Introduction
Intervertebral disc (IVD) degeneration is a common
clinical problem that may result in low back pain and physical
disability. Progressive loss of proteoglycans and disc dehydration,
the major pathological characteristics of disc degeneration, may
lead to alterations in disc structure and impaired disc function.
The outer third of the IVD consists of the annulus fibrosus and
nucleus pulposus. The latter is rich in proteoglycans, which are
necessary for the normal function of the IVD. Recent findings have
demonstrated that the number of nucleus pulposus cells is reduced
and the composition of the extracellular matrix (ECM) associated
with these cells is altered in degenerated discs (1–3).
Interleukin (IL)-1, a proinflammatory cytokine
present in the degenerating disc (4–6), is
thought to contribute significantly to the loss of ECM integrity
and nucleus pulposus cells (3).
Only one cell line is present in the nucleus pulposus, and its
constituents are responsible for secreting the content of the ECM.
Therefore, the ability of IL-1β to reduce the number of cells in
the nucleus pulposus and, thus, alter the characteristics of the
ECM, may be important in the degeneration of the disc. Growth
factors such as tumor growth factor (TGF)-β1 and insulin-like
growth factor (IGF)-1 have been shown to stimulate the
proliferation of nucleus pulposus cells in humans (7,8).
Findings of previous studies suggested that growth factors also
induce the regeneration of normal ECM in the IVD (9,10).
To determine whether there is a therapeutic role for
growth factors in individuals with disc degeneration, we
investigated the effects of IGF-1 on the IL-1β-induced loss of
nucleus pulposus cells using light microscopy, Giemsa staining,
TdT-mediated dUTP-biotin nick end-labeling (TUNEL) and flow
cytometry (FCM).
Materials and methods
Cell culture
IVDs were obtained from lumbar spines of mature New
Zealand white rabbits immediately postmortem. The nucleus pulposus
was harvested from these specimens, washed with Hank’s balanced
salt solution (HBSS), and transported to the laboratory within 30
min of harvesting. The nucleus pulposus tissue was rinsed 3 times
in HBSS and then dissected into small fragments ~1 mm3
in size. Cells were isolated from the nucleus pulposus using
sequential enzyme digestion with 0.25% trypsin for 30 min, washed
with HBSS and incubated in 0.1% collagenase type II at 37°C for 2–3
h. The cells were collected by filtering through a 200-mesh nylon
cell strainer and subjected to centrifugation of 1,000 × g for 5
min. The cells were then washed twice with phosphate-buffered
saline, resuspended and grown in medium containing Dulbecco’s
modified Eagle’s medium with Ham’s F-12 nutrient mixture (DMEM-F12)
supplemented with 10% (vol/vol) fetal bovine serum (FBS) plus 1%
penicillin and streptomycin. The culture medium was changed every
2–3 days. The phenotype of the nucleus pulposus cell was confirmed
using positive immunostaining for type II collagen and toluidine
blue staining of glycosaminoglycans. First-passage chondrocytes
were used in our experiments.
Cells were grown in 80% confluency, first-passage
chondrocytes were digested using trypsin and then transferred to
6-well plates to grow to a density of 1×105/ml in
DMEM-F12 supplemented with 10% (vol/vol) FBS. After 24 h of
adherence, the medium was changed to DMEM-F12 without FBS, and the
cells were cultured for another 24 h. At that time, the medium was
removed, and the cells were grown in DMEM-F12 containing IL-1β (100
μg/l) with or without IGF-1 (500 μg/l) for 24 h. A subset of cells
grown in DMEM-F12 without FBS for 24 h served as controls.
Animal care was carried out in accordance with the
National Institute of Health Guide for the Care and Use of
Laboratory Animals (NIH Publications no. 80–23; revised 1996) and
was approved by the Bengbu Medical College Animal Care Committee of
the Use of Laboratory Animals.
Giemsa staining
The cells were digested using trypsin and cultured
in 6-well plates, each of which contained a 1×1-cm cover glass. The
slides were removed 12 h later. The cells growing on each slide
were fixed in methanol for 2 min. Giemsa staining solution was then
applied for 10 min, and the cells were made transparent using
xylene. The cells were then covered and observed using light
microscopy.
