Introduction
Platelet-rich plasma (PRP) is a blood-derived
fraction containing high concentrations of platelets and growth
factors (1). Application of
autologous PRP has been reported to facilitate wound healing in
several fields, including facilitating bone proliferation in
orthopedic surgery, regenerating periodontal ligaments and
accelerating the wound healing process in diabetic ulcers (2–4).
Therefore, in this study, we applied autologous PRP not only to
surgical wounds, but also to various forms of ulcers.
Wound healing processes are composed of coagulation,
inflammation, migration/proliferation and remodeling phases
(5,6). The migration/proliferation of
keratinocytes is the key step in accelerating wound healing
(7,8). Cell migration/proliferation is
precisely regulated by various cell cycle regulatory proteins
(9,10). Positive cell cycle regulatory
proteins are cyclin D1, cyclin E, cyclin A and their kinase
partners, cyclin-dependent protein kinases (CDKs). Among these,
cyclin D1-CDK4/6 and cyclin E-CDK2 are the key positive regulatory
proteins in the progression of the G1/S transition phase and cyclin
A-CDK2 is important in the G2/M transition phase. Cells in chronic
skin ulcers show low cell proliferation rates through the cell
cycle and cell cycle arrest, resulting in cellular senescence.
Chronic wounds, such as diabetic foot ulcers and venous leg ulcers
show impairment of proliferation and migration of keratinocytes
into the wound, which result in delayed epithelization (8,11,12).
We have previously reported that PRP treatment induced accelerated
proliferation and migration of fibroblasts through upregulation of
cyclin E and CDK4, which is important in cell migration and
proliferation (13). However, the
effect of PRP treatment on migration and proliferation of the HaCaT
keratinocyte cell line and its clinical effectiveness in acute and
chronic ulcers remain under investigation.
In this study, we investigated the effect of PRP on
cell migration, proliferation, and expression of cell cycle
regulatory proteins in HaCaT cells and the clinical improvement of
PRP-treated ulcers.
Materials and methods
Materials
Antibodies against cell cycle regulatory proteins
(CDK2, CDK4, cyclin A, cyclin D1 and cyclin E) were obtained from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-RB antibody
was purchased from Pharmingen (BD Biosciences, San Jose, CA,
USA).
Patients
Participants with acute and chronic ulcers were
recruited through the dermatology clinic. The recruitment period
was between January 2010 and December 2010 and the study was
completed in March 2011. Exclusion criteria included the presence
of active infection, compromised immune function and coagulation
disorders. These processes were approved by the institutional
review board of Keimyung University and DongSan Hospital. Written
informed patient consent was obtained from the patient.
In total, 16 patients (means ± SD age, 60±13, 39–83
years) with various ulcers, including traumatic ulcer, livedoid
vasculitis, stasis ulcer, venous leg ulcer, burn ulcer and pressure
ulcer, were included. All patients had ulcers involving different
body parts: lower leg (7 patients), toe (4), sole (2), heel (1), hand dorsum (1) and finger (1). Ulcer grade was grade 2 (14 patients)
or grade 3 (2 patients).
PRP preparation
PRP was prepared from the patient’s own blood. A
small volume of blood (12 ml) was collected using a commercial kit
(MyCells Autologous Preparation kit®, Holon, Israel)
according to the manufacturer’s instructions.
PRP application on wound
Activated PRP was applied to the wound as a liquid
or gel form according to wound size, location and condition. Prior
to the application of PRP, necrotic tissues or eschars were
debrided using a scalpel and images were captured with a digital
camera (450D; Canon, Tokyo, Japan). Following PRP application, a
foam dressing (Allevin®, Smith and Nephew, Huntingdon,
UK) was placed on the ulcer for 48 h. Time intervals between
treatments varied from twice a week to once a week. The number of
treatments differed depending on the wound state. After each
treatment, the same dermatologist assessed clinical improvement and
epithelization rate. Overall degree of ulcer improvement was
assessed every week until complete epithelization occurred.
