Introduction
Multiple myeloma (MM) is a common cancer,
pathologically recognized by a continuous expansion of abnormal
plasma cells in marrow, which leads to progressive damage of the
bones, exerting profound influence on the life and prognosis of
these patients. At present, most of the studies concerning the
molecular mechanism of MM have focused on the activation mechanism
of osteoclasts, however little is known about the inhibition
mechanism of osteoblasts.
The chemotherapeutics currently available for MM
include vincristine, dexamethasone (DXM), melphalan and various
other medicines, but treatment with these agents may lead to the
risk of other types of malignancies of the hematological system,
such as myelodysplastic syndrome (MDS) (1). Proteasome inhibitors and bortezomib
(Velcade®), a newly developed medicine with potential
effect on MM, have been recently introduced and are widely used in
the treatment of recurrent and intractable MM (2). Their rates of efficacy, however,
range between 40 and 60%, even with acute side effects,
dose-limiting toxicity and progressive drug fastness. It has been
reported that preparations containing brucine are noticeably
effective for abirritation, anti-inflammation, immunoregulation
(3), osteoarthritis and inhibition
of the apoptosis of cartilage cells (4,5).
Yet, the role of brucine in bone metabolism in multiple myeloma
which is characterized by bone lesions requires exploration. Given
the insufficient study on the function of osteoblasts in the
mechanism of multiple myeloma, the present study aimed to explore
the nosogenesis of MM from the perspective of both osteoblasts and
osteoclasts.
Materials and methods
Cells
The MM cell line U266 was obtained from Bethune
International Peace Hospital, Shijiazhuang, China. Mouse embryonic
osteoblasts MC3T3-E1 were from the Cell Collection Center of Wuhan
University, China (CCTCC code: CRL-2593).
Differentiation of osteoblast progenitor
cells
Osteoblast progenitor cell line MC3T3-E1 was
cultured in DMEM/high glucose medium containing 10% FBS at 37°C in
5% CO2. When the cells reached a fusion level of 90%,
they were subcultured in 6-well plates.
Fully-grown fusion cells were passaged at a ratio of
1:3, and 5×104 MC3T3-E1 cells were inoculated in 6-well
plates. After 3 h, when all the cells adhered to the side of the
plate, the supernatant was collected, and the culture system
mentioned above was implemented. Three days later the sugar medium
was replaced and 6 days after the culture, cells were
collected.
Differentiation of osteoblast progenitor
cells
The MM cell line U226 was cultured at 37°C in 5%
CO2. Then 2×105/ml cells were inoculated in
flasks of 75 cm2. Three days later, a sample of the
suspended cells was centrifuged in 3 tubes (15 ml and 1000 rpm) for
5 min. The centrifuged cells were then filtered using a sterile
filter with an aperture of 0.22 μm before they were preserved at
−70°C.
The cultured osteoblast progenitor cells were
divided into 2 groups: i) the blank control in DMEM/high glucose
medium containing 10% FBS and ii) the treatment group, containing
30% MM cell supernatant. A total amount of 5×104
MC3T3-E1 cells were cultured on 6-well plates in complete medium
containing 30% of MM cell supernatant. The medium was replaced 3
days later and the cells were collected 6 days later.
MTT analysis
The cell suspension was adjusted to
1×105/ml and the cells were transferred to a 96-well
culture plate, with each well containing 200 μl. To each
experimental group, brucine of a different viscosity was added, but
not in the control group. In addition to the above two groups, a
blank control was also established (medium only but without cells).
For each group, 3 parallel wells were available and the culture was
carried out for 72 h. A total amount of 20 μl of MTT (5 mg/ml) was
added to each well and 4 h later the cells were centrifuged and the
supernatant was discarded. To each well, 150 μl of DMEM was added,
followed by a 10-min oscillation before each group was tested for
their absorbance value (A570) using an automatic
microplate reader. Finally, the cell growth inhibition rates were
calculated. Cell growth inhibition rate (%) = Acontrol
group − Aexperimental group/Acontrol
group − Ablank control × 100%.
According to the linear regression equation, the
half maximal inhibitory concentration (IC50) of brucine
was determined. The same process was followed using bortezomib.
Effects on MM bone metabolism
MC3T3-E1 cells were cultured on 6-well plates in
complete medium contaning 30% MM cell supernatant. In the
bortezomib-treated group, the 30% MM cell supernatant used was
treated with bortezomib (at the concentration of 22.4 nmol/l, which
was the IC50 value of bortezomib) for 48 h. In the
brucine-treated group, the 30% MM cell supernatant was treated with
brucine (at the concentration of 0.16 mg/ml, which was the
IC50 value of brucine) for 48 h.
