Introduction
Breast cancer occurs in approximately 99.4 of every
100,000 women in North America and is the most frequent malignancy
in women (1,2). The disease is the second leading
cause of mortality due to cancer among women (3,4).
Given its incidence and threat to survival rate, much effort has
been devoted to understanding the etiology of breast tumors.
Numerous discoveries have arisen with regard to the genetic and
molecular origins and progression of this disease. However, breast
tumor cells are, in some cases, able to evade immune surveillance,
allowing the tumor to grow and advance.
The mechanisms by which these tumor cells escape
detection remain unknown; however, previous research has confirmed
that interleukin-17 (IL-17) is significant in promoting tumor
growth (5–7). IL-17 is an inflammatory cytokine that
has functions in innate and adaptive immunity and is expressed from
Th17 cells (8). However, the
dysregulation of IL-17 is apparent in breast tumors, demonstrated
by the presence of IL-17-expressing cells within them. This
indicates that IL-17 aids these cells in escaping elimination by
immune cells, thereby promoting tumor progression (9).
In this study, murine breast cancer models were
established to analyze the expression of IL-17 within mammary
tumors. The correlation between IL-17 expression and tumor
development was investigated.
Materials and methods
Establishing mouse breast cancer
models
Female BALB/c mice, 6–8 weeks old, average body
weight 20.5±2.4 g, were purchased from the Experimental Animal
Center of Shanghai Second Military Medical University (Shanghai,
China). Mouse breast cancer cell lines MA782 (MA782/5S28102, China
Center for Type Culture Collection, Wuhan University, Hubei, China)
and 4T1 (American Type Culture Collection, Manassas, VA, USA) were
cultured in RPMI-1640 medium supplemented with 100 U/ml penicillin,
100 U/ml streptomycin and 2 M glutamine at 37°C and 5%
CO2. The cells were harvested during the logarithmic
growth phase. Mice (32) were randomly divided into four groups of
8. Mice in two groups received subcutaneous injections of MA782
cells (1×106 cells/mouse) into the mammary gland on the
left abdominal wall; mice in the other two groups received
injections of 4T1 cells (5×106 cells/mouse). A tumor 2×2
mm in size was considered to be a successfully established model.
The study was approved by the ethics committee of the School of
Medicine, Anhui University of Science and Technology.
Lymphocyte and tumor cell collection from
tumor samples
Inoculated mice were sacrificed by decapitation at
tumor-bearing weeks 1 (early) and 4 (late). Tumor tissues were
excised and minced (<3 mm3 pieces), washed with
phosphate-buffered saline (PBS), then digested in calf serum-free
medium with 0.05% collagenase IV, 100 mg/l DNase I (Sigma, St.
Louis, MO, USA) and l6 M CaCl2. Tissue pieces were
oscillated for 40 min in digestion buffer, then re-suspended in
mouse lymphocyte separation medium. The upper layer was covered
with a thin layer of RPMI-1640. Following gradient centrifugation,
the lymphocyte layer was collected and washed, then re-suspended in
culture medium to isolate tumor-infiltrating lymphocytes; the
lymphocyte separation medium and sediment layers were collected and
washed, then re-suspended in culture medium to isolate tumor
cells.
Western blotting
Tumor tissue was homogenized and treated with 100 ml
cell lysis buffer on ice for 30 min. Samples were centrifuged at
4°C and 12,000 × g for 15 min. Supernatant was transferred to
another centrifuge tube for the determination of total protein
content using the Coomassie brilliant blue microdisk (Sigma)
colorimetric method. Total protein was subjected to SDS-PAGE for
western blotting to detect IL-17 expression with anti-IL-I7
monoclonal antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) and HRP-labeled secondary antibodies. Following detection
of bands on nitrocellulose membranes, Quantity One (BioRad,
Hercules, CA, USA) was used to analyze the gray-value of the target
band. Relative expression levels of the target band were compared
to the β-actin control.
ELISA
Phorbol-12-myristate-13-acetate (PMA), anti-CD3
monoclonal antibody and anti-CD28 monoclonal antibody were added to
lymphocyte and tumor cell cultures isolated from tumor tissue and
continuously cultured for 5 days. ELISA kits were used to detect
IL-I7 expression (R&D Systems, Minneapolis, MN, USA) in
supernatants according to the manufacturer’s instructions.
