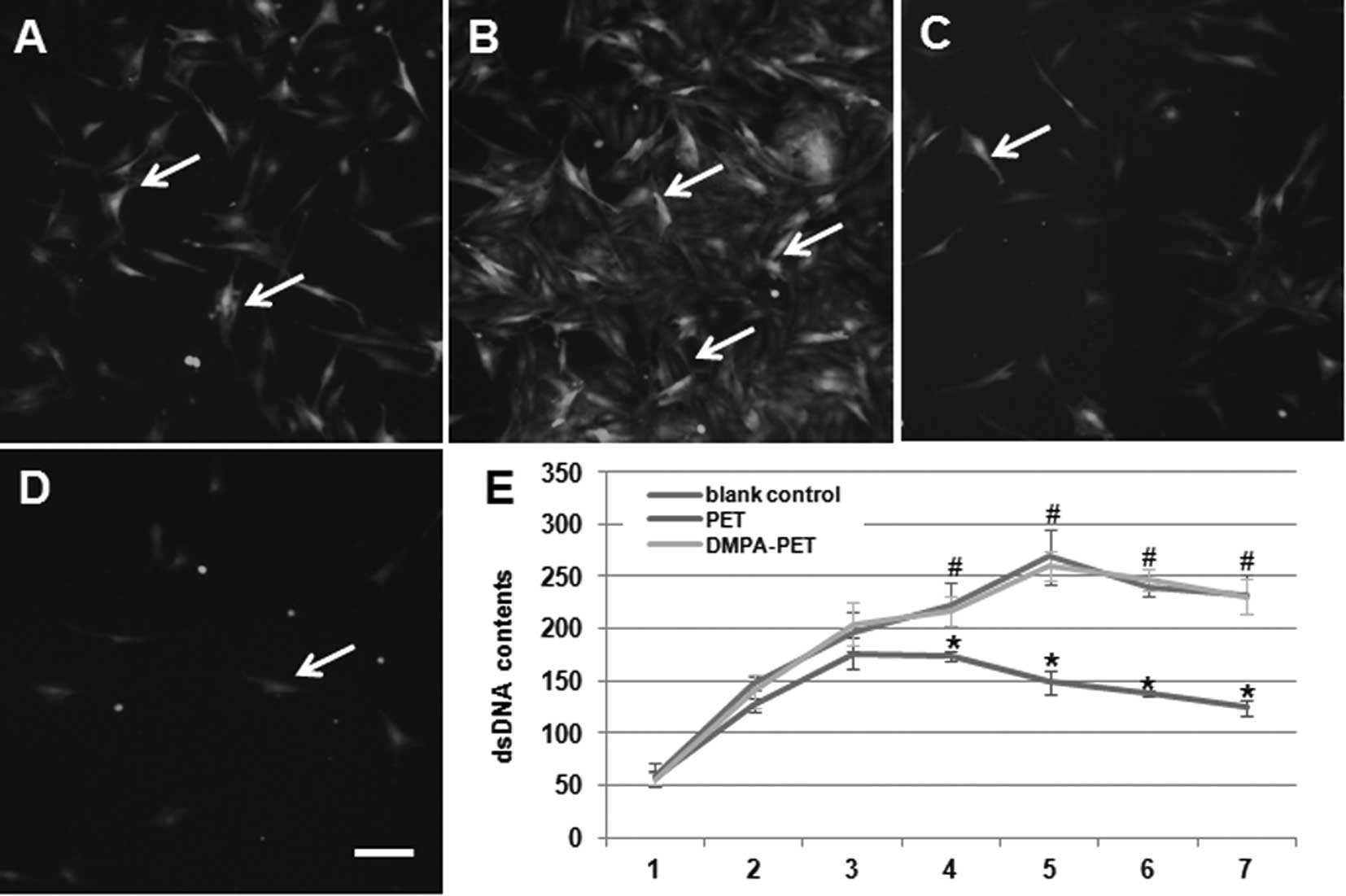 | Figure 5Fluorescence images of the BMSCs on
the different fibers: (A) DMPA-PET after 3 days, (B) DMPA-PET after
7 days, (C) PET after 3 days, (D) PET after 7 days (arrows, cells;
bar, 50μm). (E) DsDNA contents of the different groups
(*P<0.05, #P>0.05, n=10). BMSCs; bone
marrow stromal cells; PET, polyethylene terephthalate; DMPA-PET,
dimethylolpropionic acid-polyethylene terephthalate; dsDNA,
double-stranded DNA. |
Discussion
PET is commercially one of the most significant and
successful engineered polymers, and is widely used in the form of
fibers, films and molded articles (27). Due to its high plasticity,
flexibility and mechanical resistance to rupturing, PET fibers are
considered to be especially adapted to fabricate artificial
ligaments (22). The Ligament
Advanced Reinforcement System (LARS) ligament, which is made of
PET, has become one of the graft choices for ligament rupture
management, along with autografts and allografts, in a number of
countries (15). However, as PET
fibers are hydrophobic, certain clinical studies have proven that
LARS ligaments integrate poorly into the surrounding host tissue
resulting in failed ligament reconstruction after long-term
follow-up (28). Thus, a number of
studies have focused on modifying the surface of PET by grafting
functional chemical groups, including carboxylic, amide, sulfonate
and hydroxyapatite (21,29). However, since PET is
semi-crystalline in nature and does not contain chemically reactive
groups, it is resistant to straight chemical modification (27).