Cell cultures
HaCaT keratinocyte cell lines were maintained at
37°C in a humidified atmosphere of 95% air and 5% CO2 in
DMEM supplemented with 10% heat inactivated fetal bovine serum, 2
mM glutamine, 100 U/ml penicillin and 100 g/ml streptomycin. For
the experiments, cells (5×104 cells/ml) were seeded in a
culture dish and maintained in a tissue culture incubator.
Cell proliferation assay
HaCaT cells were seeded at a density of
15×104 cells/well in 6-well culture plates. The cells
were cultured using serum-free DMEM supplemented with 0 (control),
0.05, 0.5, 1 or 2% activated PRP for 3 days. The cultured cells
were assayed for proliferation using the trypan blue exclusion
method. Briefly, cells were washed with phosphate-buffered saline
and stained with trypan blue dye. Viable cells were observed as
light reflecting cells, but dead cells were observed as dark cells.
The numbers of viable cells were measured under an inverted phase
contrast microscope.
Migration assay
For the measurement of cell migration, confluent
keratinocytes, which were kept in serum-free medium for 24 h, were
wounded with a plastic micropipette tip. After washing, the medium
was replaced by serum-free medium or serum-free medium supplemented
with 0.01, 0.05, 0.5, 1 or 2% activated PRP for 24 h. Images of the
wounded area were taken every 24 h by phase-contrast microscopy
under crystal violet staining.
Western blot analysis
Whole cell extracts were prepared in lysis buffer
[10 mM Tris (pH 7.4), 5 mM EDTA, 130 mM NaCl, 1% Triton X-100,
phenylmethylsulphonyl fluoride (PMSF, 10 g/ml), aprotinin (10
g/ml), leupeptin (10 g/ml), 5 mM phenanthroline and 28 mM
benzamidine-HCl]. The protein concentration of extracts was
estimated with Bradford reagent (Bio-Rad Laboratories, Hercules,