TUNEL assay
The cells were again subjected to trypsinization and
cultured in 6-well plates, each containing a 1×1-cm cover glass.
The glass slides were removed 12 h later, and the cells growing on
them were fixed in 4% paraformaldehyde for 60 min. Methanol
containing 3% H2O2 was then applied for 5 min
to inactivate endogenous peroxidase. The cells were then exposed to
0.1% Triton X-100 at 4°C for 2 min, incubated with 50 μl of the
TUNEL reaction mixture (5 μl TdT-enzyme solution + 45 μl of
nucleotide mixture solution) in the dark at 37°C for 60 min,
exposed to 3,3′-diaminobenzidine (DAB), and then counterstained
with hematoxylin. The cells were then dehydrated using graded
ethanol and covered with a xylene-based mounting medium. The
percentage of TUNEL-positive cells from the control, IL-1β and
L-1β+ IGF-1 groups were determined by counting the
TUNEL-positive cells under 10 non-continuous low-power fields
(magnification, ×100).
Flow cytometry
Cells were cultured in DMEM-F12 containing IL-1β 100
μg/l with or without IGF-1 500 μg/l for 24 h. Cells cultured in
DMEM-F12 without FBS for 24 h served as controls. The cells were
then digested using trypsin, collected, washed in FCM buffer, and
resuspended in FCM wash buffer. To detect cell apoptosis, the cells
were incubated with 5 μl of Annexin V-FITC incubation reagent in
the dark for 15 min at 4°C, followed by incubation with propidium
iodine (PI)-PE of 10 μl for 5 min at 4°C. Samples were analyzed
within 30 min using FCM.
Statistical analysis
Data were presented as the means ± SD (n=3). Groups
of data were compared statistically using the Mann-Whitney U test.
Values were considered statistically significant when
P<0.05.
Results
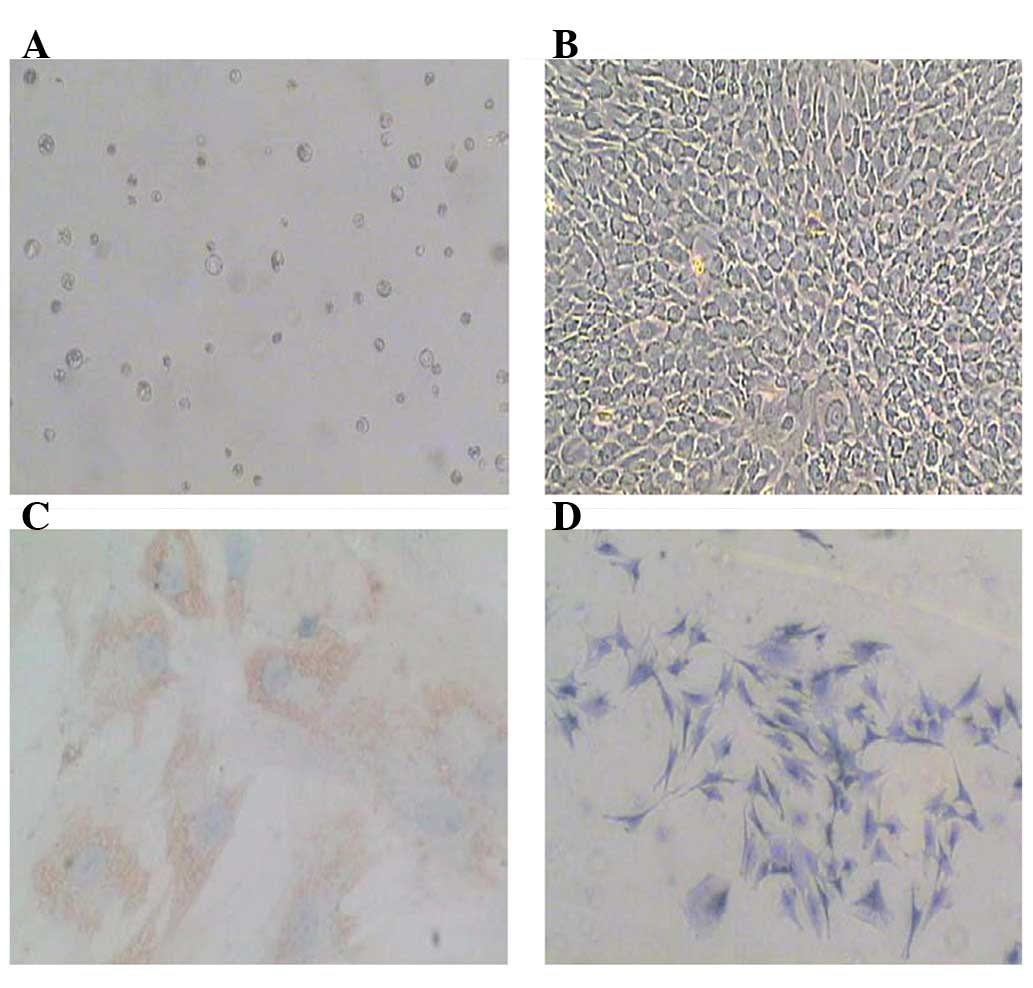
Cultivation and identification of the
nucleus pulposus cells
Primary nucleus pulposus cells were obtained by
sequential enzyme digestion and cultured in DMEM-F12 10% FBS
(Fig. 1A). Following culture for
7–10 days, the cells formed a complete monolayer (Fig. 1B). To identify the nucleus pulposus
phenotype cell, toluidine blue staining was used to identify
glycosaminoglycans and immunostaining was used to detect type II
collagen (Fig. 1C and D). The