IL-17 infusion and tumor growth
Recombinant IL-17 (50 μg/l, Pepro Tech, Rocky Hill,
NJ, USA) was added to 4T1 cells in culture; the cell proliferation
rate was analyzed after 5 days. BALB/c mice (16) were inoculated with treated 4T1
cells, then randomly divided into infusion and non-infusion groups.
Mice in the infusion group received injections of 1 μg IL-17 via
the caudal vein on days 1, 7 and 14; mice in the non-infusion group
received saline only on those days. Tumor size and volumes were
measured [tumor volume = (length × short
diameter2)/2].
Detection of CD34 expression by
immunohistochemistry
Tissues were fixed in neutral formalin, dehydrated
and embedded in paraffin wax for sectioning (slice thickness of 4
μm) by conventional methods. Sections were dewaxed with
dimethylbenzene and rehydrated, then heated for antigen retrieval.
Endogenous peroxidase activity was blocked by treatment with 3%
hydrogen peroxide solution. Sections were then sealed with
non-specific serum and placed in a wet box at room temperature.
Primary antibodies (Mena and Her-2) were added to the wet box for
incubation at 4°C overnight. Following incubation, sections were
washed with PBS three times, then treated with biotinylated
secondary antibodies and incubated at room temperature. Following
three more washes in PBS, streptococcus-avidin-peroxidase
(Zhongshang Golden Bridge Biotechnology Co., Ltd., Beijing, China)
was added to the wet box and sections were incubated at 37°C for 30
min. Staining was developed with DAB chromogen (Dako, Carpinteria,
CA, USA) and detected under a light microscope. Known positive
tissue sections were used as positive controls and PBS was used in