CA, USA) using bovine serum albumin as the standard. Equal amounts
of protein (40 μg/lane) were resolved by 6.5–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto a
nitrocellulose membrane. The membrane was then washed with
Tris-buffered saline (10 mM Tris, 150 mM NaCl) containing 0.05%
Tween-20 (TBST) and blocked in TBST containing 5% non-fat dried
milk. The membrane was further incubated with respective specific
antibodies. The membrane was continuously incubated with
appropriate secondary antibodies coupled to horseradish peroxidase
and developed in the ECL Western detection reagents (Amersham
Pharmacia Biotech, Piscataway, NJ, USA).
Results
Effect of PRP on cell
migration/proliferation rates of the HaCaT keratinocyte cell
line
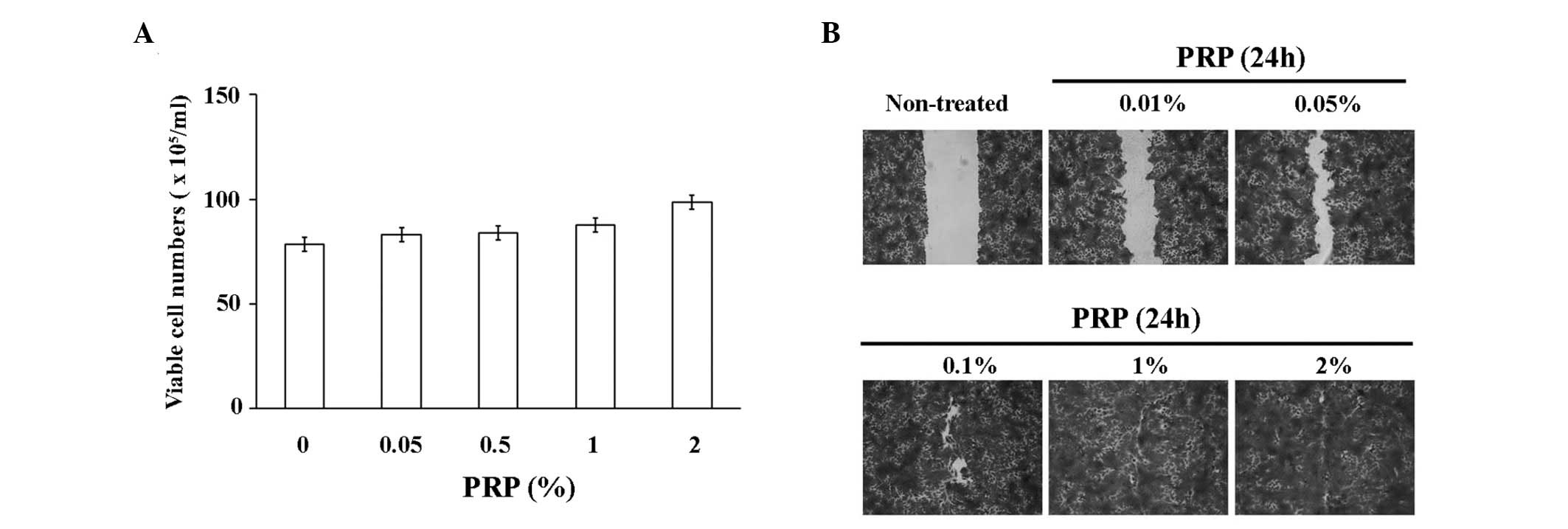
We analyzed the effect of PRP on migration and
proliferation rates of the HaCaT keratinocyte cell line. As shown
in Fig. 1A, PRP treatment resulted
in a dose-dependent increase of the proliferation rates of HaCaT
cells. In addition, PRP treatment resulted in markedly increased
rates of migration of HaCaT cells, even with a low concentration of
PRP (0.005–0.5%; Fig. 1B).
Expression of cell cycle regulatory
proteins in the HaCaT keratinocyte cell line by PRP treatment
Cell cycle regulatory proteins are important in
proliferation and migration of HaCaT keratinocyte cell lines. We
have investigated the question of whether PRP treatment promotes
the proliferative and migratory activities of HaCaT cells through
upregulation of G1/S or G2/M transition regulatory proteins. In
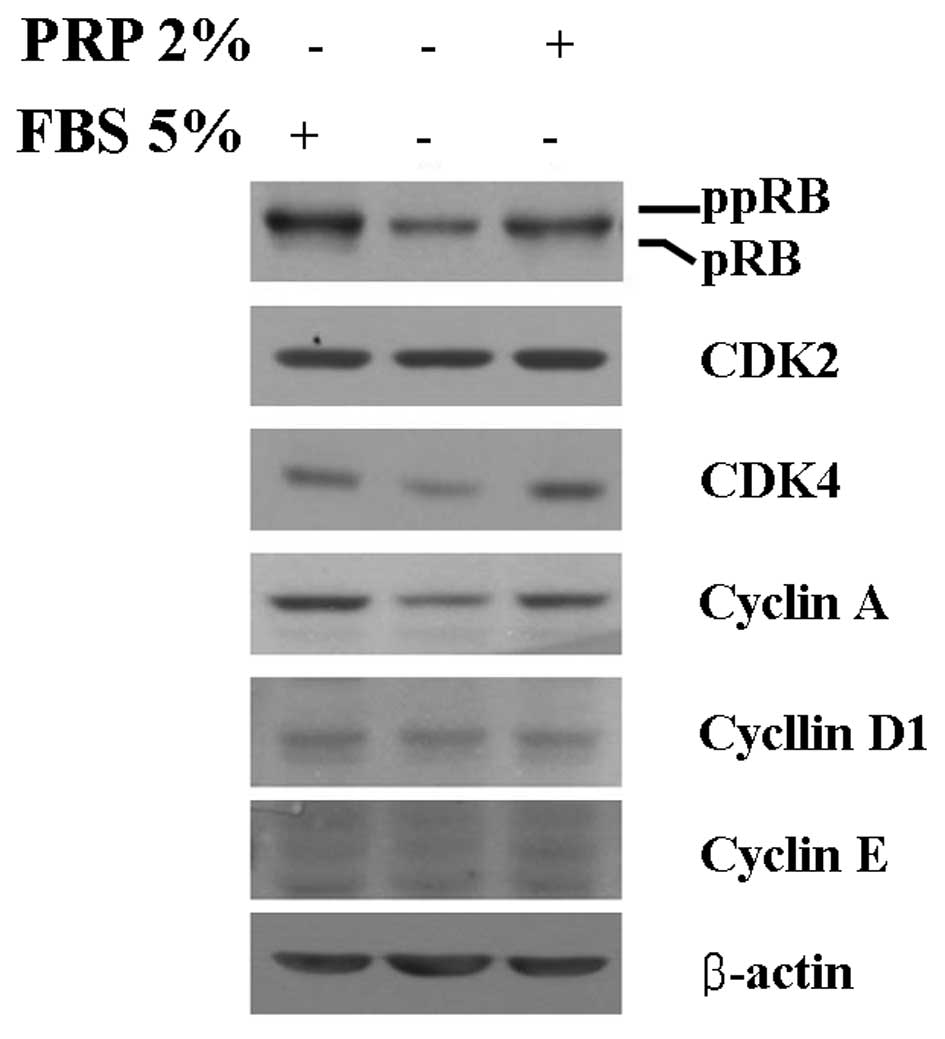
order to determine the basal expression levels of cell cycle
regulatory proteins in HaCaT cells, cells were cultured without
fetal bovine serum (FBS) for 3 days. After serum starvation for 3