In order to improve the biocompatibility of PET, we
developed a novel modification method involving 3 steps. Firstly,
we treated PET fibers with sodium hydroxide to hydrolyze certain
esters on the surface of the fibers. Secondly, the hydrolyzed PET
fibers were reacted with TDI in acetone solution with DBTDL as the
activator. Finally, the fibers were reacted with a hydrophilic
molecule, DMPA, with fluorate boron as the activator. The excess
base was removed by repeated washing with distilled water until the
pH of the solution returned to a physiological range (7.0–7.4). The
SEM images demonstrated that plenty of granules were deposited on
the surface of the fibers after DMPA modification (Fig. 1B). This observed difference was
attributed to the fragmentation of polymer chains caused by surface
etching, and the deposition of grafted components (DMPA) on the
filament surface. This is a general behavior which has been
observed in other systems (30).
This rough surface was considered to be increasingly suitable for
the adhesion of cells (31). The
FTIR data further confirmed that a number of hydrophilic groups,
including hydroxyl and carboxyl, were formed on the surface of the
DMPA-PET fibers (Fig. 2B).
Furthermore, from the co-culture with the BMSCs, we proved that the
DMPA-PET fibers demonstrated excellent cytocompatibility compared
to the original PET fibers (Fig.
5).
DMPA is a unique, trifunctional molecule, which
incorporates a hindered, tertiary carboxylic acid group and 2
reactive primary hydroxyls. As a crystalline monomer, DMPA has been
proven to be odorless and considered to provide superior
advantages, including easy control, non-toxicity and high
hydrophilicity in the formulation and processing of water soluble
resins (32–35). It can be used as a modifier for
urethane, polyester/alkyd, phenolic, amino and epoxy systems
(33). The hindered carboxyl of
DMPA makes the introduction of free acid groups easy and convenient
(34). In this study, we succeeded
in improving the hydrophilicity and cytocompatibility of PET fibers
by modifying the surface of DMPA.
In order to investigate the effects of DMPA
modification on the changes in the thermophysical and crystal
properties of the PET fibers, DSC and XRD were employed in this
study. Notably, the DSC data demonstrated that there were no
significant changes in the endothermic peaks, fusion enthalpy and
crystalline indices between the DMPA-PET and the PET fibers, which
indicated that the original thermophysical and crystal properties
of PET remained in the DMPA-modified PET fibers (Table I). These results were further
confirmed by the XRD patterns (Fig.
3B). We considered that the main reason behind these results
was that the introduction of DMPA had no effect on the crystalline
structure of the PET fibers. In line with our study, DMPA was found
to be easily built into the polymer chain without side reactions
during the process, and to not influence the natural thermophysical
and crystal properties of the final product (34).
In order to observe the effects of DMPA modification
on the mechanical strength of PET, the tensile test was applied in
this study. The ultimate tensile strength and tensile stress of one
single fiber from the DMPA-PET group were similar to those from the
PET group. Although the elongation percentage at breakage of the
DMPA-PET group was slightly higher than that of the PET group,
there was no significant difference between the 2 groups.
Similarly, the difference in the initial modulus between the 2
groups was also not significant (Fig.
