Introduction
The exact pathogenesis of ulcerative colitis (UC)
remains unknown. However, a number of factors, including the
histopathology of colonic lesions and the beneficial effects of
corticosteroid therapy, point toward immunological involvement.
Ulcerated lesions in UC are accompanied by a prominent infiltration
of inflammatory cells, including T lymphocytes, macrophages,
neutrophils and plasma cells. In immunological pathogenesis,
cytokines are considered to play critical roles. Such factors are
secreted by immune and non-immune cells. These small polypeptides
have extensive biological functions, including regulating
cell-to-cell signaling, adjusting the immune response and
regulating inflammation. These small proteins may be divided into 3
classes: promoters of inflammation and anti-inflammatory and growth
factors. In terms of UC pathogenesis, the balance between pro- and
anti-inflammatory factors is considered to be particularly
significant.
Chemotactic factors (chemokines) belong to a class
of inflammatory molecules that plays a significant role in
promoting the development of UC. Chemokines may be divided into 4
groups: CXC, CC, C and CX3C. CCL20 belongs to the CC group of
chemotactic agents. It is strongly chemotactic for lymphocytes and
recruits lymphocytes and dendritic cells (DDs) into epithelial
tissue. Immature DCs (imDCs) are selectively attracted by CCL20.
Therefore, CCL20 plays a role in the formation of mucosal lymphoid
tissue. A number of research efforts have been aimed at inhibiting
chemokines as a means of treating UC (1–7).
In the current study, dextran sulfate sodium (DSS)
was used to induce UC in mice (1,2,8,9).
Immunohistochemistry, RT-PCR and western blotting were used to
detect CCL20 in this experimental mouse model of colitis.
Immunohistochemistry was also used to detect the CCL20 levels in 65
patients with UC and in 30 normal controls. Overall, this study
examines the role of CCL20 in UC.
Patients and methods
Patients
Colonic biopsies were obtained from 65 consenting
patients with UC (34 females and 31 males; median age, 44 years;
range, 16–75 years) undergoing colonoscopy for diagnostic purposes
as approved by the Institutional Review Board of the Affiliated
Hospital of Nantong University. The diagnoses were based on
clinical and endoscopic parameters. Endoscopic appearance of the
colonic mucosa was assessed according to the criteria of Murano
et al (10): mild (n=22),
moderate (n=26) and severe (n=17). Histological disease activity
was assessed according to the criteria of Truelove and Richard:
mild (n=23), moderate (n=24) and severe (n=18). Additional colonic
biopsies were obtained as the controls from consenting normal
patients (n=30; 8 females and 22 males; median age, 42.5 years;
range, 20–65 years) undergoing endoscopy to rule out neoplastic
disease by pathological examination.
Grouping of experimental animals and
establishment of the model
A total of 40 female adult BALB/c mice (mass, 14–20
g) were purchased from the Experimental Animal Center, Medical
School of Nantong University (Nantong, China). The animals were
kept in cages with controlled temperature (23±2°C) on a 12-h
light-dark cycle and were randomly divided into 2 groups (n=20 for
each group): the control group (group C) and the UC model group
(group M). The mice in the UC model group freely drank a 5% DSS
solution for 7 days in order to develop experimental colitis
(1,2,8,9).
Mice in the control group drank distilled water for the same 7-day
span. On day 8, all mice were sacrificed and colon specimens were
collected for research purposes. This study was approved by the
Institutional Review Board of the Affiliated Hospital of Nantong
University.
