Introduction
Oral squamous cell carcinoma (OSCC) accounts for
more than 90% of oral cavity tumors. With approximately 8000
mortalities per year nationally, it constitutes approximately 3% of
all cancer cases in the United States and is one of the six most
frequent types of cancer worldwide (1). Although tobacco and alcohol are
regarded as primary risk factors, it is clear that genetic and
epigenetic factors contribute to this cancer (2). On this basis, novel molecular markers
involved in OSCC development and progress should be
investigated.
The protein encoded by the CNTN1 gene is a member of
the immunoglobulin superfamily, which includes N-CAM, L1 and
Nr-CAM. CNTN1 is a glycosylphosphatidylinositol (GPI)-anchored
neuronal membrane protein that functions as a cell adhesion
molecule (3). It mediates cell
surface interactions during nervous system development, involving
the formation of paranodal axo-glial junctions in myelinated
peripheral nerves and signaling between axons and myelinating glial
cells via its association with CNTNAP1. In addition, as a ligand of
Notch1, CNTN1 promotes Notch1 activation which is involved in
oligodendrocyte generation through the released notch intracellular
domain (NICD) and subsequent translocation to the nucleus (4). However, it is becoming increasingly
evident that certain members of this immunoglobulin superfamily
facilitate the motility, invasion and metastasis of cancer
(5,6). Additionally, the location of CNTN1 in
the 12q11-q12 chromosomal region, which is a breakpoint region in
several types of cancer, suggests that CNCT1 is involved in tumor
formation or progression.
Notably, in vitro silencing of CNTN1
expression may inhibit the invasive and metastatic ability of lung
adenocarcinoma cells (7).
Furthermore, VEGF-C/Flt-4-mediated invasion and metastasis of
cancer cells were found to be through the upregulation of the
neural cell adhesion molecule CNTN1 which activated the Src-p38
MAPK-C/EBP-dependent pathway (8).
In view of its malignant phenotype-promoting activities in cancer
cells and its growth-promoting abilities in neural cells, this
study investigated the possibility of CNTN1 as a prognostic marker
for patients with OSCC and the association between CNTN1 expression
and metastasis of OSCC in vivo. CNTN1 protein levels were
evaluated in 45 primary OSCC specimens by immunohistochemistry. Our
study demonstrates that CNTN1 protein level was markedly associated
with lymph node metastasis of patients with OSCC (P=0.006). CNTN1
expression was significantly associated with overall survival of
patients with OSCC (P=0.032; log-rank) and disease-free survival of
patients with OSCC (P=0.038; log-rank). In vitro results
revealed that CNTN1 ablation was able to inhibit the invasion
potential of OSCC cells, but not proliferation of OSCC cells. We
conclude that CNTN1 is a novel and powerful factor for the
metastasis and prognosis of OSCC patients.
Patients and methods
Patients and specimens
Patients (n=45) with stage I to IV OSCC who
underwent radical surgery at the Department of Oral and
Maxillofacial Surgery, Shanghai Ninth People's Hospital, Shanghai
Jiao Tong University School of Medicine, Shanghai, China between
January 2002 and December 2002, who had not undergone radio-or
chemotherapy, were enrolled into this prospective study. All of the
tumors were classified according to the International Union Against
Cancer (UICC) tumor/lymph node/metastasis (TNM) classification
system (9). Histological diagnoses
of OSCC were made according to the criteria of the World Health
Organization (WHO) for the histological typing of cancer (10). Patients were biopsied and
histopathologically examined at the Ninth People's Hospital,
Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Patients were prospectively evaluated (chest X-ray or thoracic CT
scan, abdominal sonography or CT scan or MRI and serum chemistry)
every 3 months for the first 2 years after surgery, every 6 months
for the following 3 years and annually thereafter. This study was
approved by the ethics committee of Shanghai Ninth People's
Hospital. Informed consent was obtained from each patient. A total
of 45 patients with follow-up periods up to 8.3 years were included
in the study. Annual follow-up data were retrieved from the medical
records. The specimens were fixed in 10%-buffered formalin and
embedded in paraffin wax. Paraffin blocks were sectioned into 4 μm
slices.