4). The PET fiber is regarded as an ideal material for the
preparation of artificial ligaments, due to its high strength and
stiffness, combined with reduced strain-to-failure (25). However, certain modified methods
have been found to cause a decrease in the mechanical properties,
including tensile strength, elongation at breakage or the elastic
modulus of PET during the processing of the modification (28–30).
In this study, we proved that our 3-step DMPA-modification method
can significantly increase the hydrophilicity of the PET fibers
without changing their mechanical strength.
In this study, we modified PET fibers by using DMPA.
The characteristics of the modified fibers were observed and
compared with those of the original PET. Based on our results, we
found that the introduction of DMPA can significantly increase the
hydrophilic group on the surface of the PET fibers, which results
in an improvement of the biocompatibility without comprising the
mechanical strength. These results demonstrate that DMPA-modified
PET fibers have significant potential as a material for the
development of artificial ligaments.
Acknowledgements
This study was supported by the National Science
Foundation of China (No. 81071457).
References
|
1
|
Söderman K, Pietilä T, Alfredson H, et al:
Anterior cruciate ligament injuries in young females playing soccer
at senior levels. Scand J Med Sci Sports. 12:65–68. 2002.PubMed/NCBI
|
|
2
|
Keene G: Arthroscopic reconstruction of
the anterior cruciate ligament. A comparison of patellar tendon
autograft and four-strand hamstring tendon autograft. Am J Sports
Med. 28:4382000.PubMed/NCBI
|
|
3
|
Mihelic R, Jurdana H, Jotanovic Z, et al:
Long-term results of anterior cruciate ligament reconstruction: a
comparison with non-operative treatment with a follow-up of 17–20
years. Int Orthop. 35:1093–1097. 2011.PubMed/NCBI
|
|
4
|
Price JS, Till SH, Bickerstaff DR, et al:
Degradation of cartilage type II collagen precedes the onset of
osteoarthritis following anterior cruciate ligament rupture.
Arthritis Rheum. 42:2390–2398. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Montgomery A: Can many anterior cruciate
ligament (ACL) ruptures heal without surgery? N Z Med J.
124:1042011.PubMed/NCBI
|
|
6
|
Vavken P and Murray MM: The potential for
primary repair of the ACL. Sports Med Arthrosc. 19:44–49. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bryant AL, Creaby MW, Newton RU and Steele
JR: Hamstring antagonist torque generated in vivo following ACL
rupture and ACL reconstruction. Knee. 17:287–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mauch C, Arnold MP, Wirries A, et al:
Anterior cruciate ligament reconstruction using quadriceps tendon
autograft for adolescents with open physes- a technical note.
Sports Med Arthrosc Rehabil Ther Technol. 3:72011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bak K, Jørgensen U, Ekstrand J and
Scavenius M: Reconstruction of anterior cruciate ligament deficient
knees in soccer players with an iliotibial band autograft. A
prospective study of 132 reconstructed knees followed for 4 (2–7)
years. Scand J Med Sci Sports. 11:16–22. 2001.PubMed/NCBI
|
|
10
|
Burks RT, Crim J, Fink BP, et al: The
effects of semitendinosus and gracilis harvest in anterior cruciate
ligament reconstruction. Arthroscopy. 21:1177–1185. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee S, Seong SC, Jo H, et al: Outcome of
anterior cruciate ligament reconstruction using quadriceps tendon
autograft. Arthroscopy. 20:795–802. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller SL and Gladstone JN: Graft
selection in anterior cruciate ligament reconstruction. Orthop Clin
North Am. 33:675–683. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akhtar MA, Bhattacharya R, Ohly N, et al:
Revision ACL reconstruction-causes of failure and graft choices. Br
J Sports Med. 45:A15–A16. 2011. View Article : Google Scholar
|
|
14
|
Díaz-de-Rada P, Barriga A, Barroso JL, et
al: Positive culture in allograft ACL-reconstruction: what to do?
Knee Surg Sports Traumatol Arthrosc. 11:219–222. 2003.PubMed/NCBI
|
|
15
|
Legnani C, Ventura A, Terzaghi C, et al:
Anterior cruciate ligament reconstruction with synthetic grafts. A
review of literature. Int Orthop. 34:465–471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsumoto H and Fujikawa K: Leeds-Keio
artificial ligament: a new concept for the anterior cruciate
ligament reconstruction of the knee. Keio J Med. 50:161–166. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bernardino S: ACL prosthesis: any promise
for the future? Knee Surg Sports Traumatol Arthrosc. 18:797–804.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pavon-Djavid G, Gamble LJ, Ciobanu M, et
al: Bioactive poly (ethylene terephthalate) fibers and fabrics:
grafting, chemical characterization, and biological assessment.