results showed that these cells expressed both type II collagen and
glycosaminoglycans, thus demonstrating the phenotype of nucleus
pulposus cells.
IL-1β-induced apoptosis of nucleus
pulposus cells
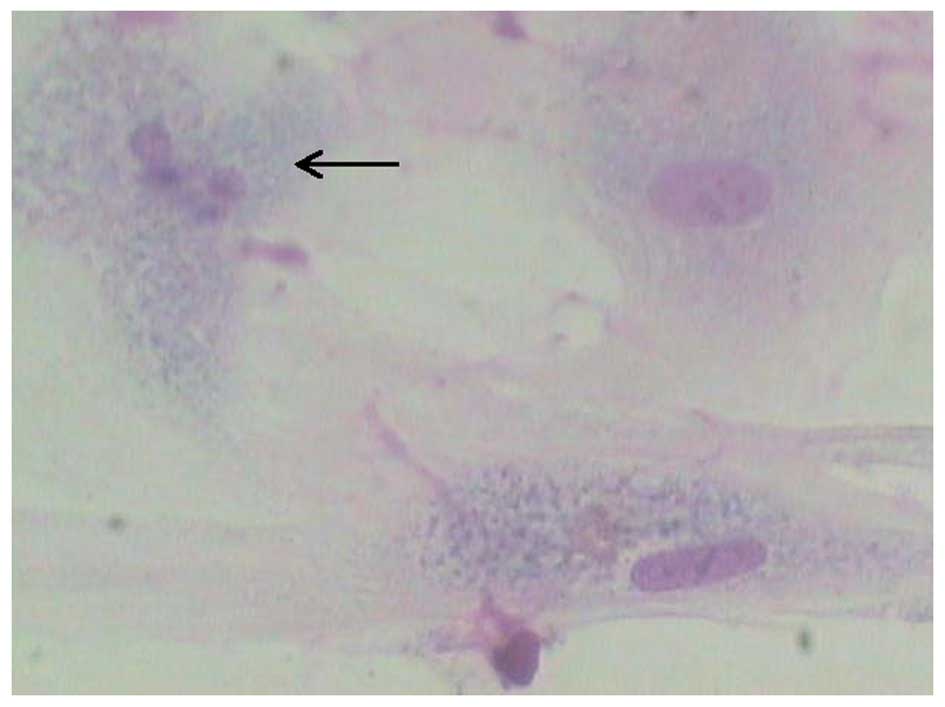
The result obtained from Giemsa staining indicates
that nuclear fragmentation occurred following culture of nucleus
pulposus cells in the presence of 100 μg/l IL-1β (Fig. 2), suggesting that IL-1β induced
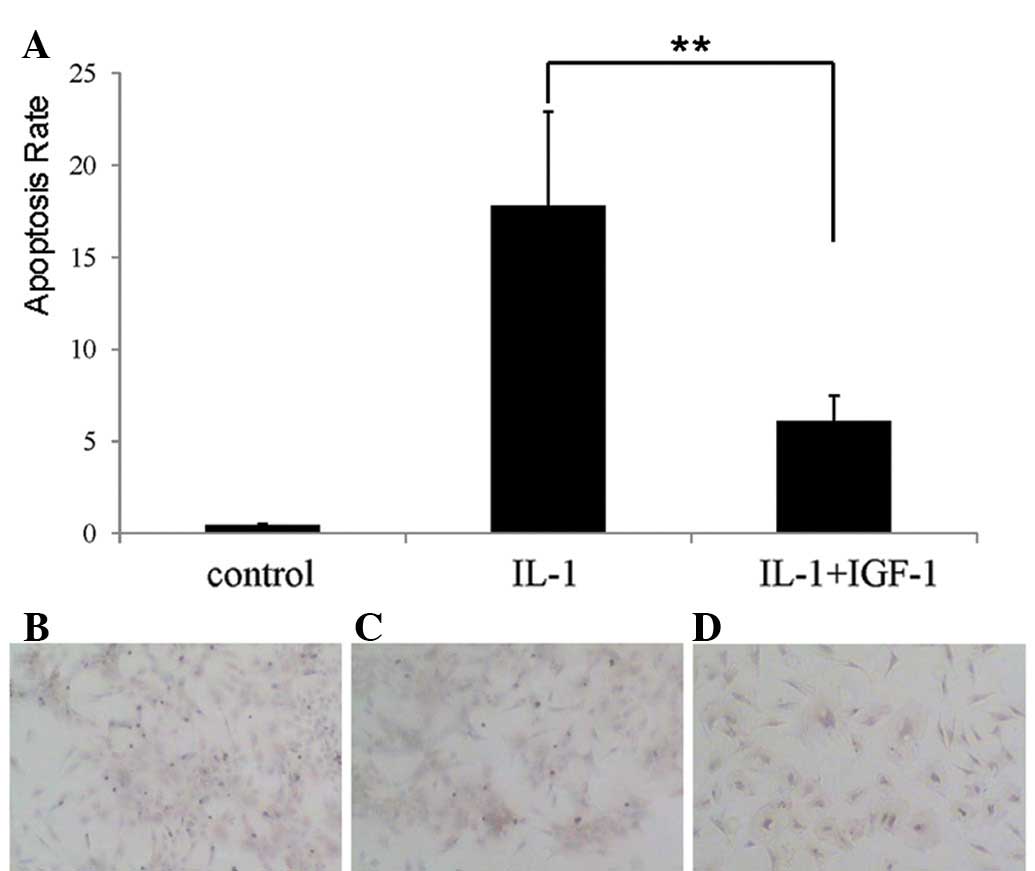
apoptosis of nucleus pulposus cells. TUNEL staining also showed
that the percentage of TUNEL-positive cells was markedly greater in
the IL-1β-treated group than that in the controls (Fig. 3A-C).
IGF-1 reduced IL-1β-induced apoptosis of
nucleus pulposus cells
To determine whether IGF-1 affected IL-1β-induced
apoptosis of nucleus pulposus cells, both 500 μg/l IGF-1 and 100
μg/l IL-1β were added into culture medium simultaneously. The
result of TUNEL indicated that IL-1β-induced apoptosis was
significantly suppressed (P<0.01) in the presence of IGF-1
(Fig. 3A and D), suggesting that
IGF-1 inhibits apoptosis of nucleus pulposus cells induced by
IL-1β.