Cell lines
The human HNSCC cell lines Tca, Tca-M, Tb, Tca/CDDP
(kindly provided by the Shanghai Ninth People's Hospital, Shanghai,
China), TSCC (kindly provided by Wuhan University, School of
Medicine, China), OSC-4, NB and NT (kindly provided by Kochi
University, School of Medicine, Japan) were cultured in RPMI-1640
medium (Gibco-BRL, Carlsbad, CA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Gibco-BRL), penicillin
(100 U/ml) and streptomycin (100 μg/ml) at 37°C in a humidified 5%
CO2 atmosphere. CAL27 (American Type Culture Collection,
Manassas, VA, USA) was cultured in Dulbecco's modified Eagle is
medium (DMEM; Gibco BRL) supplemented with 10% heat-inactivated FBS
(Gibco BRL), penicillin (100 U/ml) and streptomycin (100 μg/ml) at
37°C in a humidified 5% CO2 atmosphere.
Immunohistochemistry
The avidin-biotin complex (ABC) technique was
performed using a Vectastain Elite ABC kit (Vector Laboratories,
Inc., Burlingame, CA, USA). Briefly, paraffin-embedded tissue
sections were dewaxed and rehydrated using xylene and a series of
graded alcohols. To determine antigenicity, slides were steamed
with 10 mmol/l citrate buffer (pH 6.0; DAKO/Cytomation, Glostrup,
Denmark) for 20 min. Endogenous peroxidase activity was quenched by
immersing the slides in 3% hydrogen peroxide in double-distilled
water for 20 min. Tissue sections were blocked with 10% normal
horse serum for 30 min at room temperature. The slides were then
incubated with monoclonal anti-CNTN1 antibody at 1:100 dilution
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C
overnight. Each section was treated with biotinylated-secondary
antibody for 30 min at room temperature. Diaminobenzidine was used
as the chromogen for the immunoperoxidase reaction and the slides
were counterstained with Mayer's hematoxylin (DAKO/Cytomation).
Sections were thoroughly washed, glass covered and analysed by
light microscopy, using a magnification of up to ×400. For the
immunohistochemical assessment of CNTN1 expression, the frequency
of cytoplasmic staining was evaluated using a semiquantitative
score: 0–1, from negativity to positivity in <50% (low
expression); 2, positivity in >50% (high expression). The score
of each lesion was the average of the indices generated by two
observers (W.C. and H.M.W.) blinded to the clinical information.
The differences between the two observers were <10% in almost
all cases.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
High quality total RNA (2 μg) was directly processed
to cDNA using the reverse transcription kit (Promega, Madison, WI,
USA), following the manufacturer's instructions, in a total volume
of 25 μl. The primer sequences used were: CNTN1, forward:
5′-CAACAAAACCATATCCTGCTGA-3′; reverse:
5′-AGATCACTGCCTATGTCCACCT-3′; β-actin, forward:
5′-TCACCCACACTGTGCCCATCTACGA-3′; reverse:
5′-CAGCGGAACCGCTCATTGCCAATGG-3′; Each primer was added at a final
concentration of 0.5 μM to a 15 μl reaction mixture in PCR buffer,
containing 1 μl cDNA, 0.25 mM of each dNTPs, 1.5 mM
MgCl2 and 2.5 units Taq DNA polymerase. An initial
denaturation was conducted for 5 min at 94°C and 35 cycles were
performed with the following PCR program: denaturation at 94°C for
30 sec, annealing at 45 for 60°C for CNTN1 for 30 sec and 55°C for
β-actin for 30 sec, elongation at 72°C for 30 sec, followed by a
final extension of 5 min at 72°C. Ethidium bromide-stained bands
were visualized by UV transillumination and the fluorescence
intensity was quantified using the FR-200 system (Shanghai FURI
Science and Technology Co., Ltd., Shanghai, China). RT-PCR data
were from at least three independent experiments.
Small interfering RNA (siRNA)
knockdown
Two siRNAs against CNTN1 were designed and
chemically synthesized (Shanghai GenePharma Co., Ltd., Shanghai,
China). The siRNA which had a greater silencing effect was selected
for further study, with the following sequence: CNTN1-siRNA_1697:
5′-GGUCCUUCAAUGGCUAUGUTT-3′ and 5′-ACA UAGCAUUGAAGGACCTT-3′ for
nucleotides 1697–1718. In addition, a negative control, siRNA_NC;
5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACG UGACACGUUCGGAGAATT-3′ was
also synthesized. The in vitro transient transfection was
performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions.
Proliferation assay
Proliferation assays were performed to analyze the
proliferation potential of transient-transfected si-RNA_1697,
siRNA_NC and Lipofectamine 2000 only into cells by using the
Cell-Counting kit (CCK)-8 (Dojindo, Kumamoto, Japan). The cells
were harvested and plated onto 96-well plates at 1×103
cells per well and maintained at 37°C in a humidified incubator. At
the indicated times, 10 μl of the CCK-8 solution were added into
the triplicate wells and incubated for 1 h and the absorbance at
450 nm was measured to calculate the number of vital cells in each
well. Cells were performed in triplicate and the mean [± standard
deviation (SD)] optical density (OD) was reported.
