Introduction
In 1991 Van der Bruggen et al(1) isolated the first melanoma-associated
antigen gene (MAGE) from melanoma cells using gene
transfection. This gene, MAGE-A1, was identified as a testis
tumor antigen gene. To date, more than 30 MAGE family
members have been discovered, including MAGE-A, B,
C, D, E, F, G, H,
L2, Necidin, I and J. Proteins in the
MAGE family share an amino acid sequence containing a MAGE homology
domain (MHD) and may be subdivided into groups I and II according
to their protein expression patterns. Group I proteins, including
MAGEs A, B and C, are expressed in many types of tumor tissues, but
are not expressed in normal tissues, with the exception of placenta
and adult testis (2).
MAGE-A encodes a tumor-specific antigen that
is highly expressed (3–7) in the majority of tumor tissues.
Currently, the MAGE-A subfamily includes MAGE-A1 through A15. All
known genes of this subfamily are localized on chromosome Xq28.
Each has a length of approximately 4.5 kb, contains three exons and
encodes a protein approximately 309–319 amino acids long (369 amino
acids for MAGE-A10) (8). The
majority of the studies on this subfamily have focused on MAGE-A1
and MAGE-A3; particularly their involvement in tumorigenesis and
their potential as therapeutic targets. The expression patterns and
functions of most MAGE-A family members remain unclear, but it is
likely that some of them also promote tumor development. Indeed,
recent evidence demonstrates expression of both MAGE-A10 and
MAGE-A11 in breast tumors (9).
To clarify the expression and role of MAGE-A11 in
breast cancer, we compared MAGE-A11 protein expression by
immunohistochemistry in breast cancer tissues and tumor-adjacent
normal tissues. Further, MAGE-A11 expression was evaluated for
potential correlations with the clinicopathological features of
breast carcinomas. Additionally, we extended recent findings
(9) by transfecting breast cancer
cell lines with MAGE-A11 in order to determine the effect of its
expression on tumor proliferation.
Materials and methods
Research participants
Tissues were collected from 100 patients who were
undergoing surgery to remove breast tumors at The Affiliated
Hospital of Yancheng Health Vocational and Technical College,
Yancheng, China, between 2010 and 2011. Samples included cancer
tissues and corresponding tumor-adjacent tissues (>5 cm from
cancer tissue edge). Fine needle aspiration cytology (FNAC) and
frozen section (FS) were performed prior to surgery in order to
confirm cancer. Patients had not received any radiotherapy,
chemotherapy or endocrine therapy. The mean age of patients was
53.9±9.6 years, with ages ranging from 32–70 years. Samples
included 78 cases of invasive ductal carcinoma and 22 cases of
invasive lobular carcinoma; there were 4 cases of grade I, 69 cases
of grade II and 27 cases of grade III cancer. TNM staging was
performed according to the UICC in 1997: 16 cases were in stage I,
66 in stage II and 18 in stage III (10). Other tumor characteristics that
were evaluated included tumor volume (28 cases <2
cm3, 24 cases 2–5 cm3 and 48 cases >5
cm3) and metastasis (43 cases with lymph node
metastasis, 57 without). Additionally, expression of breast cancer
markers was investigated; these included estrogen receptor α (ER-α)
(66 cases were positive), ER-β (44 cases were positive), human
epidermal growth factor receptor 2 (HER-2) (51 cases were negative,
49 were positive), progestational hormone receptor (PR) (52 cases
were positive) and amplification in breast cancer 1 (AIB-1) (54
cases were positive).
Experimental methods
Materials
Human breast cancer MCF-7 cell strains were
purchased from Kunming Cell Bank (China Academy of Sciences).
Lipofectamine 2000 transfection kits were purchased from Invitrogen
(Carlsbad, CA, USA). Transfection plasmids (pCMV-AC-GFP) were
purchased from Origene Technologies, Inc. (Rockville, MD, USA).
Rabbit anti-human MAGE-A11 monoclonal antibodies were purchased
from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and
immunohistochemistry kits and DAB chromogenic agents were purchased
from Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing,
China). Rabbit anti-human MAGE-A11 polyclonal antibodies or rabbit
anti-human β-actin (internal reference) monoclonal antibodies were
purchased from Sigma (St. Louis, MO, USA). Electrochemistry
light-emitting reagents were from Thermo Scientific (Rockford, IL,
USA). The study was approved by the ethics committee of Yancheng
Health Vocational and Technical College, Yancheng, China.