Disease activity index (DAI) and
histological disease score
The DAI was determined by an investigator blinded to
the experiment by scoring the extent of body weight loss, stool
hemoccult positivity or gross bleeding and stool consistency, in
accordance with the method described by Murano et al
(10) (Table I). For histology, the rectum was
fixed in 10% neutral buffered formalin and 4-mm specimens were
subjected to hematoxylin and eosin (H&E) staining. Randomly
selected fields (n=15) magnified at ×100 were inspected and graded
by a pathologist blinded to the treatment instructions (Table II) (5). The mean score in each section was
calculated.
 | Table IDisease activity index. |
Table I
Disease activity index.
| Score | Weight loss (%) | Stool
consistencya | Occult/gross
bleeding |
|---|
| 0 | (−) | Normal | Normal |
| 1 | 1–5 | | |
| 2 | 5–10 | Loose | Guaiac (+) |
| 3 | 11–15 | | |
| 4 | >15 | Diarrhea | Gross bleeding |
 | Table IIHistological disease score. |
Table II
Histological disease score.
| Grade | Characteristic |
|---|
| 0 | Normal colonic
mucosa |
| 1 | Loss of one-third of
the crypts |
| 2 | Loss of two-thirds of
the crypts |
| 3 | Lamina propria
covered with a single layer of epithelium and mild inflammatory
cell infiltration is present |
| 4 | Erosions and marked
inflammatory cell infiltration are present |
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA from the colonic mucosa was extracted
according to standard TRIzol RNA isolation instructions and
evaluated with a spectrophotometer for quantity and purity. First
strand cDNA was synthesized from 1 μg total RNA in a 25-μl reaction
volume, containing 4 μl 5X First-Strand Buffer, 0.5 μl ribonuclease
inhibitor, 2 μl dNTP mix, 2 μl DTT and 1 μl MMLV Reverse
Transcriptase (Sangon Biotech, Shanghai, China). The RT reaction
was carried out for 60 min at 37°C. PCR products were obtained from
5 μl of each cDNA sample in the presence of 2.5 μl 10X PCR buffer
with MgCl2, 1 μl dNTPs, 2 μl sense and antisense primers
(each) and 1 μl Taq (Sangon Biotech). The housekeeping gene,
glyceraldehyde 3-phosphate dehydrogenase (GAPDH), was used as the
internal control. The primer sequences for CCL20 were: sense,
5′-AGCAGCAGCAACT ACGACT-3′; and antisense, 5′-TCTTAGGCTGAGGAGG
TTCA-3′. The primers for GAPDH were: sense, 5′-ATGGG
AAGCTGGTCATCAAC-3′; and antisense, 5′-TTCAGCTCT GGGATGCCT-3′. The
sizes of the amplified products were 202 bp for CCL20 and 484 bp
for GAPDH.
The amplification was performed under the following
conditions: for CCL20, 34 cycles with an annealing temperature of
56°C for 30 sec; and for GAPDH, 30 cycles with an annealing
temperature of 58°C for 30 sec. Denaturation and extension
conditions were 94°C for 30 sec and 72°C for 40 sec, respectively.
PCR products were separated by electrophoresis on a 1.5% agarose
gel, stained with ethidium bromide (EB) and analyzed with the Gel
Doc 2000 system (Bio-Rad, Hercules, CA, USA). The integrated
density of the bands was used as a quantitative parameter. CCL20
mRNA levels were expressed as the ratio of band optical intensity
to GAPDH. All experiments were performed at least twice and the
reported results were reproducible.
Immunohistochemistry
Immunohistochemistry was performed in order to
examine the CCL20 protein expression in the colonic mucosa of UC
patients and in a mouse model. Briefly, colonic mucosa samples were
isolated and immediately fixed in 10% pH-neutral phosphate-buffered
formalin. The fixed tissues were then embedded in paraffin and kept
until use. Paraffin sections (4 μm) were cut, deparaffinized and
hydrated. A Universal Immuno-enzyme Polymer method (Elivison
staining) was employed for immunohistochemical staining. Anti-CCL20
polyclonal antibody (R&D Systems, Minneapolis, MN, USA;
dilution, 15 μg/ml) was used as the primary antibody for 1-h
incubation at room temperature. Briefly, staining intensity was
scored as 0 (negative), 1 (weak), 2 (medium) or 3 (strong). The
extent of staining was scored as 0 (0%), 1 (1–25%), 2 (26–50%), 3
(51–75%) or 4 (76–100%) according to the percentage of the positive
staining area, in relation to the whole carcinoma area.