Invasion assays
A total of 1×105 various cells in 750 μl
serum-free RPMI-1640 medium were plated onto a BD BioCoat™
Matrigel™ Invasion Chamber (8 μm pore size; BD Biosciences,
Franklin Lakes, NJ, USA) and the lower chamber was immediately
filled with 750 μl of RPMI-1640 medium with 10% FBS as a
chemoattractant. After 48 h of incubation in a humidified
atmosphere containing 5% CO2 at 37°C, the non-invading
cells were removed from the upper surface of the membrane by a
cotton swab and the membranes were then fixed with methanol and
stained with 0.5% crystal violet. Invading cells were captured and
counted in five random non-overlapping fields under a light
microscope (magnification, ×100).
Statistical analysis
The associations between CNTN1 expression status and
clinicopathological parameters were analyzed using the Mann-Whitney
U test and the Kruskal-Wallis test for categorical variables. The
probability of overall survival and disease-free survival by CNTN1
protein expression was determined using the Kaplan-Meier method.
Paired t-test was used for analysis of the in vitro studies.
Analyses were conducted using SAS 9.1.3 software. The tests were
two-sided, and P<0.05 was considered to indicate a statistically
significant difference.
Results
Correlation between CNTN1 expression and
patient characteristics
To identify the association between CNTN1 expression
and clinicopathological factors, the expression of CNTN1 was
analyzed in 45 primary OSCCs by immunohistochemistry. Positive
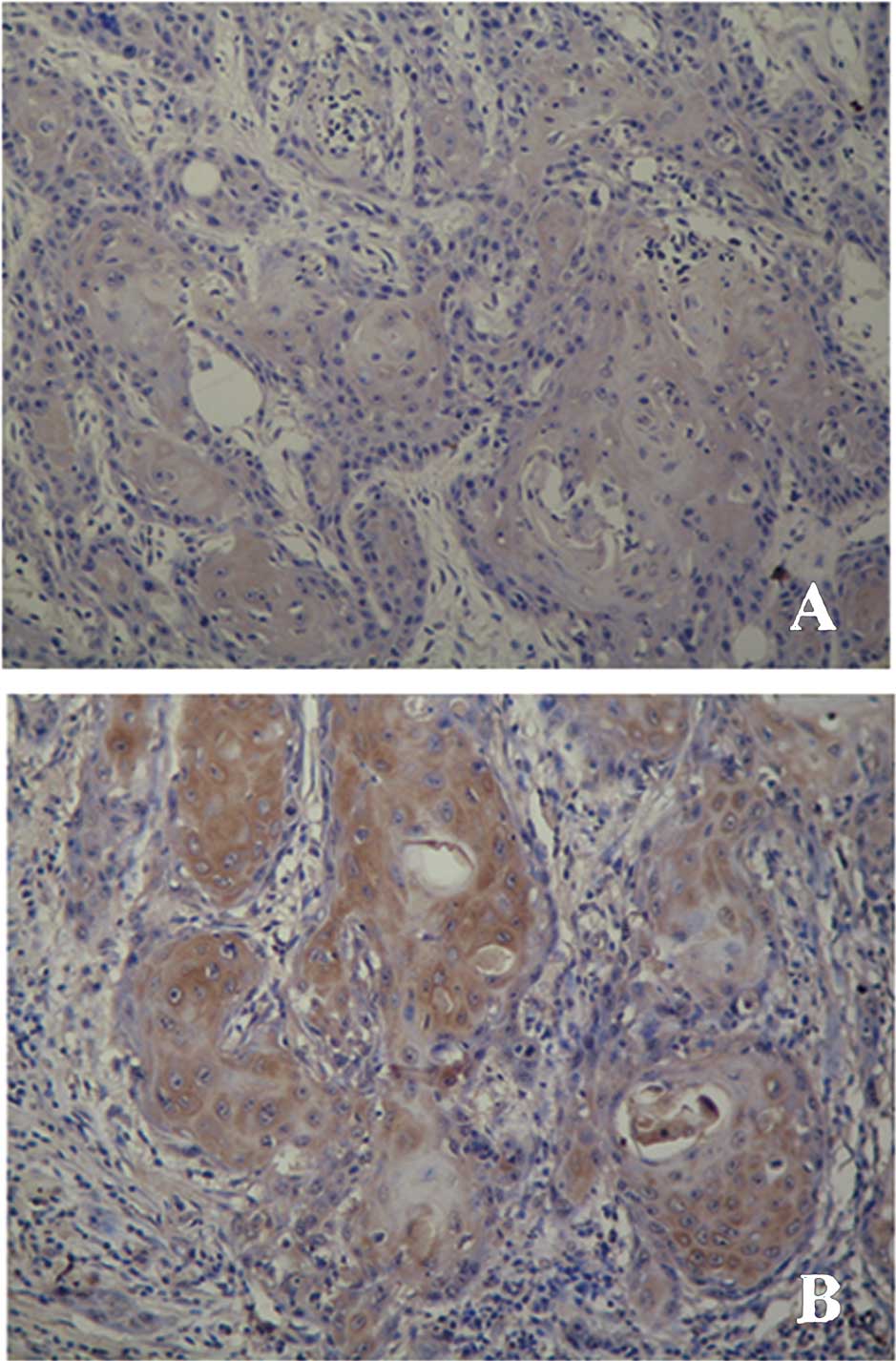
lesions demonstrated clearly membrane or cytoplasmic localization
of the CNTN1 protein (Fig. 1),
which is consistent with its function in cells. Distribution of the
CNTN1 expression status and associations with general