days, cells were cultured under 5% FBS or 2% (v/v) PRP with DMEM
(Fig. 2). Expression levels of
G1/S transition regulatory proteins, namely, ppRb, CDK4 and cyclin
A, were decreased in serum-starved HaCaT cells. Notably, 2% PRP
induced increased expression of cyclin A and CDK4 in HaCaT cells,
resulting in increased expression of ppRb. Markedly increased
expression of cyclin D1, cyclin E and CDK4 was not observed.
Wound healing effect of PRP on acute and
chronic skin ulcers
Based on the laboratory data that support the
enhancing effect of PRP on wound healing, we performed a clinical
application of PRP to acute and chronic wounds and evaluated its
clinical wound healing effect. The clinical characteristics and
outcomes of patients are summarized in Table I.
 | Table IList of PRP-treated acute and chronic
patients. |
Table I
List of PRP-treated acute and chronic
patients.
| No. | Age/gender | Diagnosis | Duration of disease
(months) | Site | Ulcer stage | Days of
epithelialization (completeness, %) |
|---|
| 1 | 55/F | Stasis ulcer | 36 | Lt lower leg | II | 24 (90) |
| 2 | 62/M | Stasis ulcer | 2 | Lt ant, shin | II | 24 (90) |
| 3 | 63/M | DM foot ulcer | 12 | Lt toe | II | 5 (60) |
| 4 | 51/M | DM foot ulcer | 18 | Lt sole | III | 20 (95) |
| 5 | 47/F | DM foot ulcer | 2 | Lt heel | II | 22 (90) |
| 6 | 63/M | DM foot ulcer | 4 | Rt 1st toe tip | II | 6 (90) |
| 7 | 63/F | Venous leg ulcer | 7 | Lt med malleolus | II | 20 (95) |
| 8 | 69/F | Traumatic ulcer | 5 | Rt lower leg | II | 20 (70) |
| 9 | 66/F | Traumatic ulcer | 2 | Rt thumb | II | 7 (90) |
| 10 | 39/M | Livedoid
vasculitis | 2 | Lt med malleolus | II | 21 (100) |
| 11 | 83/M | Claw’s foot | 4 | MIP joint, 3rd
toe | II | 23 (95) |
| 12 | 69/M | Dehiscence | 1 | Rt sole | III | 4 (80) |