Subsequently, the sum of the intensity and extent scores was
regarded as the final staining score for CCL20. A final score ≥3
was considered positive.
Western blotting
Western blotting was performed on whole cell
lysates. Aliquots of total protein (20 μg per lane) were
electrophoresed on 10% SDS-polyacrylamide gradient gels and
transferred onto nitrocellulose membranes (Millipore, Billerica,
MA, USA). The membranes were incubated for 8 h at room temperature
with anti-CCL20 mAb (R&D Systems, Alexis Biochemicals, San
Diego, CA, USA). Following washing with rinsing buffer, the
membranes were incubated with 1:15,000 diluted horseradish
peroxidase-conjugated anti-mouse immunoglobulin antibody (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), followed by
development with enhanced chemiluminescence reagents (Amersham
Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK).
Statistical analysis
Data are presented as the means ± standard error
(SEM) and analyzed with STATA 7.0 by ANOVA and t-tests between
groups. P<0.05 was considered to indicate a statistically
significant result.
Results
DAI
Control group mice had normal diets, activities and
bowel movements. Their coats were healthy and overall body quality
was slightly increased. Model group mice began to appear anorexic
from day 1 and at the same time, their activity decreased, their
hair stood vertically, they had abnormal stools and lost weight. By
day 3, the mice in the model group began experiencing gross
bleeding, occult blood production and more pronounced weight loss.
Compared with the control group, the DAI of the model group was
significantly higher (3.48±0.44 versus 0.88±0.22, P<0.05) at day
7 (Table III).
 | Table IIIDAI of DSS group compared with control
group (P<0.05). |
Table III
DAI of DSS group compared with control
group (P<0.05).
| Day | Control group
DAI | DSS group DAI |
|---|
| 1 | 1 | 1 |
| 2 | 1 | 1 |
| 3 | 1.45 | 3.2 |
| 4 | 1 | 2.55 |
| 5 | 1 | 2.96 |
| 6 | 1 | 3.05 |
| 7 | 1 | 3.5 |
Histological disease score
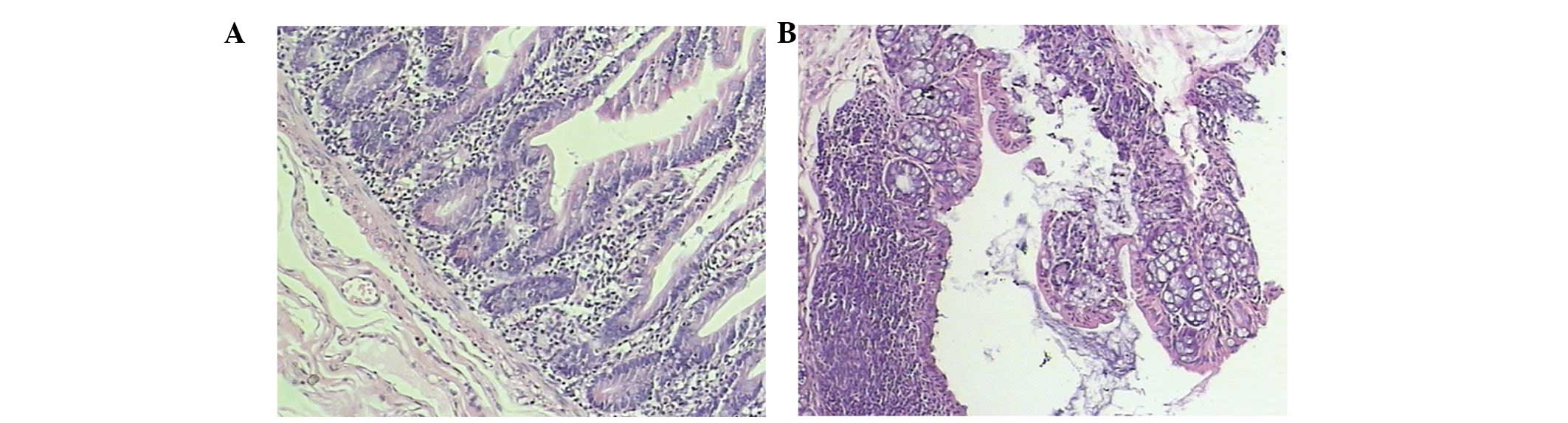
The colonic mucosal epithelium in the control mice
was normal and complete. The inherent layered glands were normal
and the submucosa revealed only a few inflammatory cells that had
infiltrated. However, there was no evidence of erosion or ulcer
formation. The colonic mucosa in the model mice was damaged and
lost. There was epithelial erosion, ulcer formation and the
inherent layered glands were deformed. Additionally, the mucosa was
disordered and the submucosa exhibited a high degree of lymphocyte
and mononuclear cell infiltration (Fig. 1). The histological scores of colons
from mice in the model group were significantly higher than those
in the control group (3.35±0.43 versus 0.92±0.29, P<0.05;
Table IV).