clinicopathological parameters are shown in Table I. Of 45 patients enrolled in this
study, 12 (26.7%) patients had regional lymph node metastasis to a
certain extent. Furthermore, 9 (75%) of 12 (26.7%) patients with
regional lymph node metastasis revealed a high expression level of
CNTN1, whereas 22 (66.7%) of 33 (73.33%) patients without regional
lymph node metastasis revealed a low expression level of CNTN1. A
significantly high expression level of CNTN1 was found to be
markedly association with patients who had regional lymph node
metastasis. Additionally, no statistically significant correlation
was found between CNTN1 protein level and other clinicopathological
factors, including age (P=0.063), gender (P=0.064), clinical TNM
classification (P=0.069), pathological grade (P=0.527) and tumor
recurrence (P=0.640).
 | Table ICorrelation between CNTN1 expression
and patient characteristics. |
Table I
Correlation between CNTN1 expression
and patient characteristics.
| CNTN1 expression (No.
of cases) | |
|---|
|
| |
|---|
| Characteristics | Low | High | P-value |
|---|
| Age at diagnosis |
| <60 years | 20 | 8 | 0.063 |
| ≥60 years | 7 | 10 | |
| Gender |
| Female | 8 | 11 | 0.064 |
| Male | 19 | 7 | |
| TNM
classification |
| I or II | 16 | 3 | 0.069 |
| III or IV | 11 | 15 | |
| Regional
metastasis |
| With | 3 | 9 | 0.006 |
| Without | 24 | 9 | |
| Recurrence |
| With | 3 | 1 | 0.640 |
| Without | 24 | 17 | |
| Histological
grading |
| I | 11 | 5 | 0.527 |
| II | 16 | 13 | |
| III | 0 | 0 | |
CNTN1 expression and patient
survival
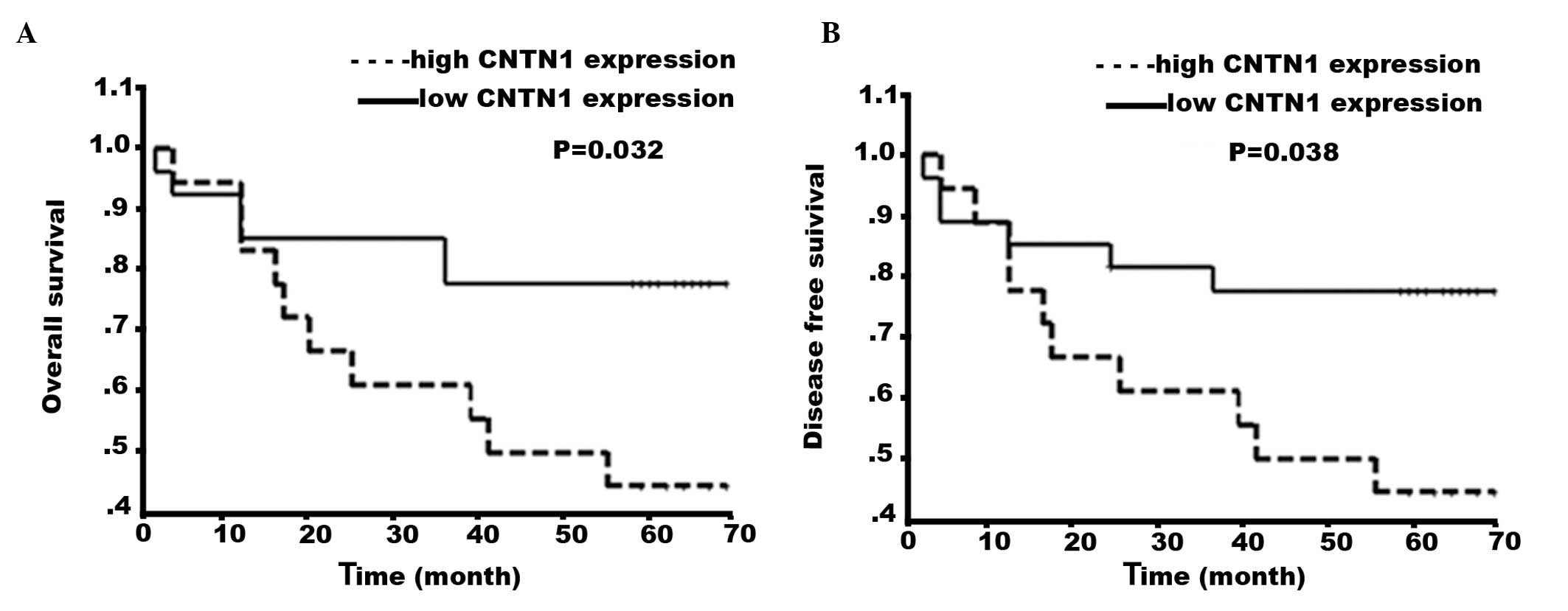
To determine whether CNTN1 protein expression is a
prognostic marker for patients with OSCC, the overall and the
disease-free survival of OSCC were calculated using the log-rank
test and curves were constructed using the Kaplan-Meier method