Biomacromolecules. 8:3317–3325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bolton CW and Bruchman B: Mechanical and
biological properties of the GORE-TEX expanded
polytetrafluoroethylene (PTFE) prosthetic ligament. Aktuel Probl
Chir Orthop. 26:40–51. 1983.PubMed/NCBI
|
|
20
|
Lavoie P, Fletcher J and Duval N: Patient
satisfaction needs as related to knee stability and objective
findings after ACL reconstruction using the LARS artificial
ligament. Knee. 7:157–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou J, Ciobanu M, Pavon-Djavid G, et al:
Morphology and adhesion of human fibroblast cells cultured on
bioactive polymer grafted ligament prosthesis. Conf Proc IEEE Eng
Med Biol So. 5115–5118. 2007.PubMed/NCBI
|
|
22
|
Ventura A, Terzaghi C, Legnani C, et al:
Synthetic grafts for anterior cruciate ligament rupture: 19-year
outcome study. Knee. 17:108–113. 2010.PubMed/NCBI
|
|
23
|
Carson L, Kelly-Brown C, Stewart M, et al:
Synthesis and characterization of chitosan-carbon nanotube
composites. Mater Lett. 63:617–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hadjizadeh A, Ajji A and Bureau MN:
Nano/micro electro-spun polyethylene terephthalate fibrous mat
preparation and characterization. J Mech Behav Biomed Mater.
4:340–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lechat C, Bunsell A, Davies P and Piant A:
Mechanical behaviour of polyethylene terephthalate and polyethylene
naphthalate fibres under cyclic loading. J Mater Sci. 41:1745–1756.
2006. View Article : Google Scholar
|
|
26
|
Peng CH, Bai BS and Chen YF: Study on the
preparation of Mn-Zn soft magnetic ferrite powders from waste Zn-Mn
dry batteries. Waste Manag. 28:326–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abdolahifard M, Hajir Bahrami S and Malek
R: Surface modification of PET fabric by graft copolymerization
with acrylic acid and its antibacterial properties. ISRN Organic
Chemistry. Article ID 2654152011.PubMed/NCBI
|
|
28
|
Guidoin MF, Marois Y, Bejui J, et al:
Analysis of retrieved polymer fiber based replacements for the ACL.
Biomaterials. 21:2461–2474. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li H, Ge Y, Wu Y, et al: Hydroxyapatite
coating enhances polyethylene terephthalate artificial ligament
graft osseointegration in the bone tunnel. Int Orthop.
35:1561–1567. 2011. View Article : Google Scholar
|
|
30
|
Gupta B, Revagade N, Atthoff B, et al:
Radiation-induced graft modification of knitted poly(ethylene
terephthalate) fabric for collagen immobilization. Polym Adv
Technol. 18:281–285. 2007. View
Article : Google Scholar
|
|
31
|
Pezzatini S, Morbidelli L, Gristina R, et
al: A nanoscale fluorocarbon coating on PET surfaces improves the
adhesion and growth of cultured coronary endothelial cells.
Nanotechnology. 19:2751012008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zia KM, Zuber M, Barikani M, et al:
Surface characteristics of chitin-based shape memory polyurethane
elastomers. Colloids Surf B Biointerfaces. 72:248–252. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Narayan R, Chattopadhyay DK, Sreedhar B,
et al: Synthesis and characterization of crosslinked polyurethane
dispersions based on hydroxylated polyesters. J Appl Polym Sci.
99:368–380. 2006. View Article : Google Scholar
|
|
34
|
Rahman MM, Kim EY and Lee WK: Effect of
DMPA-clay-POSS content on thermal and mechanical properties of
nanostructured ionomeric polyurethanes. J Nanosci Nanotechnol.
10:6981–6985. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ren Z, Ma D, Wang Y and Zhao G: Molecular
structure and hydrogen bonds in solid dimethylol propionic acid
(DMPA). Spectrochim Acta A Mol Biomol Spectrosc. 59:2713–2722.
2003. View Article : Google Scholar : PubMed/NCBI
|