 | Table IVHistological disease score. |
Table IV
Histological disease score.
| Group (n=10) | Histological disease
score (mean ± SD) |
|---|
| Control | 0.92±0.29 |
| DSS | 3.35±0.43a |
RT-PCR
CCL20 mRNA was expressed in the colons of all mice
in the model and control groups (Fig.
2). However, in the model group, CCL20 mRNA expression was
significantly higher than that in the control group and this
expression positively correlated with the degree of inflammation
(P<0.01).
Immunohistochemistry in experimental
colitis in mice and UC patients
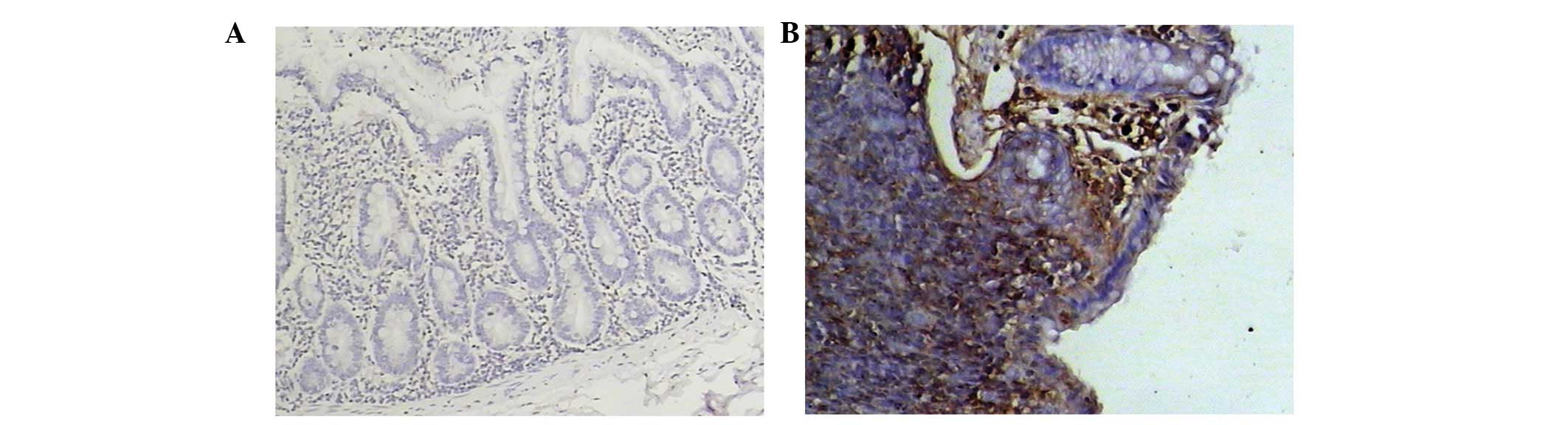
CCL20 was either weakly expressed or not expressed
at all in the control mice. However, in the model group mice, CCL20
expression was high (Fig. 3).
CCL20 protein expression was exclusively localized to the mucosal
epithelium covering the lymphoid follicles.
Immunohistochemical scores of CCL20 in the model
group were significantly higher than those in the control group and
the scores correlated with inflammation degree (P<0.01).