(Fig. 2). The median follow-up of
patients was 5.0 years (range, 0.2–8.3). During the follow-up of 45
patients, 16 patients (35.56%) succumbed to OSCC. Of these 16
patients, 6 patients (37.5%) demonstrated a low protein level of
CNTN1, while 10 patients (62.5%) demonstrated a high protein level
of CNTN1. The survival of patients with OSCC was calculated from
the time of radical surgery until the end of the follow-up. The
disease-free survival rate at 3 and 5 years after radical surgery
for the whole cohort of patients was 71.11 and 64.44%,
respectively. Survival analysis revealed that CNTN1 expression was
significantly associated with overall survival of patients with
OSCC (P=0.032; log-rank) and disease-free survival of patients with
OSCC (P=0.038; log-rank).
Effect of CNTN1 ablation on cell
proliferation of OSCC cell lines
To evaluate whether CNTN1 promotes malignant
phenotypes of OSCC cells, we firstly assessed the effect of CNTN1
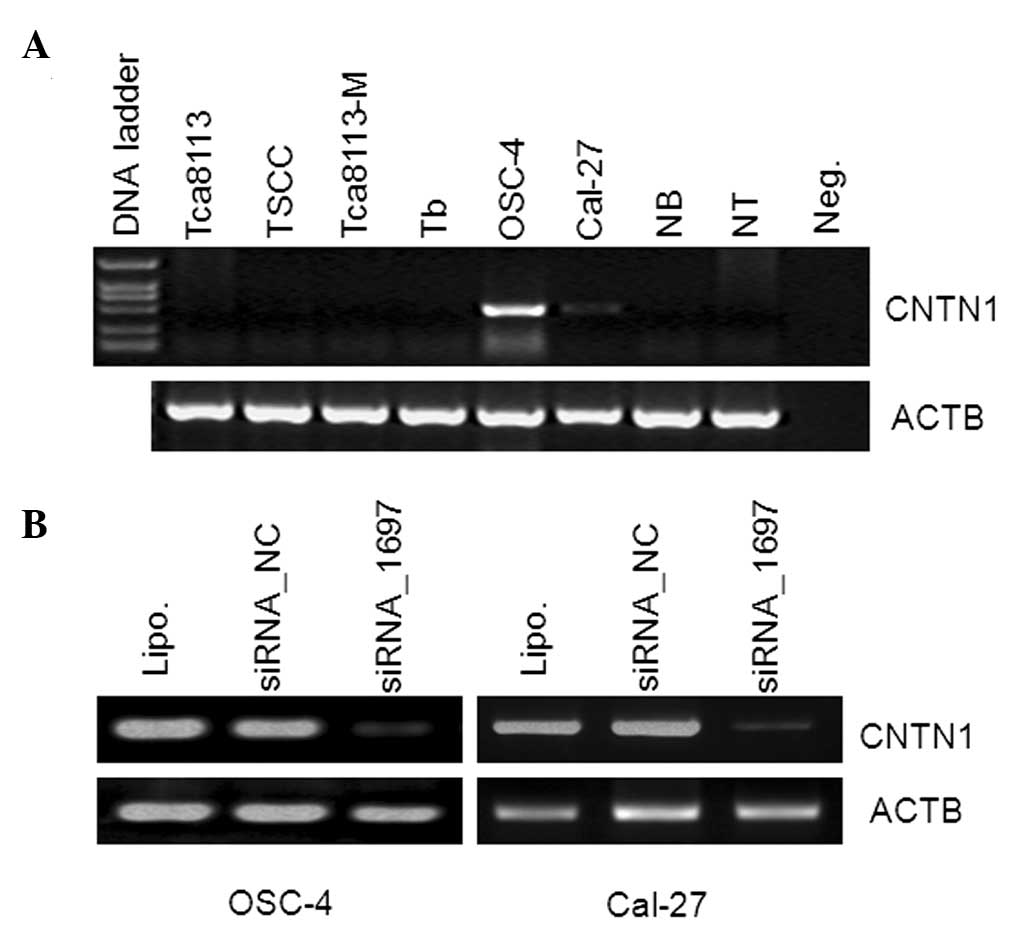
ablation on cell proliferation of OSCC cell lines. Based on the
CNTN1 expression pattern in OSCC-derived cell lines (Fig. 3A), we transiently transfected
target CNTN1 siRNA, negative control siRNA_NC and Lipofectamine
2000-only to OSC-4 and Cal-27 cells, all of which have endogenous
CNTN1 mRNA expression (Fig. 3B).
However, our results demonstrated that silencing CNTN1 did not
remarkably inhibit the proliferation of OSCC cells compared to
cells transfected with siRNA_NC control (Fig. 4A). Furthermore, in vitro
invasion assays were performed to determine the effect of CNTN1 on
cell invasion using a BD BioCoat Matrigel Invasion Chamber. The
Matrigel matrix served as a reconstituted basement membrane in
vitro. Moreover, the number of cells invading through the