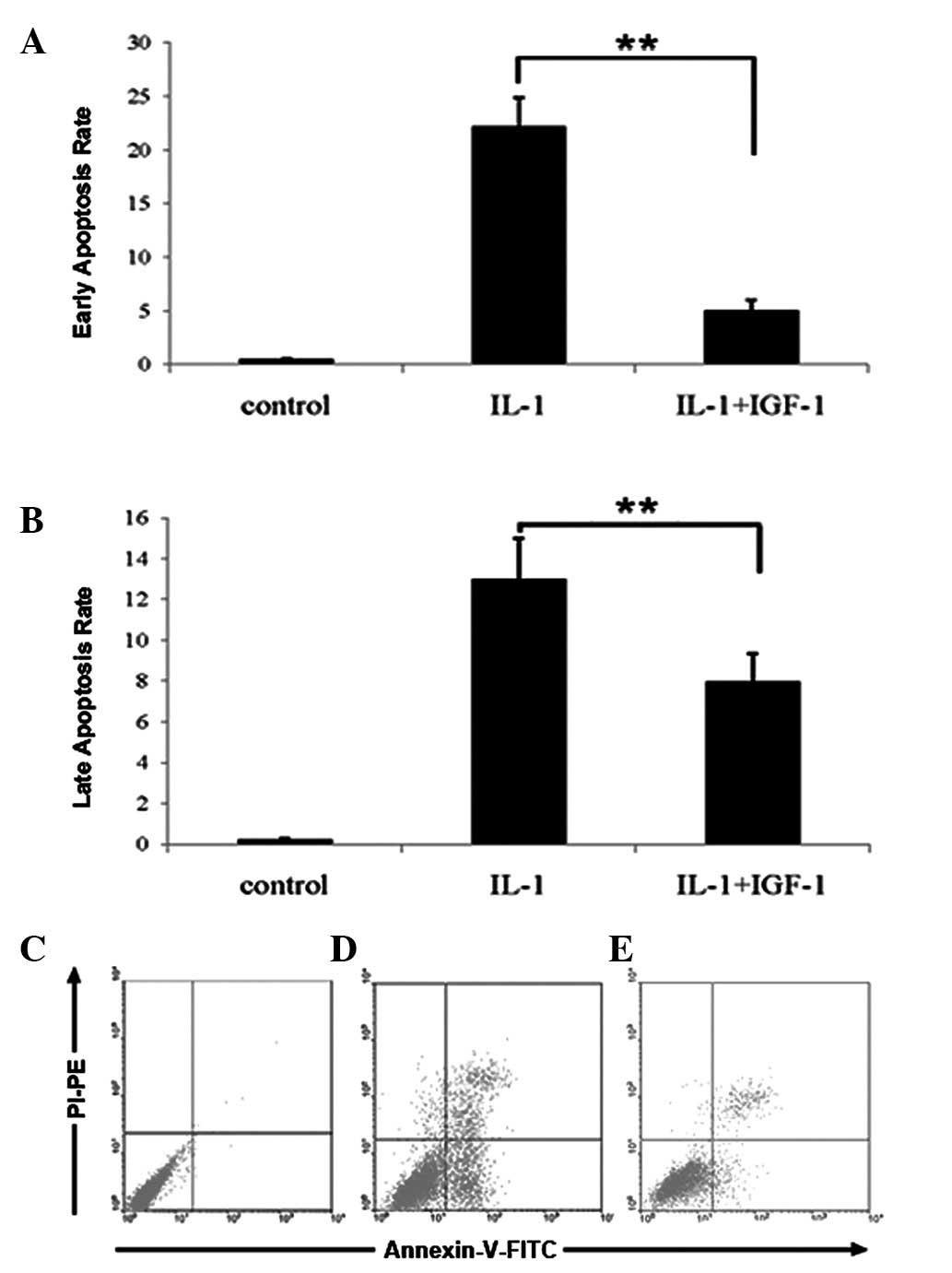
The results of TUNEL were confirmed by FCM. The
percentage of nucleus pulposus cells with signs of early and late
stages of apoptosis was significantly higher in the IL-1β group
compared with the controls. However, the treatment of IGF-1 reduced
IL-1β-induced apoptosis of nucleus pulposus cells (Fig. 4A-C, P<0.01).