| 13 | 60/M | Open wound | 2 | Rt ant shin | II | 17 (85) |
| 14 | 44/M | Pressure ulcer | 2 | Lt lat malleolus | II | 35 (100) |
| 15 | 73/M | Burn | 2 | Hand | II | 10 (90) |
| 16 | 54/M | Burn | 3 | Lt toe and heel | II | 30 (100) |
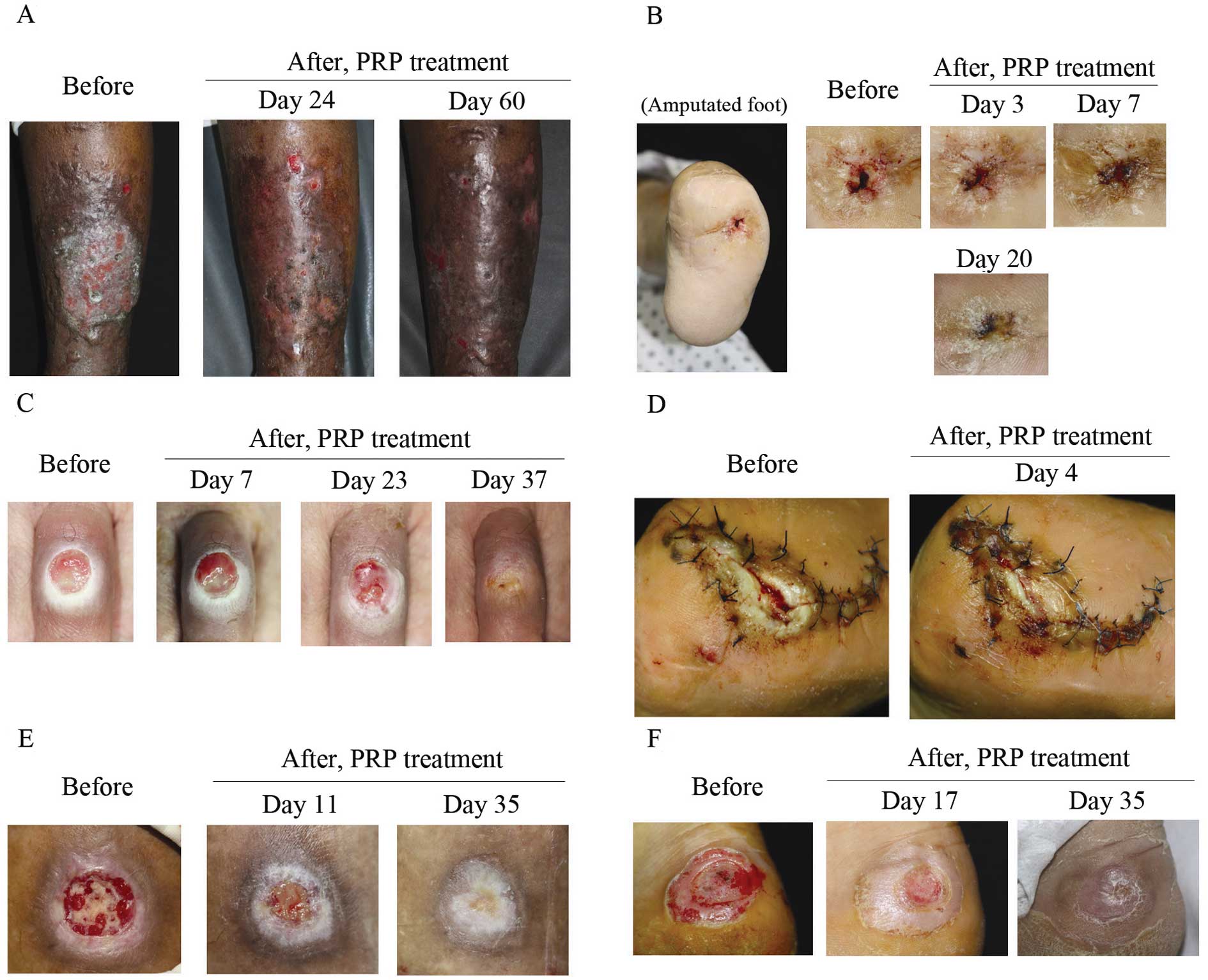
Eleven patients with chronic ulcers (cases 1–11)
presented with stasis ulcer, diabetic ulcer, venous leg ulcer,
livedoid vasculitis, claw foot and traumatic ulcer. The mean period
of the disease was 8.54 months. However, in 15.18 days, 9 patients
showed 90–100% epithelization. Case 2 presented with large-sized
stasis ulcers and had been refractory to standard treatment for 2
months. However, almost a complete epithelization was shown in 24
days after 3 PRP treatments at 3-day intervals (Fig. 3A). In case 4, the patient had been
suffering from a penetrating non-adhesion deep ulcer on an
amputated foot for 18 months (Fig.
3B). The wound had not been healed by sutures. Thus, we applied
PRP gel (6 times) on the ulcer and observed a complete
epithelization 20 days after treatment. In case 11, a round-shaped
ulcer on the middle interphalangeal joint of the left 3rd toe
following corn treatment using cryotherapy (Fig. 3C) also showed epithelization in 23
days after treatment 3 times with PRP gel solution.
In 5 patients with acute ulcers (cases 12–16), an
80–100% epithelization rate was achieved in 20 days. These wounds
had occurred for an average of 2 months, including special
indications such as dehiscence (case 12, Fig. 3D), open wound, pressure ulcer (case
14, Fig. 3E) and burn wound (case
16, Fig. 3F). Three months after
the treatment, the majority of the patients (90%), including acute
and chronic patients, evaluated the ulcer appearance as having good