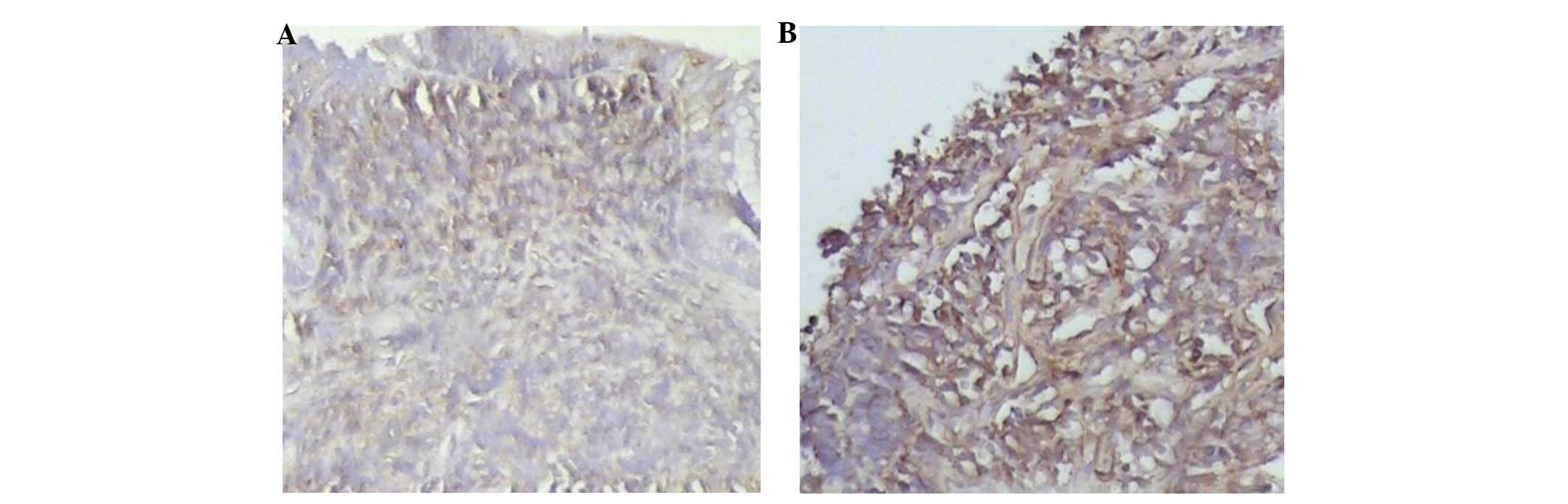
In normal colonic mucosa organization, CCL20 was
either weakly expressed or not expressed at all. In the bowel
mucosa of patients with UC, the CCL20 expression level was
4.52±1.75 points (Fig. 4B) and
this was significantly higher than the levels in the normal control
group mice (0.56±0.15 points; Fig.
4A). This difference was statistically significant (P<0.01).
CCL20 expression in UC increased significantly with the degree of
inflammation.