RT-PCR
Total RNA was extracted from a total amount of
5–10×106 of marrow mononuclear cells using TRIzol
one-step method (TRIzol is a new total RNA extraction reagent) and
then were tested for their concentration and purity with a UV
spectrophotometer. The primer sequences of alkaline phosphatase
(ALP), osteocalcin (OC), osteoprotegerin (OPG) and receptor
activator of nuclear factor κB ligand (RANKL) are shown in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primers | Sequences |
|---|
| ALP |
| P1 |
5′-CCAGCAGGTTTCTCTCTTGG-3′ |
| P2 |
5′-CCAGCAGGTTTCTCTCTTGG-3′ |
| OC |
| P1 |
5′-AAGCAGGAGGGCAATAAGGT-3′ |
| P2 |
5′-TGCCAGAGTTTGGCTTTAGG-3′ |
| OPG |
| P1 |
5′-CTGCCTGGGAAGAAGATCAG-3′ |
| P2 |
5′-TTGTGAAGCTGTGCAGGAAC-3′ |
| RANKL |
| P1 |
5′-AGCCGAGACTACGGCAAGTA-3′ |
| P2 |
5′-GCGCTCGAAAGTACAGGAAC-3′ |
The 50-μl PCR reaction system included a total
amount of 2 μl DNA, 1 μl dNTP at 2.5 mmol/l, 2.5 U Taq Plus
Polymerase, 5 μl 10× buffer and 2.5 μl primer (10 μmol/l).
The reaction conditions included an initial
denaturation at 94°C for 5 min, followed by 32 cycles of 94°C for
20 sec, 56°C for 20 sec and 72°C for 20 sec. The PCR products were
detected by agarose gel electrophoresis, followed by chart
recording using ImageMaster UDS Gel analysis software. The process
was repeated three times. The PCR amplification length was 239 bp
(ALP), 212 bp (OC), 226 bp (OPG) and 208 bp (RANKL).
Statistical analysis
SPSS 11.5 was adopted for the data analysis with all
the results expressed as means ± standard deviation (SD). ANOVA was
chosen for pair-wise comparison.
Results
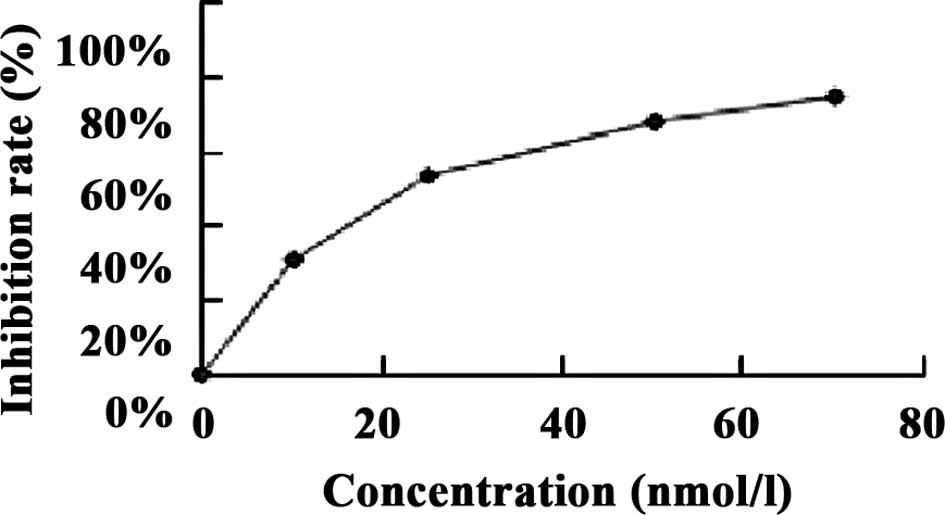
The results showed that the IC50 of
brucine in the U266 cell line at 48 h was 47.84×104
nmol/l, and that of bortezomib was 22.4 nmol/l (Fig. 1).