transwell membrane in OSC-4 transfected with siRNA_1697 and Cal-27
transfected with siRNA_1697 was significantly lower than those
transfected with siRNA_NC, respectively (P<0.01) (Fig. 4B).
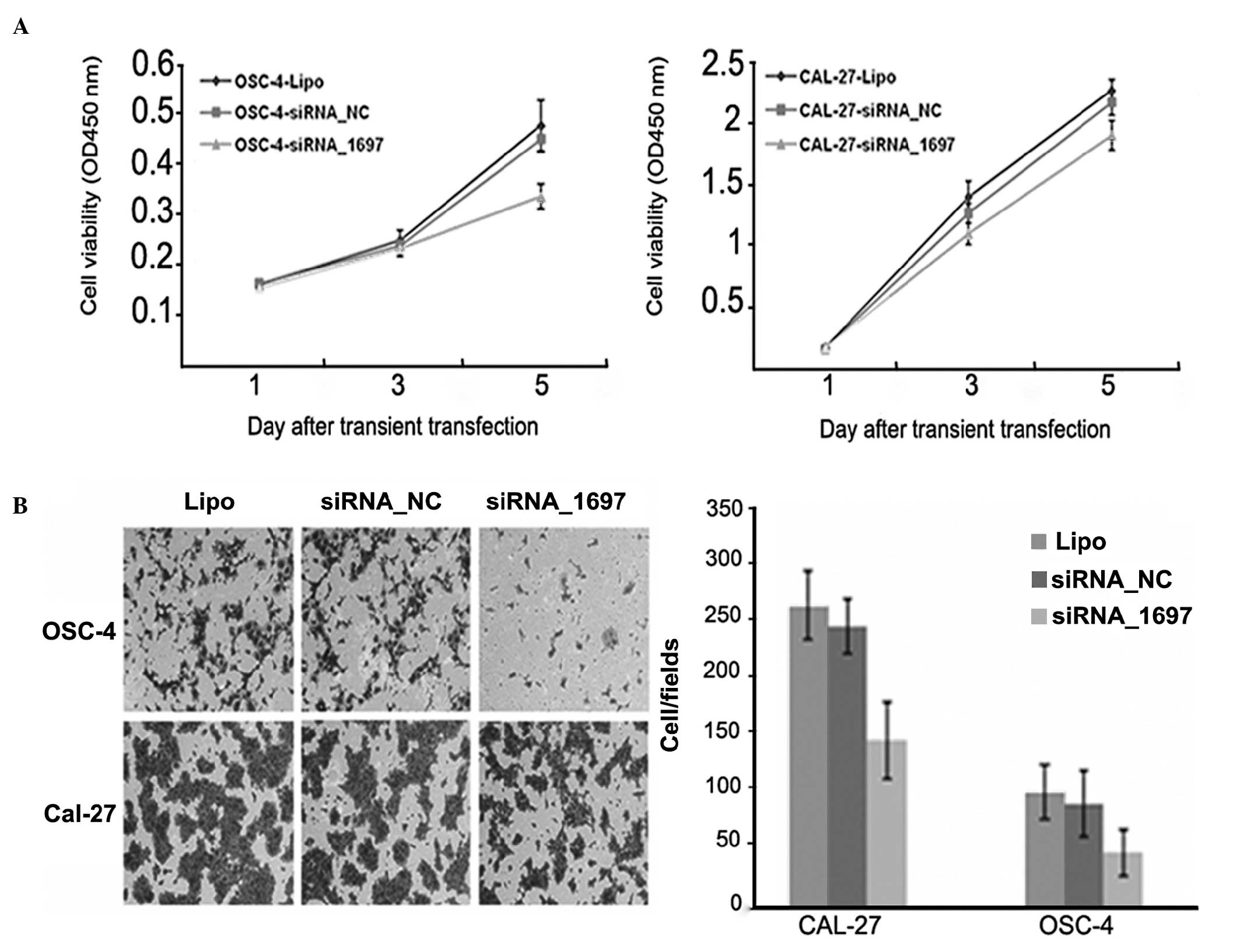 | Figure 4Proliferation and invasive ability of
OSCC cells after suppression of CNTN1 expression. (A) Proliferation
of OSCC cells was not markedly inhibited after CNTN1 siRNA,
compared to cells transfected with siRNA_NC control (left panel,
OSCC-4; right panel, Cal-27 cells). (B) Suppression of CNTN1
expression decreased the invasive ability of OSCC cells. Left
panel, in vitro invasion assays; right panel, number of
cells invading through the transwell membrane in OSC-4 transfected
with siRNA_1697 and Cal-27 transfected with siRNA_1697 were
significantly lower than those transfected with siRNA_NC,
respectively (P<0.01, two-tailed Student's t-test). Lipo,
Lipofectamine 2000; OSCC, oral squamous cell carcinoma; CNTN1,
contactin 1; siRNA, small interfering RNA. |
Discussion
As one of the most common types of epithelial
cancer, the incidence of oral squamous cell carcinoma (OSCC) is the
sixth highest worldwide (11).
Traditional treatments, including radical surgery, radiotherapy and
chemotherapy, have not sufficiently improved the five-year survival
rate of patients with this disease in more than two decades.
Moreover, the development of OSCC is evolutionary and characterized
by multistep carcinogenic processes, in which the activation of
oncogenes and inactivation of tumor suppressor genes are key
features leading to OSCC progression. Considering these factors,
the identification of useful predictors or targets of OSCC for
diagnosis, therapy and prognosis is promising. Although numerous
studies have been conducted, few useful molecular predictors or
targets for OSCC have been identified.