place of primary antibodies as a negative control. CD34 staining
appears as brownish-yellow or sepia-toned endothelial cell
membranes or cytoplasm. Neovascularization is usually expressed as
tumor microvascular density (MVD), as reported by Weidner (10). The region with the most dense
microvascular distribution under ×100 light microscopy was assessed
by counting the number of vessels expressing CD34 in 5 individual
visual fields. The mean value is expressed as MVD in units of count
at ×200 magnification. Positively-stained single endothelial cells
or endothelial cell clusters that were significantly separated from
the neighboring microvessels were counted; all blood vessels with
lumens greater than 8 red blood cells and a thicker muscular layer
were not counted.
Statistical analysis
SPSS 17.0 software was used for statistical
analysis. Data are expressed as mean ± SEM. The analysis was
performed with a two-sided test, with the α level equal to 0.05 and
P<0.05 considered to indicate statistically significant
differences.
Results
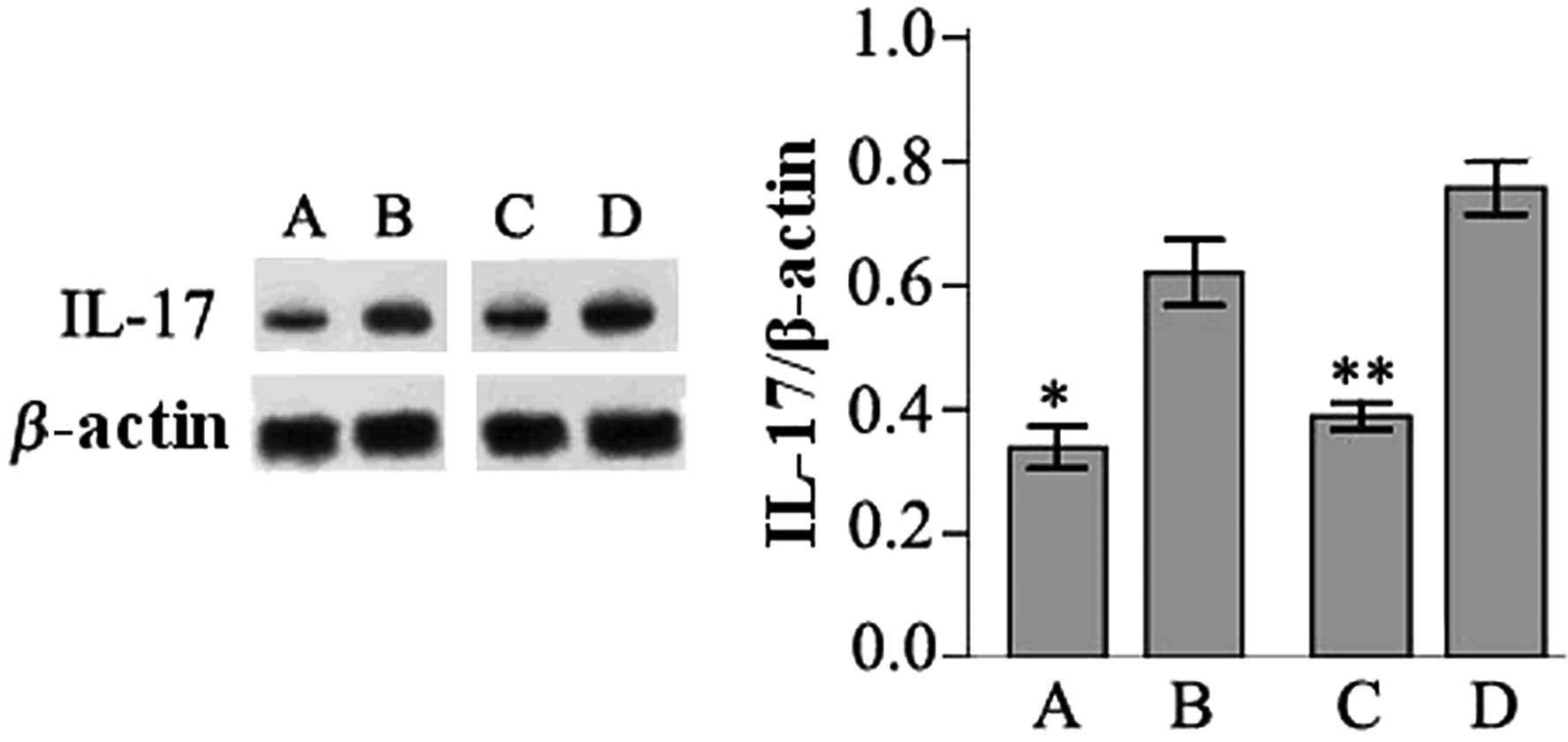
IL-17 expression in tumoral tissue
To determine whether IL-17 expression is
dysregulated in breast tumors, tumor tissues from mice inoculated
with MA782 or 4T1 breast cancer cells were subjected to western
blotting. IL-17 expression was detected in tumor samples from mice
inoculated with MA782 cells and 4T1 cells, as well as in early and
late stage tumors. However, the expression levels of IL-17 were
significantly higher in late than in early stage tumor tissues
(P<0.05; Fig. 1).
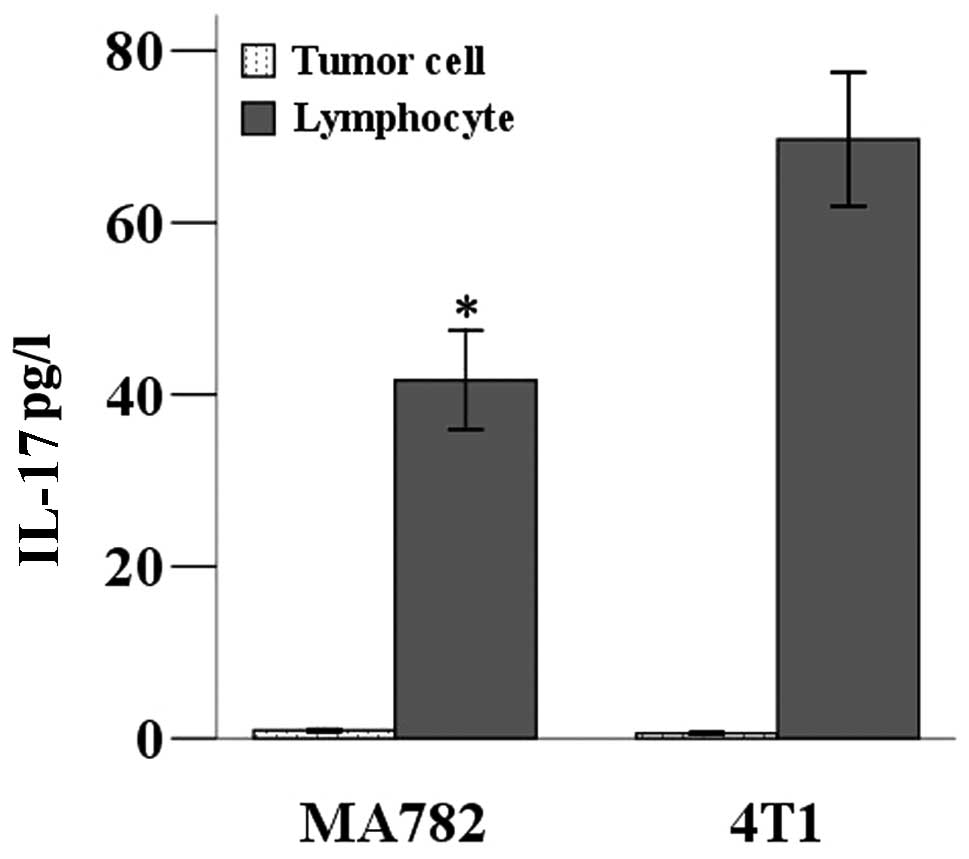
IL-17 is expressed by CD4+ T helper
cells, namely Th17 cells (6,11).