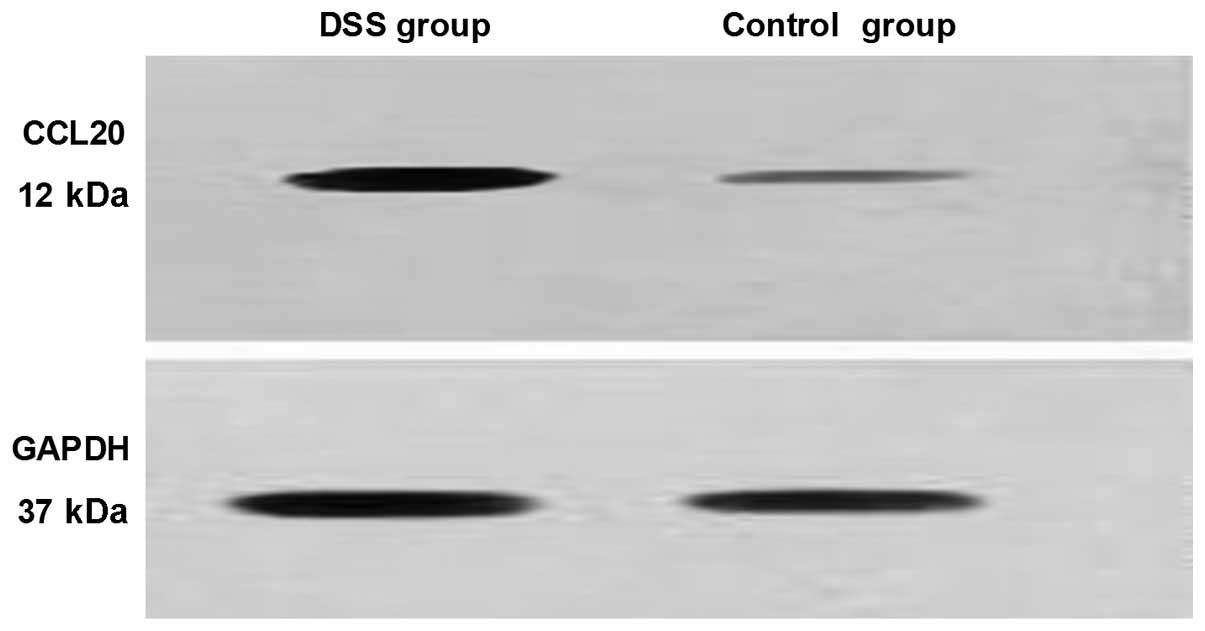
Western blotting
CCL20 was either weakly expressed or not expressed
at all in the control group. However, in the model group, CCL20 was
expressed at high levels (Fig. 5).
That is, CCL20 protein expression in the model group was
significantly higher than that in the control group and positively
correlated with the degree of inflammation (P<0.01).
Discussion
Chemokines are a relatively recently identified
family of approximately 40 chemotactic, 7–10-kDa peptides, which
have been implicated in the pathophysiology of UC. The 40
chemokines identified in humans are classified into 4 families,
designated CXC, CC, C and CX3C, where X is another amino acid,
depending upon the spacing of the 2 N-terminal cysteine residues.
Chemokines attract inflammatory cells to a particular location and
activate them. Chemokines are produced by a wide variety of cells,
including the inflammatory cells present in UC lesions,
fibroblasts, endothelial cells and epithelial cells, all of which
are abundant in the gastrointestinal system (3–5,10–18).
The main functions of intestinal epithelial cells
are absorption and secretion in order to keep the intestinal
microenvironment stable. The intestinal immune system is delicately
balanced with factors that promote and hinder inflammatory
responses. If this balance is disrupted, the extensive,
non-specific activation of inflammatory cells results in the
production and release of destructive immune molecules and
inflammatory factors (19). These
factors include activated macrophages and T lymphocytes,
chemotactic factors that promote inflammation and other factors
involved in the expression of class II MHC molecules (6). Under normal circumstances, such
inflammation is inhibited by interleukin (IL)-4, IL-1 receptor
antagonists, IL-10 and transforming growth factor (TGF)-β 1.
However, in diseased states, these factors cannot fulfill their
biological roles (20). In the
case of inflammation or infection by pathogenic microorganisms,
epithelial cells attract neutrophils, lymphocytes and other
inflammatory cells to the bowel mucosa. Additionally, surface
cytokines release factors, including IL-1α and tumor necrosis
factor (TNF) α. The activation of NF-κB further raises CCL20 levels
and the expression of CCR6 (21).
For similar reasons, UC results in significantly increased levels
of CCL20. As a result, imDC chemotactic lymphocytes gather in the
bowel wall and worsen the inflammation. DCs are antigen cells that
have strong antigen processing functions. Dendritic progenitor
cells exist in the blood circulation and also settle in the
gastrointestinal tract, epithelial tissue of the respiratory tract,
urinary and reproductive systems, the heart, liver, kidney and in
other essential organs. In the presence of various stimulants, DCs
gradually mature through the lymphatic and blood circulation to the
lymph nodes (14). Mature DCs
(mDCs) activate T lymphocytes to induce immune responses and lead
to the expression of significant stimulatory molecules, including
CD80, CD86, CD83, CD54 and CD40 (7,14,22–24).