CNTN1 is a novel member of the contactin subgroup of
the immunoglobulin superfamily which also includes contactin-2, 5
and 6. The well-known role of these proteins and ligands is the
repulsive guidance of nerve axons, regulating neurite extension in
a mouse neuroblastoma cell line and primary hippocampal neurons
(12–14). In addition, mutations in the CNTN1
gene causing a familial type of lethal congenital myopathy have
been reported (15). In addition
to its regulatory role in the nervous system, CNTN1 functions as a
glycosylphosphatidylinositol anchor neural cell adhesion molecule
(NCAM), which is involved in tumor cell adhesion, invasion and
metastasis (16–18). CNTN1 was first described as a
metastasis-promoting oncogenic protein by Su et al (7,8).
Suppression of CNTN1 expression abolished the ability of lung
cancer cells to invade and metastasize by activating RhoA, but not
Cdc42 or Rac1, suggesting that CNTN1 is a key regulator of invasion
and metastasis in lung adenocarcinoma (7). Considering the particularity of OSCC
in epidemiology, which is notably different from other cancer
types, as well as the different molecule signatures in invasion and
metastasis, in this study, we investigated whether CNTN1 is a major
factor promoting OSCC progression and metastasis. Experimental
results demonstrated that CNTN1 expression is markedly associated
with regional lymph node metastasis (P=0.006) in patients with OSCC
and silencing CNTN1 decreased the invasion potential of OSCC cells,
confirming that CNTN1 is a powerful factor involved in invasion and
metastasis in OSCC. Notably, CNTN1 ablation exerted little effect
on the proliferation of OSCC cells, indicating that CNTN1 promotes
malignant phenotypes of OSCC by exclusively activating the
metastastic potential.