to excellent improvement and the remaining patients (10%) rated the
improvement as moderate.
Discussion
Platelet-rich plasma (PRP) is an autologous
preparation of platelets concentrated in plasma. PRP contains
>30 bioactive proteins including PDGF, TGF-β, VEGF, EGF and
bFGF, which have fundamental roles in wound healing (14). These factors are known to regulate
processes such as cell migration, attachment, proliferation and
differentiation, and promote extracellular matrix (ECM)
accumulation by binding to specific cell surface receptors
(15). Thus, autologous PRP
therapy may be a promising agent for acceleration of the wound
healing process through high concentrations of growth factors.
Keratinocytes are important in epithelization during
the wound healing process (8,16).
In a number of studies, critical defects were found in epithelial
cell migration in chronic skin ulcers. Promoting epithelial cell
migration enhances wound healing not only in chronic skin ulcers
but also in acute ulcers. In this study, we attempted to
investigate the effect of PRP on keratinocyte activation,
migration, proliferation and expression of cell cycle regulatory
proteins. Our data clearly indicated that even an extremely low
concentration (0.5%) of PRP accelerated the migration of HaCaT
cells. In addition, cell proliferation rates of PRP-treated HaCaT
cells were increased by the upregulation of CDK4 and cyclin A
expression. Thus, PRP-induced keratinocyte migration and cell
proliferation contribute to the rapid wound healing process in
chronic and acute wounds. In our previous study, we identified
PRP-induced cell cycle promotion in human skin fibroblasts, which
is indicative of the wound healing effect of chronic ulcers
(13). Thus, PRP exerts cell cycle
progression in keratinocytes and fibroblasts.