Immunohistochemistry
MAGE-A11 expression was detected in breast cancer
sections by immunohistochemistry using the
streptavidin-biotin-horseradish peroxidase complex method (SP
method). Briefly, tissues were formalin-fixed, dehydrated and
paraffin-embedded for sectioning at 4 μm. Tissue slices were
dewaxed and rehydrated for antigen repair at a high temperature in
a microwave. Cooled sections were treated with 3% hydrogen peroxide
solution to block endogenous peroxidase activity, sealed with
non-specific serum, then placed in a wet box and incubated at room
temperature. Primary antibodies were added to the wet box, and
sections were incubated at 4°C overnight. Biotin-labeled rabbit
anti-human MAGE-A11 polyclonal antibodies were added as the
secondary antibody and placed at room temperature. Following three
washes in PBS, streptococcus avidin-peroxidase was added to
sections for incubation for 30 min at 37°C. After washing with PBS
three times, DAB chromogen was applied according to the
manufacturer’s instructions in order to develop color. Sections
were counterstained with hematoxylin, dehydrated and mounted. Known
positive tissue slices were used as a positive control and PBS was
used as a negative control in place of primary antibodies.
Sections were examined under a light microscope.
Protein staining appeared as yellow to brown granules in the
cytoplasm and nucleus. To assess protein expression, 10 visual
fields were selected at high power. Staining intensity was divided
into four classes: uncolored (a score of 0), pale yellow (a score
of 1), yellow (a score of 2) and brown-yellow (a score of 3).
Additionally, staining frequency was divided into five classes
according to the proportion of positively-stained cancer cells out
of all tumor cells: ≤5% positive was scored as 0, 6–25% was scored
as 1, 26–50% as 2, 51–75% as 3 and ≥76% as 4. Scores for staining
intensity and staining frequency were added for each case; total
scores of 0 were considered (−), total scores of 1–2 as (+), total
scores of 3–5 as (++) and total scores of 6–7 as (+++).
Cells and cell culture
Human breast cancer MCF-7 cells were cultured in
RPMI-1640 culture solution containing 10% fetal bovine serum, 100
U/ml penicillin, 100 μg/ml streptomycin and 8% NaHCO3 at
37°C under 5% CO2 conditions.
MAGE-A11 transfection
pCMV-AC-MAGE-A11-GFP expression plasmid and null
vector pCMV-AC-GFP (control) were transfected into MCF-7 cells
during exponential growth, according to the methods included with
the Lipofectamine 2000 transfection kits. Subsequent experiments
were performed 48 h later.
Western blotting
MCF-7 cells were collected 48 h following
transfection and washed twice with PBS. Suspensions were
centrifuged and supernatants were discarded. Cell pellets were
sonicated in order to quantify the total proteins with
bicinchoninic acid (BCA). Samples of total protein (30 μg/well)
were loaded on a 10% gel for SDS-PAGE. Proteins were transferred to
polyvinylidene difluoro ethylene film (PVDF). The membrane was
washed with 5% non-fat milk powder at 4°C overnight. Rabbit
anti-human MAGE-A11 polyclonal antibody (1:1000) or rabbit
anti-human β-actin (reference control) monoclonal antibody were
added and incubated with the membrane at room temperature for 1 h.
After the membrane was washed three times in PBS, 1:3000
horseradish peroxidase-labeled anti-rabbit secondary antibody was
added at room temperature for 1 h. Additional PBS washes were
performed prior to the addition of the electrochemical luminescence
reagent. Infrared fluorescence scan imagery was used to develop and
analyze blots. The relative molecular mass of MAGE-A11 protein
carrying green fluorescent protein (GFP) labels was
7×104. The experiment was repeated three times.
MTT colorimetry
MCF-7 cells were inoculated in 6 cm petri dishes. At
60% confluence, cells were transfected with the
pCMV-AC-MAGE-A11-GFP expression plasmid or null vector
(pCMV-AC-GFP) as mentioned previously. Forty-eight hours after
transfection, cells were inoculated in 96-well plates at a density
of 5000 cells/well and incubated overnight to promote adherence.
Adherent cells were continuously cultured for 0, 24 or 48 h; at the
end of each time point, 10 μl of MTT (100 mg/ml) was added per
well. Subsequently, absorbance (OD) values were measured for each
well at a 490-nm wavelength using a Thermo Labsystems colorimeter.
Proliferation rates were calculated 24 and 48 h after cell
adherence according to the following formula: cellular
proliferation rate = average OD value in experimental group/average
D value of 0 h group × 100%. The experiment was repeated three
times.