Our data demonstrated that CNTN1 expression was
significantly associated with the overall survival of patients with
OSCC (P=0.032; log-rank test) and disease-free survival of patients
with OSCC (P=0.038; log-rank test) by univariate analysis. Thus,
CNTN1 may be a novel predictor of clinical outcome for patients
with OSCC.
Acknowledgements
This work was supported by the Project of Science
and Technology Commission of Shanghai Municipality (Grant nos.
10410711200, 08140902100 and 11495802000) and the Key Project of
Science and Technology Commission of Shanghai Municipality (Grant
no. 03JC14052).
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
Statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
2
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
|
|
3
|
Falk J, Bonnon C, Girault JA and
Faivre-Sarrailh C: F3/contactin, a neuronal cell adhesion molecule
implicated in axogenesis and myelination. Biol Cell. 94:327–334.
2002.
|
|
4
|
Hu QD, Ang BT, Karsak M, et al:
F3/contactin acts as a functional ligand for Notch during
oligodendrocyte maturation. Cell. 115:163–175. 2003.
|
|
5
|
Prag S, Lepekin EA, Kolkova K, et al: NCAM
regulates cell motility. J Cell Sci. 115:283–292. 2002.
|
|
6
|
Shtutman M, Levina E, Ohouo P, Baig M and
Roninson IB: Cell adhesion molecule L1 disrupts
E-cadherin-containing adherens junctions and increases scattering
and motility of MCF7 breast carcinoma cells. Cancer Res.
66:11370–11380. 2006.
|
|
7
|
Su JL, Yang CY, Shih JY, et al: Knockdown
of contactin-1 expression suppresses invasion and metastasis of
lung adenocarcinoma. Cancer Res. 66:2553–2561. 2006.
|
|
8
|
Su JL, Yang PC, Shih JY, et al: The
VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells.
Cancer Cell. 9:209–223. 2006.
|
|
9
|
Sobin LH and Fleming ID: TNM
Classification of Malignant Tumors, fifth edition (1997). Union
Internationale Contre le Cancer and the American Joint Committee on
Cancer. Cancer. 80:1803–1804. 1997.
|
|
10
|
Pindborg JJ, Reichart PA, Smith CJ, et al:
Histological Typing of Cancer and Precancer of the Oral Mucosa. 2nd
edition. Springer-Verlag; Berlin: pp. 24–40. 1997
|
|
11
|
Mignogna MD, Fedele S and Lo Russo L: The
World Cancer Report and the burden of oral cancer. Eur J Cancer
Prev. 13:139–142. 2004.
|
|
12
|
Reid RA, Bronson DD, Young KM and Hemperly
JJ: Identification and characterization of the human cell adhesion
molecule contactin. Brain Res Mol Brain Res. 21:1–8. 1994.
|
|
13
|
Mikami T, Yasunaga D and Kitagawa H:
Contactin-1 is a functional receptor for neuroregulatory
chondroitin sulfate-E. J Biol Chem. 284:4494–4499. 2009.
|
|
14
|
Eckerich C, Zapf S and Ulbricht U:
Contactin is expressed in human astrocytic gliomas and mediates
repulsive effects. Glia. 53:1–12. 2006.
|
|
15
|
Compton AG, Albrecht DE and Seto JT:
Mutations in contactin-1, a neural adhesion and neuromuscular
junction protein, cause a familial form of lethal congenital
myopathy. Am J Hum Genet. 83:714–724. 2008.
|
|
16
|
Lehembre F, Yilmaz M and Wicki A:
NCAM-induced focal adhesion assembly: a functional switch upon loss
of E-cadherin. EMBO J. 27:2603–2615. 2008.
|
|
17
|
Van Kilsdonk JW, Wilting RH and Bergers M:
Attenuation of melanoma invasion by a secreted variant of activated
leukocyte cell adhesion molecule. Cancer Res. 68:3671–3679.
2008.
|
|
18
|
Gavert N, Sheffer M and Raveh S:
Expression of L1-CAM and ADAM10 in human colon cancer cells induces
metastasis. Cancer Res. 67:7703–7712. 2007.
|