Discussion
Proinflammatory cytokines including IL-1β, IL-6,
prostaglandin E2 and TNF are important in the mechanism underlying
IVD (11–13). These cytokines are able to induce
the production of factors associated with inflammation, pain and
disc matrix catabolism in the nucleus pulposus (14). IL-1β upregulates the expression of
MMP-3 and MMP-9, which may contribute to the
catabolism of the disc matrix (12), thereby playing an important role in
the pathological degradation of the disc. Moreover, investigators
found a simultaneous reduction in matrix synthesis factors
(aggrecan, type II collagen, and Sox9) and an increase in
inflammatory cytokine (IL-1β and TNF) levels during disc
degeneration (15). These
observations indicate that proinflammatory cytokines stimulate the
degradation of the ECM surrounding the nucleus pulposis, which may
lead to disc degeneration.
The late stage of disc degeneration is characterized
by a reduction in the number of nucleus pulposis cells, and IL-1β
is thought to contribute significantly to this loss. Thus,
IL-1β-induced nucleus pulposus cell apoptosis may also be involved
in disc degeneration. Our findings have shown that IL-1β was able
to induce apoptosis of nucleus pulposus cells. It has been proven
that IGF-1 is efficient in stimulating the proliferation of human
nucleus pulposus cells (9,16). Results of a recent study have
indicated that exogenous and autocrine growth factors such as
platelet-derived growth factor, basic fibroblast growth factor, and
IGF-I stimulate the proliferation of human IVD through the MEK/ERK
and PI-3K/Akt pathways (8).
Findings of an in vivo study indicated that age-related disc
degeneration is associated with downregulation of the expression of
IGF-1 (17), while findings of an
in vitro study demonstrated that IGF-1-dependent
proteoglycan synthesis decreased with age (9). Taken together, these results suggest
that IGF-1 likely contributes to the development of clinical
interventions for disc degeneration. In the present study, the
results from TUNEL and FCM indicated that the rate of apoptosis is
particularly high in nucleus pulposus cells in the presence of
IL-1β compared with the controls. However, when treated with IGF-1,
the apoptosis of nucleus pulposus cells induced by IL-1β was
reduced significantly, suggesting that IGF-1 reverses IL-1β-induced
apoptosis of nucleus pulposus cells in vitro. The results
from FCM also suggest that IL-1β induced both the early and late
stages of apoptosis of nucleus pulposus cells and that the
apoptosis was suppressed by IGF-1. These results were similar to
those of a study suggesting that anabolic cytokines such as TGF and
IGF-1 likely have a fundamental role in the prevention of
degenerative disc disease (18,19),
maintainance of ECM synthesis, and prevention of disc
degeneration.
In conclusion, findings of this study have
demonstrated that IGF-1 reverses IL-1β-induced apoptosis of nucleus
pulposus cells. Thus, IGF-1 is a potentially appropriate target for
the development of treatments for individuals with disc
degenerative disease.
Acknowledgements
This study was supported by the Key Research
Foundation of the Education Bureau of Anhui Province, China
(KJ2011A204).
References
|
1
|
Freemont AJ: The cellular pathobiology of
the degenerate intervertebral disc and discogenic back pain.
Rheumatology (Oxford). 48:5–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith LJ, Nerurkar NL, Choi KS, et al:
Degeneration and regeneration of the intervertebral disc: lessons
from development. Dis Model Mech. 4:31–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le Maitre CL, Freemont AJ and Hoyland JA:
The role of interleukin-1 in the pathogenesis of human
intervertebral disc degeneration. Arthritis Res Ther. 7:R732–R745.