Colony-forming assay
MCF-7 cells were inoculated in 6-cm petri dishes. At
60% confluence, cells were transfected with pCMV-AC-MAGE-A11-GFP
expression plasmid or the null vector pCMV-AC-GFP, as mentioned
previously. Forty-eight hours later, cells were inoculated in 10-cm
petri dishes at a density of 3000 cells/dish and incubated
overnight for adherence. G418 was added at a final concentration of
600 pg/ml for selection; the solution was replaced once a week. Two
weeks later, Wright-Giemsa staining was performed and cell colonies
(diameter >1 mm) were counted. The experiment was repeated three
times with three culture dishes per group; the mean number of
colonies was determined.
Statistical methods
SPSS 17.0 statistical software was used for
statistical analysis. A χ2 test was used to compare
expression of MAGE-A11 protein; for measurement data, two-tailed
Student’s t-test was used. P<0.05 was considered to indicate a
statistically significant difference.
Results
MAGE-A11 expression in breast cancer and
corresponding tumor-adjacent tissue
Immunohistochemistry of MAGE-A11 in breast cancer
and normal adjacent tissues showed that, of 100 cases, the protein
was expressed in 56.0% of breast cancer samples and 0.0% of normal
adjacent tissues (Table I). This
difference in expression rate was significantly different
(P<0.05).
 | Table IExpression of MAGE-A11 in breast
cancer and adjacent normal tissue [n (%)]. |
Table I
Expression of MAGE-A11 in breast
cancer and adjacent normal tissue [n (%)].
| Groups | n | − | + | ++ | +++ |
|---|
| Breast cancer
tissues | 100 | 44 (44.0) | 23 (23.0) | 20 (20.0) | 13 (13.0) |
| Adjacent normal
tissues | 100 | 100 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 200 | 144 (72.0) | 23 (11.5) | 20 (10.0) | 13 (6.5) |
MAGE-A11 expression and
clinicopathological features of breast cancer
MAGE-A11 expression in breast tumors was related to
expression of both HER-2 and ER-β (P<0.05), but there were no
correlations with patient age, pathology, differentiation, clinical
staging, tumor size or lymphatic metastasis, or ER-α, PR or AIB-1
expression (Table II).
 | Table IICorrelation between expression of
MAGE-A11 protein and clinicopathological parameters in breast
cancer [n (%)]. |
Table II
Correlation between expression of
MAGE-A11 protein and clinicopathological parameters in breast