The ANOVA results between the target bands and the
ratios to the GRAPH gene for the 4 groups were examined for OC,
ALP, OPG and RANKL. The results showed that the P-values for the
comparisons between the treated and the other 4 groups (control,
supernatant (30%)-, bortezomib- and brucine-treated) were <0.05,
indicating a significant difference (Table II).
 | Table IIDifferences in ALP, OC, OPG and RANKL
of the 4 cell groups. |
Table II
Differences in ALP, OC, OPG and RANKL
of the 4 cell groups.
| Groups | ALP | OC | OPG | RANKL |
|---|
| Blank control | 0.8166±0.0108 | 0.9786±0.0034 | 0.8646±0.0032 | 0.2466±0.0054 |
| Supernatant
(30%)-treated | 0.0956±0.0019a | 0.1888±0.0023a | 0.1557±0.0030a | 1.0820±0.0056a |
|
Bortezomib-treated | 0.4830±0.0082a,b | 0.6018±0.0008a,b | 0.6211±0.0010a,b | 0.7474±0.0014a,b |
| Brucine-treated | 0.5277±0.0034a,b,c | 0.6833±0.0052a,b,c | 0.6782±0.0086a,b,c | 0.6890±0.0063a,b,c |
Brucine- and bortezomib-treated groups were
significantly different in regards to levels of ALP, OC, OPG and
RANKL (P<0.05), with the first three variables of the
brucine-treated group larger than those of the bortezomib-treated
group, while RANKL of the brucine-treated group was lower than that
of the bortezomib-treated group, indicating that brucine is
superior in its effects on MM.
Discussion
Extensive data indicate that the major effector
cells that induce MM are osteoclasts (6), derived from mononuclear phagocyte
system and are highly differentiated multiple-core cells capable of
reabsorbing sclerotin (7).
Osteoclasts in the marrow of MM patients, when stimulated by
malignant plasma or other cells in the marrow microenvironment,
increase in number and activity causing more active osteolysis
(8). Additionally, where primary
bone loss occurs, new born formation decreases or even disappears,
causing an imbalance between osteolysis, which progresses rapidly,
and subsequently osteogenesis decreases (or even disappears). These
are exactly the major physiological features of MM. Studies of the
mechanism of MM have focused mainly on the increase in osteoclasts
both in amount and in activity while research on the role of
osteoblasts in MM have received less attention. This study aimed to
explore the nosogenesis of MM from two aspects: osteoclasts and
osteoblasts (9).
The latest discovery that the OPG/RANKL/RANK system
plays a vital role in osteoclastogenesis is a significant
breakthrough in the field of bone physiology (10–12).
The human body contains various cytokines and hormones which exert
effects on bone metabolism by regulating the OPG/RANKL ratio in the
microenvironment of marrow. Furthermore, the OPG/RANKL/RANK system
was recognized only in recent years to be an important signaling
pathway in the process of osteoclast differentiation (13,14).
In brief, RANKL, which is expressed on the surface of
osteoblast/stromal cells, binds to RANK on osteoclast precursors or
osteoclasts (15), and promotes
osteoclastogenesis and bone resorption. However, OPG which is
expressed by osteoblasts/stromal cells, strongly inhibits bone
resorption by binding to its ligand RANKL and thereby blocks the
interaction between RANKL and RANK. The main function of OPG is to
inhibit the differentiation of osteoclasts and the resorption
activity of mature osteoclasts and induce its apoptosis while its
ligand, RANKL or OPGL, contributes to the differentiation of
osteoclasts, vitalizes mature osteoclasts and prevents their
apoptosis.
Therefore, the OPG/RANKL ratio serves as an
important lever in keeping the balance between bone resorption and
formation, and is also a significant indicator of bone remodeling.
Furthermore, their balance determines the activity of osteoclasts
and the resorption of mediated bone (16,17).
By secreting both OPG and RANKL, two key elements, osteoblasts
regulate the activity of osteoclasts and play a critical role in MM
(18).
The culture model of osteoblasts in vitro has
shown that the differentiation of osteogenic progenitor cells (OPC)
fall into three stages: cell proliferation, bone matrix maturation
and bone matrix mineralization (19–21).
In the second stage, i.e., bone matrix maturation, the ALP gene
expression level reaches its peak and in the last stage, bone
matrix mineralization, OC gene expression reaches a noticeable
higher level (22).
The present study revealed that the mRNA expression
of ALP, OC and OPG of an osteoblast cell line, after having been
cultured in complete medium containing 30% multiple myeloma cell
supernatant, decreased noticeably to much lower levels than those
of the blank control group; however the mRNA of RANKL alone
increased, which points to the conclusion that by breaking the
balance between OPG and RANKL, multiple myeloma cells promote the
differentiation of osteoclasts, vitalize their activity, prevent
their apoptosis and inhibit bone generation. The brucine-treated
group, however, showed quite different results. The mRNA expression
of ALP, OC and OPG significantly increased while the mRNA of RANKL
decreased, indicating that brucine inhibits the differentiation of
osteoclasts, and promotes their apoptosis and bone generation. In
addition, it shows that brucine is superior to bortezomib in
regards to the therapeutic effect on MM.