To determine whether the tumor cells were expressing IL-17, or
whether other IL-17-expressing cells had infiltrated the tumors,
IL-17 expression levels from cell cultures in which the breast
cancer cells had been separated from lymphocytes in the tumor
tissue were assessed. After 5 days in separate cultures, the
concentrations of IL-17 in the supernatants from each culture were
tested by ELISA. Low levels of IL-17 were secreted into the
supernatant by tumor cells; however, lymphocytes from the tumor
tissues secreted a higher level of IL-17. A higher level of IL-17
was detected in the supernatant of lymphocytes from tumors of mice
inoculated with 4T1 cells than those inoculated with MA782
(P<0.05; Fig. 2).
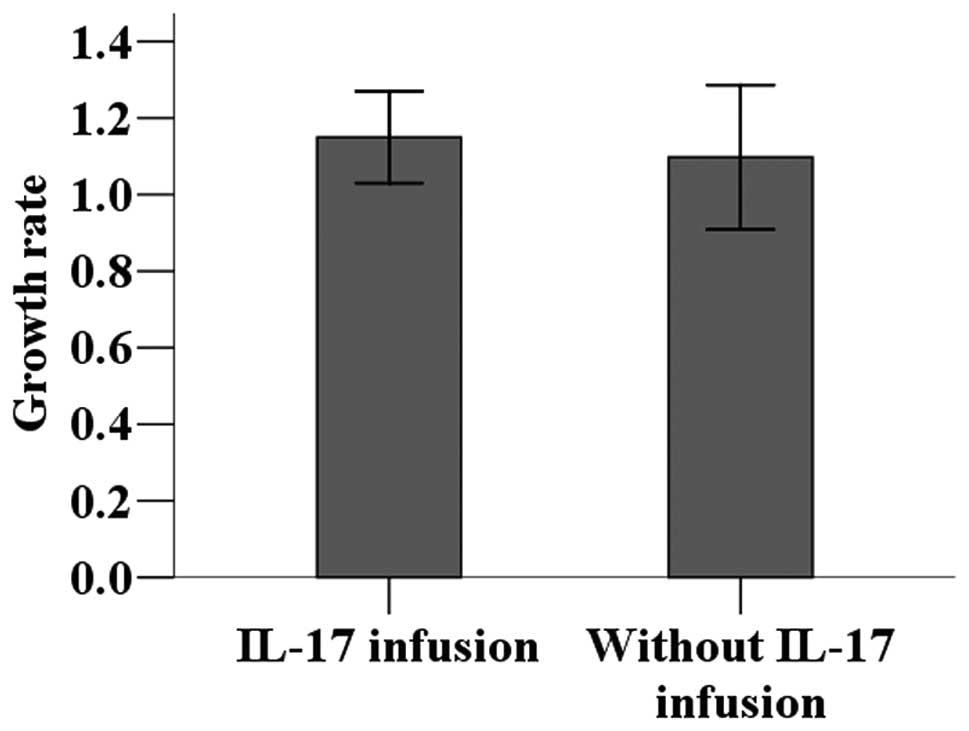
Effect of IL-17 infusion on proliferation
of tumor cells
The 4T1 breast cancer cell line was cultured with or
without recombinant IL-17 to determine its effect on tumor cell
proliferation. No statistically significant difference was observed
in the tumor cell proliferation rate between culture systems with
and without IL-17 (P>0.05; Fig.