cancer [n (%)].
| Clinicopathological
characteristics | n | − | + | ++ | +++ | χ2 | P-value |
|---|
| Age (years) |
| <55 | 53 | 26 (49.1) | 12 (22.6) | 7 (13.2) | 8 (15.1) | 3.643 | 0.303 |
| ≥55 | 47 | 18 (38.3) | 11 (23.4) | 13 (27.7) | 5 (10.6) | | |
| Pathological
type |
| Invasive ductal
carcinoma | 78 | 34 (43.6) | 17 (21.8) | 16 (20.5) | 11 (14.1) | 0.616 | 0.893 |
| Lobular
carcinoma | 22 | 10 (45.5) | 6 (27.3) | 4 (18.2) | 2 (9.1) | | |
| Histological
grade |
| Grade I | 4 | 4 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8.159 | 0.227 |
| Grade II | 69 | 31 (44.9) | 17 (24.6) | 14 (20.3) | 7 (10.1) | | |
| Grade III | 27 | 9 (33.3) | 6 (22.2) | 6 (22.2) | 6 (22.2) | | |
| TNM staging |
| Stage I | 16 | 10 (62.5) | 3 (18.8) | 3 (18.8) | 0 (0.0) | 5.089 | 0.532 |
| Stage II | 66 | 28 (42.4) | 16 (24.2) | 12 (18.2) | 10 (15.2) | | |
| Stage III | 18 | 6 (33.3) | 5 (22.2) | 5 (27.8) | 3 (16.7) | | |
| Carcinoma diameter
(cm3) |
| ≤2 | 28 | 14 (50.0) | 5 (17.9) | 5 (17.9) | 4 (14.3) | 1.359 | 0.968 |
| 2–5 | 24 | 11 (45.8) | 6 (25.0) | 4 (16.7) | 3 (12.5) | | |
| ≥5 | 48 | 19 (39.6) | 12 (25.0) | 11 (22.9) | 6 (12.5) | | |
| Lymph node
metastasis |
| Yes | 34 | 15 (44.1) | 9 (26.5) | 6 (17.6) | 4 (11.8) | 1.177 | 0.759 |
| No | 66 | 29 (43.9) | 14 (21.2) | 14 (21.2) | 9 (13.6) | | |
| ER-α |
| Negative | 34 | 10 (40.0) | 10 (40.0) | 3 (12.0) | 2 (8.0) | 0.473 | 0.925 |
| Positive | 66 | 2 (13.3) | 3 (20.0) | 4 (26.7) | 6 (40.0) | | |
| ER-β |
| Negative | 56 | 35 (62.5) | 13 (23.2) | 5 (8.9) | 3 (5.4) | 23.421 | 0.001 |
| Positive | 44 | 9 (20.5) | 10 (22.7) | 15 (34.1) | 10 (22.7) | | |
| HER-2 |
| Negative | 51 | 34 (66.7) | 12 (23.5) | 5 (9.8) | 0 (0.0) | 31.107 | 0.001 |
| Positive | 49 | 10 (20.4) | 11 (22.4) | 15 (30.6) | 13 (26.5) | | |
| PR |
| Negative | 48 | 19 (39.6) | 11 (22.9) | 11 (22.9) | 7 (14.6) | 0.980 | 0.806 |
| Positive | 52 | 25 (48.1) | 12 (23.1) | 9 (17.3) | 6 (11.5) | | |
| AIB-1 |
| Negative | 46 | 16 (34.8) | 10 (21.7) | 14 (30.4) | 6 (13.0) | 6.342 | 0.096 |
| Positive | 54 | 28 (51.9) | 13 (24.1) | 6 (11.1) | 7 (13.0) | | |
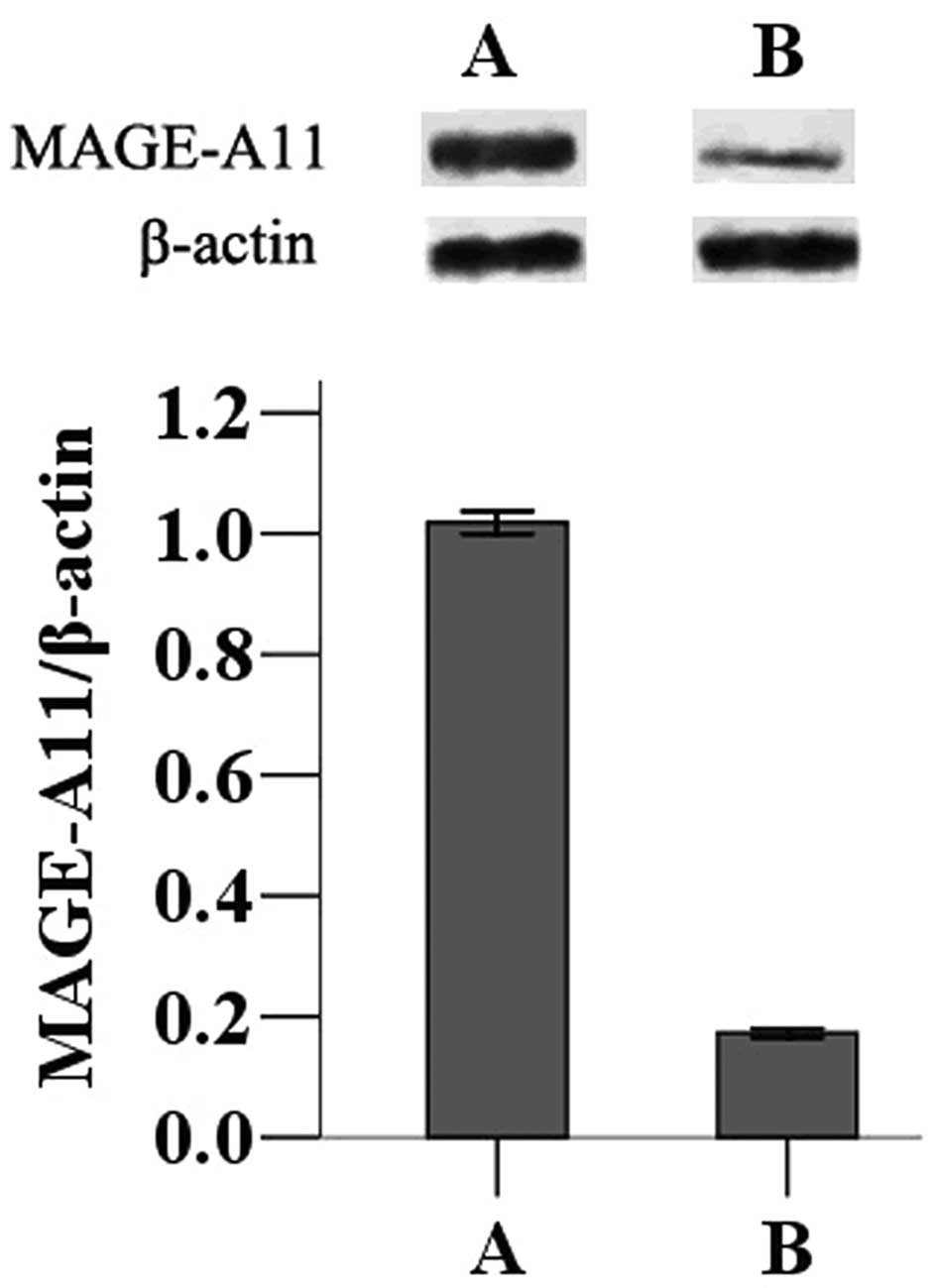
Effect of MAGE-A11 in transfected MCF-7
cells
The MAGE-A11 gene was transfected into MCF-7 cells
to determine the effects of its expression on tumor progression.