In this study, we investigated the effect of PRP on
keratinocyte migration and proliferation, key steps for wound
healing in vitro. It is important to note that its clinical
effects are not only on chronic ulcers, but also apply to various
ulcers such as venous leg ulcer, stasis dermatitis, burn ulcer and
acute traumatic ulcer. Clear effects of PRP enhancing
re-epithelization of chronic and acute wounds have also been
revealed. In the patients, periods of epithelization were shortened
from the expected time through enhanced migration and proliferation
of keratinocytes by PRP.
We have demonstrated that PRP treatment induced
increased rates of cell proliferation and cell migration of HaCaT
cells, and showed 90–100% epithelization in 15.18 days in chronic
ulcers, and 80–100% epithelization was achieved between 4 to 20
days in acute ulcers. Promotion of wound healing in the skin by
shortening the epithelization process occurs through upregulation
of cyclin A and CDK4 expression in cells, thereby promoting
proliferation and migration of keratinocytes.
Acknowledgements
This study was supported by the Research Promoting
Grant from the Keimyung University Dongsan Medical Center in
2011.
References
|
1
|
Arora NS, Ramanayake T, Ren YF and Romanos
GE: Platelet-rich plasma: a literature review. Implant Dent.
18:303–310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Azzena B, Mazzoleni F, Abatangelo G, Zavan
B and Vindigni V: Autologous platelet-rich plasma as an adipocyte
in vivo delivery system: case report. Aesthetic Plast Surg.
32:155–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klaassen MA and Pietrzak WS: Platelet-rich
plasma application and heterotopic bone formation following total
hip arthroplasty. J Invest Surg. 24:257–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akingboye AA, Giddins S, Gamston P, Tucker
A, Navsaria H and Kyriakides C: Application of autologous
derived-platelet rich plasma gel in the treatment of chronic wound
ulcer: diabetic foot ulcer. J Extra Corpor Technol. 42:20–29.
2010.PubMed/NCBI
|
|
5
|
Behm B, Babilas P, Landthaler M and
Schreml S: Cytokines, chemokines and growth factors in wound
healing. J Eur Acad Dermatol Venereol. 26:812–820. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: Growth factors and cytokines in wound
healing. Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang X, Teng Y, Hou N, Fan X, Cheng X, Li
J, Wang L, Wang Y, Wu X and Yang X: Delayed re-epithelialization in
Ppm1a gene-deficient mice is mediated by enhanced activation of
Smad2. J Biol Chem. 286:42267–42273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raja, Sivamani K, Garcia MS and Isseroff
RR: Wound re-epithelialization: modulating keratinocyte migration
in wound healing. Front Biosci. 12:2849–2868. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neganova I and Lako M: G1 to S phase cell
cycle transition in somatic and embryonic stem cells. J Anat.
213:30–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Donjerkovic D and Scott DW: Regulation of
the G1 phase of the mammalian cell cycle. Cell Res. 10:1–16. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Henderson EA: The potential effect of
fibroblast senescence on wound healing and the chronic wound
environment. J Wound Care. 15:315–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suter MM, Schulze K, Bergman W, Welle M,
Roosje P and Muller EJ: The keratinocyte in epidermal renewal and
defence. Vet Dermatol. 20:515–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho JW, Kim SA and Lee KS: Platelet-rich
plasma induces increased expression of G1 cell cycle regulators,
type I collagen, and matrix metalloproteinase-1 in human skin
fibroblasts. Int J Mol Med. 29:32–36. 2012.PubMed/NCBI
|
|
14
|
Prakash S and Thakur A: Platelet
concentrates: past, present and future. J Maxillofac Oral Sur.
10:45–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ueda M and Nishino Y: Cell-based cytokine
therapy for skin rejuvenation. J Craniofac Surg. 21:1861–1866.
2010. View Article : Google Scholar
|
|
16
|
Pullar CE and Isseroff RR: Cyclic AMP
mediates keratinocyte directional migration in an electric field. J
Cell Sci. 118:2023–2034. 2005. View Article : Google Scholar : PubMed/NCBI
|