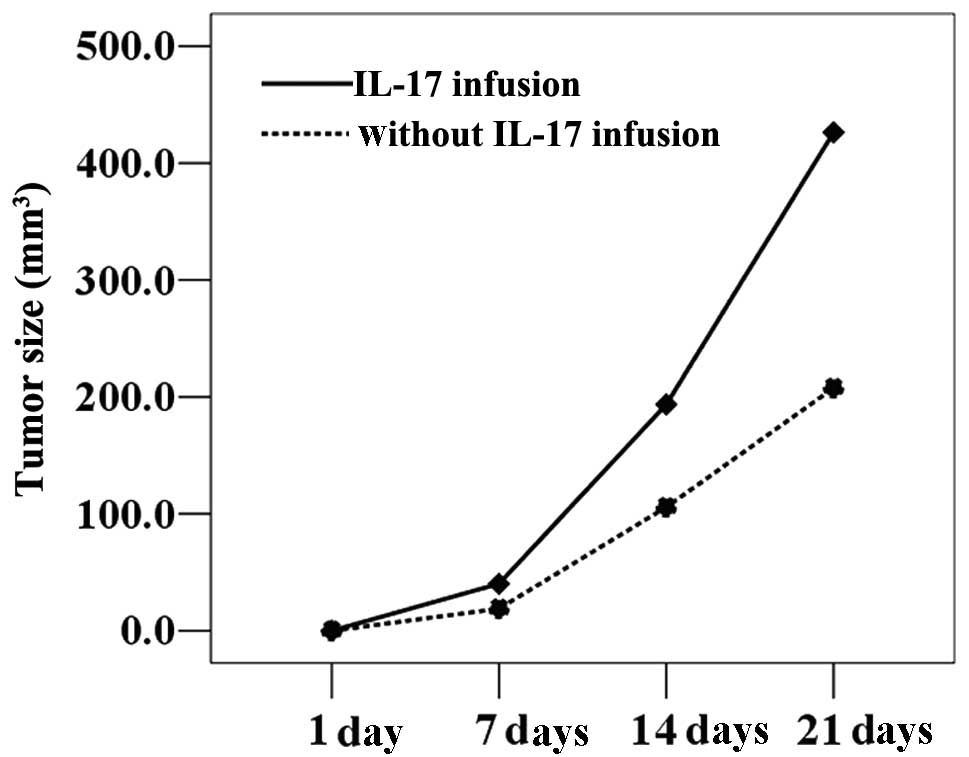
3). 4T1 cells cultured in the presence of IL-17 were injected
into BALB/c mice, which were then divided into infusion and
non-infusion groups. The infusion group received injections of
recombinant IL-17; the non-infusion groups received injections of
saline only. Tumor growth was significantly accelerated in
tumor-bearing mice following intravenous infusion of IL-17,
resulting in significantly larger tumor volumes compared with those
receiving saline only (P<0.05; Fig.
4).