Following transfection of the pCMV-AC-MAGE-A11-GFP expression
plasmid or the pCMV-AC-GFP control plasmid, western blot analysis
was used to measure expression in MCF-7 cells. Cells transfected
with pCMV-AC-MAGE-A11-GFP displayed higher expression of MAGE-A11
than those transfected with the control plasmid (Fig. 1).
Subsequently, MTT colorimetry was used to determine
proliferation rates of MCF-7 cells transfected with either the
MAGE-A11 expression construct or the control plasmid. Transfection
of pCMV-AC-MAGE-A11-GFP expression plasmid resulted in a
significantly higher proliferation rate than did transfection of
the null vector (P<0.05). Furthermore, a colony-forming assay
demonstrated that, after transfecting the pCMV-AC-MAGE-A11-GFP
expression plasmid, MCF-7 cells formed significantly more colonies
than cells not overexpressing MAGE-A11 (Fig. 2; P<0.05).
Discussion
Many studies have described the expression of MAGE-A
family members in tumors. However, the majority of these have
relied on RT-PCR, gene microarray and RNA in situ
hybridization (11,12), as the detection of specific protein
members of this family is difficult. High conservation of protein
sequences among MAGE-A family members creates challenges in the
development of specific antibodies. The availability of a specific
antibody for MAGE-A11 allowed us to perform protein expression
studies of this family member.
We found that the majority of breast cancers
expressed MAGE-A11, while none of the normal adjacent breast
tissues expressed the protein. Therefore, MAGE-A11, similarly to
other MAGEs, appears to be a tumor-specific antigen in breast
cancer. Additionally, the expression of MAGE-A11 within breast
tumors was correlated with the expression of both HER-2 and ER-β.
These results confirm recent findings (9). HER-2 expression is an important index
of poor prognosis for breast cancer patients (13), therefore, MAGE-A11 expression may
also indicate poorer prognosis for breast cancer patients.
Similarly, ER is commonly used as diagnostic indicator for, and may
help to guide the treatment of, breast cancer patients. For
example, patients with ER-positive tumors often receive tamoxifen
endocrine therapy (14). The
correlation of ER-β expression with MAGE-A11 expression may
indicate the usefulness of MAGE-A11 as a diagnostic indicator.
However, the lack of correlation between MAGE-A11 and ER-α
expression requires further research.
Our study builds on the previous report of MAGE-A11
expression in breast tumors by evaluating the effects of MAGE-A11
overexpression in breast cancer cells. Transfection of a
GFP-MAGE-A11 construct into MCF-7 breast cancer cells resulted in
increased proliferation of cells and higher rates of
colony-formation. Previous research may hint at the mechanism by
which MAGE-A11 promotes tumor cell proliferation. MAGE-A11
reportedly is capable of combining with a specific amino acid
radical sequence FXXLF at the amino terminus of the androgen
receptor (AR), increasing AR transcriptional activity (15) by phosphorylation and ubiquitination
of the epithelial growth factor-dependent MAGE-A11. Overexpression
of MAGE-A11 appears to promote the growth of prostate cancer by
AR-dependent cell proliferation (16). A similar phenomenon may occur in
breast and other tumors.