2005.
|
|
4
|
Le Maitre CL, Freemont AJ and Hoyland JA:
A preliminary in vitro study into the use of IL-1Ra gene therapy
for the inhibition of intervertebral disc degeneration. Int J Exp
Pathol. 87:17–28. 2006.PubMed/NCBI
|
|
5
|
Smith LJ, Chiaro JA, Nerurkar NL, et al:
Nucleus pulposus cells synthesize a functional extracellular matrix
and respond to inflammatory cytokine challenge following long-term
agarose culture. Eur Cell Mater. 22:291–301. 2011.
|
|
6
|
Studer RK, Vo N, Sowa G, et al: Human
nucleus pulposus cells react to IL-6: independent actions and
amplification of response to IL-1 and TNF-α. Spine (Phila Pa 1976).
36:593–599. 2011.PubMed/NCBI
|
|
7
|
Zhang R, Ruan D and Zhang C: Effects of
TGF-beta1 and IGF-1 on proliferation of human nucleus pulposus
cells in medium with different serum concentrations. J Orthop Surg
Res. 1:92006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pratsinis H, Constantinou V, Pavlakis K,
et al: Exogenous and autocrine growth factors stimulate human
intervertebral disc cell proliferation via the ERK and Akt
pathways. J Orthop Res. 30:958–964. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okuda S, Myoui A, Ariga K, et al:
Mechanisms of age-related decline in insulin-like growth factor-I
dependent proteoglycan synthesis in rat intervertebral disc cells.
Spine (Phila Pa 1976). 26:2421–2426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Osada R, Ohshima H, Ishihara H, et al:
Autocrine/paracrine mechanism of insulin-like growth factor-1
secretion, and the effect of insulin-like growth factor-1 on
proteoglycan synthesis in bovine intervertebral discs. J Orthop
Res. 14:690–699. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Erwin WM, Islam D, Inman RD, et al:
Notochordal cells protect nucleus pulposus cells from degradation
and apoptosis: implications for the mechanisms of intervertebral
disc degeneration. Arthritis Res Ther. 13:R2152011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Millward-Sadler SJ, Costello PW, Freemont
AJ and Hoyland JA: Regulation of catabolic gene expression in
normal and degenerate human intervertebral disc cells: implications
for the pathogenesis of intervertebral disc degeneration. Arthritis
Res Ther. 11:R652009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park JY, Kuh SU, Park HS and Kim KS:
Comparative expression of matrix-associated genes and inflammatory
cytokines-associated genes according to disc degeneration: analysis
of living human nucleus pulposus. J Spinal Disord Tech. 24:352–357.
2011. View Article : Google Scholar
|
|
14
|
Studer RK, Aboka AM, Gilbertson LG, et al:
p38 MAPK inhibition in nucleus pulposus cells: a potential target
for treating intervertebral disc degeneration. Spine (Phila Pa
1976). 32:2827–2833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, An HS, Toofanfard M, et al:
Low-dose interleukin-1 partially counteracts osteogenic
protein-1-induced proteoglycan synthesis by adult bovine
intervertebral disk cells. Am J Phys Med Rehabil. 84:322–329. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mavrogonatou E and Kletsas D: Effect of
varying osmotic conditions on the response of bovine nucleus
pulposus cells to growth factors and the activation of the ERK and
Akt pathways. J Orthop Res. 28:1276–1282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Murakami H, Yoon ST, Attallah-Wasif ES, et
al: The expression of anabolic cytokines in intervertebral discs in
age-related degeneration. Spine (Phila Pa 1976). 31:1770–1774.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okuda S, Myoui A, Ariga K, et al:
Mechanisms of age-related decline in insulin-like growth factor-I
dependent proteoglycan synthesis in rat intervertebral disc cells.
Spine (Phila Pa 1976). 26:2421–2426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Le Maitre CL, Richardson SM, Baird P, et
al: Expression of receptors for putative anabolic growth factors in
human intervertebral disc: implications for repair and regeneration
of the disc. J Pathol. 207:445–452. 2005.PubMed/NCBI
|
|
20
|
Jiang F, Frederick TJ and Wood TL: IGF-I
synergizes with FGF-2 to stimulate oligodendrocyte progenitor entry
into the cell cycle. Dev Biol. 232:414–423. 2001. View Article : Google Scholar : PubMed/NCBI
|