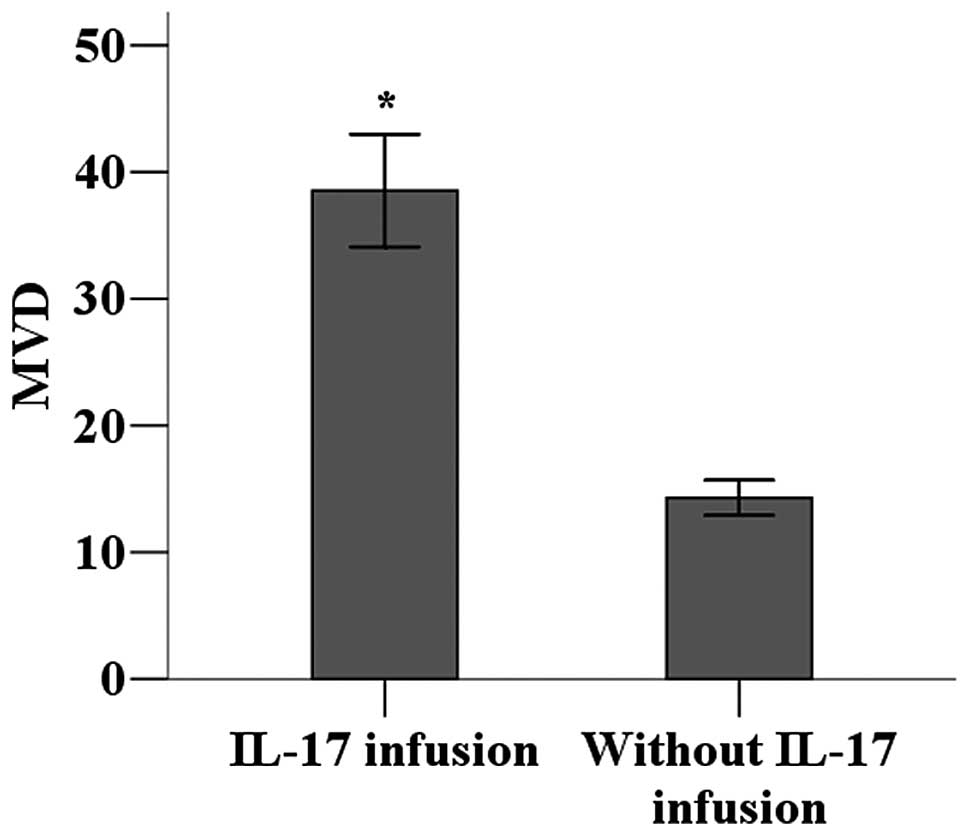
Effects of IL-17 on angiogenesis in tumor
tissues
The vascularization of tumor environments with
higher levels of IL-17 was investigated. Immunohistochemistry was
used to detect CD34 expression in tumor tissues to calculate MVD as
a measure of angiogenesis. MVD was significantly higher in the
tumor tissues of mice receiving IL-17 infusions compared with those
receiving only saline infusions (P<0.05; Fig. 5).
Discussion
IL-17 is a cytokine that is mainly secreted by Th17
cells during the inflammatory response (6,11).
Studies have shown that IL-17 promotes the release of IL-6, IL-8,
prostaglandin E2 (PGE2), IL-1®, transforming growth
factor (TNF) and some chemotactic factors, as well as the local
aggregation of inflammatory cells (12). Previous studies have indicated that
IL-17 is involved in the development and progression of numerous
inflammatory diseases, including rheumatoid arthritis, systemic
lupus erythematosus and certain respiratory diseases. Additionally,
IL-17 is expressed at different levels in a number of tumors. Zhang
et al(13) reported that
IL-17 is present in the tumor tissues and serum of gastric cancer
patients, with IL-17 expression levels correlating with the
clinical stage of tumors. Similarly, the levels of IL-17 in the
tumors of patients with colorectal cancer are significantly higher
compared with those in normal tissues (14). In mouse models of non-small cell
lung cancer, IL-17 transfection promotes tumor growth (15). Other studies suggest that IL-17 is
involved in the development and progression of prostate, ovarian,
choriocarcinoma and cutaneous T-cell tumors. The present study
demonstrates that, in mouse breast cancer models, IL-17 expression
is detected in tumor tissues at the early and late stages and IL-17
levels in late tumors are significantly higher compared with those
in early tumors.
Shime et al(16) reported that tumor cells promote
CD4+ T cells to release IL-17 through the release of
lactic acid. Kryczek et al(17) confirmed that IL-2 in the tumor
environment regulates the balance between Treg cells and the
secretion of IL-17. The present study revealed that IL-17 is mainly
expressed by tumor-infiltrating lymphocytes, while tumor cells
express little IL-17, indicating that IL-17 expression in tumors is
the result of tumor microenvironment effects. However, it remains
unclear how IL-17 expression in tumor tissues affects the
occurrence and development of tumors. In the present study, in
vitro exposure to IL-17 did not promote the proliferation of
tumor cells; however, in vivo intravenous infusion of IL-17
in tumor-bearing mice significantly promoted tumor growth. This
suggests that IL-17 indirectly promotes tumor growth. Furthermore,
we demonstrated that vascular density significantly increased in
tumors from mice receiving an intravenous infusion of IL-17.