Although the causes may be different, the process of inflammation
is uniform, involving the CD4 T cell differentiation of auxiliary T
cells (1) and Th1 or Th2. UC is
usually thought to occur via gut mucosal inflammation mediated by
Th2. It has been previously demonstrated that DC infiltration was
positively correlated with the quantity and severity of the
inflammatory response (13).
The current study demonstrates that, in the
DSS-induced colitis mouse model, CCL20 is overexpressed. By
contrast, CCL20 levels were low or undetectable in the control
mice. In UC patients, CCL20 was also highly expressed and revealed
a positive correlation with the degree of inflammation. The
overexpression of CCL20 is a significant factor in the occurrence
and development of UC by promoting imDCs in the colonic mucosa by
CCR6 and facilitating lymphocytes to lead to the damage of colonic
mucosa. Thus, CCL20 reflects the degree and severity of UC disease,
suggesting that it is means of monitoring the degree of
inflammation. It is possible that altering CCL20 may become an
effective treatment method for patients with UC.
In conclusion, CCL20 appears to play a significant
role in UC. CCL20 activates DC lymphocytes that then lead directly
to the pathological changes of UC. CCL20 reflects the degree of
inflammation, which may be used to evaluate disease severity and is
a potential therapeutic target.
Acknowledgements
This study was supported by the Natural Science
Foundation of Nantong University (10Z061).
Abbreviations:
|
UC
|
ulcerative colitis
|
|
DSS
|
dextran sulfate sodium
|
|
DAI
|
disease activity index
|
|
H&E
|
hematoxylin and eosin
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
References
|
1
|
Stevceva L, Pavli P, Husband AJ and Doe
WF: The inflammatory infiltrate in the acute stage of the dextran
sulphate sodium induced colitis: B cell response differs depending
on the percentage of DSS used to induce it. BMC Clin Pathol.
1:32001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bennink RJ, Hamann J, de Bruin K, ten Kate
FJ, van Deventer SJ and te Velde AA: Dedicated pinhole SPECT of
intestinal neutrophil recruitment in a mouse model of dextran
sulfate sodium-induced colitis. J Nucl Med. 46:526–531.
2005.PubMed/NCBI
|
|
3
|
Hyun JG, Lee G, Brown JB, et al:
Anti-interferon-inducible chemokine, CXCL10, reduces colitis by
impairing T helper-1 induction and recruitment in mice. Inflamm
Bowel Dis. 11:799–805. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rivera-Nieves J, Ho J, Bamias G, et al:
Antibody blockade of CCL25/CCR9 ameliorates early but not late
chronic murine ileitis. Gastroenterology. 131:1518–1529. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Farooq SM, Stillie R, Svensson M, Svanborg
C, Strieter RM and Stadnyk AW: Therapeutic effect of blocking CXCR2
on neutrophil recruitment and dextran sodium sulfate-induced
colitis. J Pharmacol Exp Ther. 329:123–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
MacDermott RP: Alterations of the mucosal
immune system in inflammatory bowel disease. J Gastroenterol.
31:907–916. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee HJ, Choi SC, Lee MH, et al: Increased
expression of MIP-3alpha/CCL20 in peripheral blood mononuclear
cells from patients with ulcerative colitis and its down-regulation
by sulfasalazine and glucocorticoid treatment. Inflamm Bowel Dis.
11:1070–1079. 2005. View Article : Google Scholar
|
|
8
|
ten Hove T, Drillenburg P, Wijnholds J, Te
Velde AA and van Deventer SJ: Differential susceptibility of
multidrug resistance protein-1 deficient mice to DSS and
TNBS-induced colitis. Dig Dis Sci. 47:2056–2063. 2002.PubMed/NCBI
|
|
9
|
te Velde AA, de Kort F, Sterrenburg E, et
al: Comparative analysis of colonic gene expression of three
experimental colitis models mimicking inflammatory bowel disease.