In conclusion, in breast cancer tissue expression of
MAGE-A11 protein is related to the expression of HER-2 and ER-β.
Expression of this tumor-specific antigen promotes proliferation of
human breast cancer cells in vitro. These results indicate
that MAGE-A11 may be a new treatment target for breast cancer due
to its effects on the occurrence and development of breast
cancer.
References
|
1
|
Van der Bruggen P, Traversari C, Chomez P,
Lurquin C, De Plaen E, Van den Eynde BJ, Knuth A and Boon T: A gene
encoding an antigen recognized by cytolytic T lymphocytes on a
human melanoma. Science. 254:1643–1647. 1991.
|
|
2
|
Ohman Forslund K and Nordqvist K: The
melanoma antigen genes - any clues to their functions in normal
tissues? Exp Cell Res. 265:185–194. 2001.PubMed/NCBI
|
|
3
|
Peikert T, Specks U, Farver C, Erzurum SC
and Comhair SA: Melanoma antigen A4 is expressed in non-small cell
lung cancers and promotes apoptosis. Cancer Res. 66:4693–4700.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bergeron A, Picard V, LaRue H, Harel F,
Hovington H, Lacombe L and Fradet Y: High frequency of MAGE-A4 and
MAGE-A9 expression in high-risk bladder cancer. Int J Cancer.
125:1365–1371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bellati F, Napoletano C, Tarquini E,
Palaia I, Landi R, Manci N, Spagnoli G, Rughetti A, Panici PB and
Nuti M: Cancer testis antigen expression in primary and recurrent
vulvar cancer: association with prognostic factors. Eur J Cancer.
43:2621–2627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim KH, Choi JS, Kim IJ, Ku JL and Park
JG: Promoter hypomethylation and reactivation of MAGE-A1 and
MAGE-A3 genes in colorectal cancer cell lines and cancer tissues.
World J Gastroenterol. 12:5651–5657. 2006.PubMed/NCBI
|
|
7
|
Napoletano C, Bellati F, Tarquini E, et
al: MAGE-A and NY-ESO-1 expression in cervical cancer: prognostic
factors and effects of chemotherapy. Am J Obstet Gynecol.
198:99.e1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vatolin S, Abdullaev Z, Pack SD, et al:
Conditional expression of the CTCF-paralogous transcriptional
factor BORIS in normal cells results in demethylation and
derepression of MAGE-A, and reactivation of other
cancer-testisgenes. Cancer Res. 65:7751–7762. 2005.
|
|
9
|
Lian Y, Sang M, Ding C, Zhou X, Fan X, Xu
Y, Lu W and Shan B: Expressions of MAGE-A10 and MAGE-A11 in breast
cancers and their prognostic significance: a retrospective clinical
study. J Cancer Res Clin Oncol. 138:519–527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto H, Morimoto T, Sano M, Fukuda M,
Ueno E, Tajima T and Iwase T: Proposals for revisions of UICC TNM
system (1997) - breast cancer. Gan To Kagaku Ryoho. 25:1087–1093.
1998.(In Japanese).
|
|
11
|
Sugita M, Geraci M, Gao B, et al: Combined
use of oligonucleotide and tissue microarrays identifies
cancer/testis antigens as biomarkers in lung carcinoma. Cancer Res.
62:3971–3979. 2002.PubMed/NCBI
|
|
12
|
Kufer P, Zippelius A, Lutterbüse R, et al:
Heterogeneous expression of MAGE-A genes in occult disseminated
tumor cells: a novel multimarker reverse transcription-polymerase
chain reaction for diagnosis of micrometastatic disease. Cancer
Res. 62:251–261. 2002.
|
|
13
|
Demonty G, Bernard-Marty C, Puglisi F,
Mancini I and Piccart M: Progress and new standards of care in the
management of HER-2 positive breast cancer. Eur J Cancer.
43:497–509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Green CA, Peter MB, Speirs V and Shaaban
AM: The potential role of ER beta isoforms in the clinical
management of breast cancer. Histopathology. 53:374–380. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bai S, He B and Wilson EM: Melanoma
antigen gene protein MAGE-11 regulates androgen receptor function
by modulating the interdomain interaction. Mol Cell Biol.
25:1238–1257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bai S and Wilson EM:
Epidermal-growth-factor-dependent phosphorylation and
ubiquitinylation of MAGE-11 regulates its interaction with the
androgen receptor. Mol Cell Biol. 28:1947–1963. 2008. View Article : Google Scholar : PubMed/NCBI
|