Therefore, IL-17 may accelerate the progression of tumors by
promoting microvessel formation in tumor tissues.
In summary, in murine breast cancer models, tumor
tissue expresses IL-17, with increasing expression levels in more
advanced tumors. IL-17 may promote tumor growth by promoting
microvessel formation in tumor tissues.
Acknowledgements
This study was supported by Science Fund of Anhui
Educational Department (Grant No. KJ2012A080).
References
|
1
|
Wright SE: Immunotherapy of breast cancer.
Expert Opin Biol Ther. 12:479–490. 2012. View Article : Google Scholar
|
|
2
|
World Health Organization. http://www.who.int/cancer/detection/breastcancer/en/index1.html.
Accessed April 2012
|
|
3
|
Peng J, Sengupta S and Jordan VC:
Potential of selective estrogen receptor modulators as treatments
and preventives of breast cancer. Anticancer Agents Med Chem.
9:481–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Howard JH and Bland KI: Current management
and treatment strategies for breast cancer. Curr Opin Obstet
Gynecol. 24:44–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Novitskiy SV, Pickup MW, Gorska AE, et al:
TGF-β Receptor II loss promotes mammary carcinoma progression by
Th17 dependent mechanisms. Cancer Discov. 1:430–441. 2011.
|
|
6
|
Afzali B, Lombardi G, Lechler RI and Lord
GM: The role of T helper 17 (Th17) and regulatory T cells (Treg) in
human organ transplantation and autoimmune disease. Clin Exp
Immunol. 148:32–46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang L, Qi Y, Hu J, Tang L, Zhao S and
Shan B: Expression of Th17 cells in breast cancer tissue and its
association with clinical parameters. Cell Biochem Biophys.
62:153–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pappu R, Ramirez-Carrozzi V and Sambandam
A: The interleukin-17 cytokine family: critical players in host
defence and inflammatory diseases. Immunology. 134:8–16. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu X, Mulcahy LA, Mohammed RA, et al:
IL-17 expression by breast-cancer-associated macrophages: IL-17
promotes invasiveness of breast cancer cell lines. Breast Cancer
Res. 10:R952008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weidner N: Current pathologic methods for
measuring intratumoral microvessel density within breast carcinoma
and other solid tumors. Breast Cancer Res Treat. 36:169–180. 1995.
View Article : Google Scholar
|
|
11
|
Harrington LE, Haaon RD, Mangan PR, Turner
H, Murphy TL, Murphy KM and Weaver CT: Interleukin 17-producing
CD4+ effector T cells develop via a lineage distinct from the T
helper type 1 and 2 lineages. Nat Immunol. 6:1123–1132. 2005.
|
|
12
|
Fossiez F, Banchereau J, Murray R, Van
Kooten C, Garrone P and Lebecque S: Interleukin-17. Int Rev
Immunol. 16:541–551. 1998. View Article : Google Scholar
|
|
13
|
Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma
L, Xue X, Wei G, Liu X and Fang G: The prevalence of Th17 cells in
patients with gastric cancer. Biochem Biophys Res Commun.
374:533–537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Le Gouvello S, Bastuji-Garin S, Aloulou N,
et al: High prevalence of Foxp3 and IL-17 in MMR-proficient
colorectal carcinomas. Gut. 57:772–779. 2008.PubMed/NCBI
|
|
15
|
Numasaki M, Watanabe M, Suzuki T, et al:
IL-17 enhances the net angiogenic activity and in vivo growth of
human non-small cell lung cancer in SCID mice through promoting
CXCR-2-dependent angiogenesis. J Immunol. 175:6177–6189. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shime H, Yabu M, Akazawa T, Kodama K,
Matsumoto M, Seya T and Inoue N: Tumor-secreted lactic acid
promotes IL-23/IL-17 proinflammatory pathway. J Immunol.
180:7175–7183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kryczek I, Wei S, Zou L, Altuwaijri S,
Szeliga W, Kolls J, Chang A and Zou W: Cutting ease: Thl7 and
regulatory T cell dynamics and the regulation by IL-2 in the tumor
microenvironment. J Immunol. 178:6730–6733. 2007. View Article : Google Scholar : PubMed/NCBI
|