Inflamm Bowel Dis. 13:325–330. 2007.PubMed/NCBI
|
|
10
|
Murano M, Maemura K, Hirata I, et al:
Therapeutic effect of intracolonically administered nuclear factor
kappa B (p65) antisense oligonucleotide on mouse dextran sulphate
sodium (DSS)-induced colitis. Clin Exp Immunol. 120:51–58. 2000.
View Article : Google Scholar
|
|
11
|
Boirivant M, Fuss IJ, Ferroni L, De
Pascale M and Strober W: Oral administration of recombinant cholera
toxin subunit B inhibits IL-12-mediated murine experimental
(trinitrobenzene sulfonic acid) colitis. J Immunol. 166:3522–3532.
2001. View Article : Google Scholar
|
|
12
|
Koga H, Sakisaka S, Ohishi M, et al:
Expression of cyclooxygenase-2 in human hepatocellular carcinoma:
relevance to tumor dedifferentiation. Hepatology. 29:688–696. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Banks C, Bateman A, Payne R, Johnson P and
Sheron N: Chemokine expression in IBD. Mucosal chemokine expression
is unselectively increased in both ulcerative colitis and Crohn’s
disease. J Pathol. 199:28–35. 2003.PubMed/NCBI
|
|
14
|
Watanabe S, Yamakawa M, Hiroaki T, Kawata
S and Kimura O: Correlation of dendritic cell infiltration with
active crypt inflammation in ulcerative colitis. Clin Immunol.
122:288–297. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Uguccioni M, Gionchetti P, Robbiani DF, et
al: Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in
ulcerative colitis. Am J Pathol. 155:331–336. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
MacDermott RP, Sanderson IR and Reinecker
HC: The central role of chemokines (chemotactic cytokines) in the
immunopathogenesis of ulcerative colitis and Crohn’s disease.
Inflamm Bowel Dis. 4:54–67. 1998.PubMed/NCBI
|
|
17
|
Buanne P, Di Carlo E, Caputi L, et al:
Crucial pathophysiological role of CXCR2 in experimental ulcerative
colitis in mice. J Leukoc Biol. 82:1239–1246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zimmerman NP, Vongsa RA, Wendt MK and
Dwinell MB: Chemokines and chemokine receptors in mucosal
homeostasis at the intestinal epithelial barrier in inflammatory
bowel disease. Inflamm Bowel Dis. 14:1000–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sartor RB: Cytokines in intestinal
inflammation: pathophysiological and clinical considerations.
Gastroenterology. 106:533–539. 1994.PubMed/NCBI
|
|
20
|
Ashwood P, Harvey R, Verjee T,
Wolstencroft R, Thompson RP and Powell JJ: Functional interactions
between mucosal IL-1, IL-ra and TGF-beta 1 in ulcerative colitis.
Inflamm Res. 53:53–59. 2004.PubMed/NCBI
|
|
21
|
Izadpanah A, Dwinell MB, Eckmann L, Varki
NM and Kagnoff MF: Regulated MIP-3alpha/CCL20 production by human
intestinal epithelium: mechanism for modulating mucosal immunity.
Am J Physiol Gastrointest Liver Physiol. 280:G710–G719.
2001.PubMed/NCBI
|
|
22
|
Jin Y, Fuller L, Ciancio G, et al: Antigen
presentation and immune regulatory capacity of immature and
mature-enriched antigen presenting (dendritic) cells derived from
human bone marrow. Hum Immunol. 65:93–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaser A, Ludwiczek O, Holzmann S, et al:
Increased expression of CCL20 in human inflammatory bowel disease.
J Clin Immunol. 24:74–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He C, Zhang SL, Hu CJ, Tong DW and Li YZ:
Higher levels of CCL20 expression on peripheral blood mononuclear
cells of chinese patients with inflammatory bowel disease. Immunol
Invest. 39:16–26. 2010. View Article : Google Scholar : PubMed/NCBI
|