In conclusion, this study explored the manner in
which brucine affects osteoblasts and osteoclasts, (two key
secretions of osteoblasts), in MM to regulate the differentiation
and apoptosis of osteoclasts. Furthermore, it established a cell
model in which MM cells inhibit early osteogenic differentiation
paving the way for further study of the gene mechanism in which
marrow tumors inhibit osteoblasts. At the same time, it revealed
that brucine is superior to bortezomib in regards to the
therapeutic effect on MM, which points out a new approach to the
cure of MM.
In this research, RT-PCR was adopted to measure the
amount of the elements at the gene level. In subsequent studies,
real-time PCR detection methods may be an option for achieving
better credibility and western blot analysis may be used to test
protein levels.
Acknowledgements
This study was supported by the Scientific Research
Projects of Shanxi Province Returned Students (2008, 10, 99).
References
|
1
|
Rao PS, Ramanadham M and Prasad MN:
Anti-proliferative and cytotoxic effects of Strychnos
nux-vomica root extract on human multiple myeloma cell
line-RPMI 8226. Food Chem Toxicol. 47:283–288. 2008.
|
|
2
|
Spisek R, Charalambous A, Mazumder A, et
al: Bortezomib enhances dendritic cell (DC)-mediated induction of
immunity to human myeloma via exposure of cell surface heat shock
protein 90 on dying tumor cells: therapeutic implications. Blood.
109:4839–4845. 2007. View Article : Google Scholar
|
|
3
|
Arron JR and Choi Y: Bone versus immune
system. Nature. 408:535–536. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang HW, Sharp TV, Koumi A, et al:
Characterization of an anti-apoptotic glycoprotein encoded by
Kaposi’s sarcoma-associated herpesvirus which resembles a spliced
variant of human survivin. EMBO J. 21:2602–2615. 2002.PubMed/NCBI
|
|
5
|
Shen Y, Devgan G, Darnell JE Jr and
Bromberg JF: Constitutively activated Stat3 protects fibroblasts
from serum withdrawal and UV-induced apoptosis and antagonizes the
proapoptotic effects of activated Stat1. Proc Natl Acad Sci USA.
98:1543–1548. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan LQ, Xie H, Luo XH, et al: Taurine
transporter is expressed in osteoblasts. Amino Acids. 31:157–163.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanzaki H, Chiba M, Shimizu Y and Mitani
H: Dual regulation of osteoclast differentiation by periodontal
ligament cells through RANKL stimulation and OPG inhibition. J Dent
Res. 80:887–891. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khosla S: Minireview: the OPG/RANKL/RANK
system. Endocrinology. 142:5050–5055. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giuliani N, Colla S and Rizzoli V: New
insight in the mechanism of osteoclast activation and formation in
multiple myeloma: focus on the receptor activator of NF-kappaB
ligand (RANKL). Exp Hematol. 32:685–691. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giuliani N, Colla S, Morandi F,
Barille-Nion S and Rizzoli V: Lack of receptor activator of nuclear
factor-κB ligand (RANKL) expression and functional production by
human multiple myeloma cells. Haematologica. 90:275–278. 2005.
|
|
12
|
Heider U, Zavrski I, Jakob C, et al:
Expression of receptor activator of NF-κB ligand (RANKL) mRNA in
human multiple myeloma cells. J Cancer Res Clin Oncol. 130:469–474.
2004.
|
|
13
|
Jakob F, Seefried L and Ebert R:
Pathophysiology of bone metabolism. Internist (Berl). 49:1159–1160.
2008.(In German).
|
|
14
|
Anastasilakis AD, Toulis KA, Polyzos SA
and Terpos E: RANKL inhibition for the management of patients with
benign metabolic bone disorders. Expert Opin Investig Drugs.
18:1085–1102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eghbali-Fatourechi G, Khosla S, Sanyal A,
Boyle WJ, Lacey DL and Riggs BL: Role of Rank Ligand in mediating
increased bone resorption in early post-menopausal women. J Clin
Invest. 111:1221–1230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
von Knoch F, Jaquiery C, Kowalsky M, et
al: Effects of bisphosphonates on proliferation and osteoblast
differentiation of human bone marrow stromal cells. Biomaterials.
26:6941–6949. 2005.PubMed/NCBI
|
|
17
|
Giner M, Rios MA, Montoya MA, et al:
RANKL/OPG in primary cultures of osteoblasts from post-menopausal
women. Differences between osteoporotic hip fractures and
osteoarthritis. J Steroid Biochem Mol Biol. 113:46–51. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wada N, Maeda H, Tanabe K, et al:
Periodontal ligament cells secrete the factor that inhibits
osteoclastic differentiation and function: the factor is
osteoprotegerin/osteoclastogenesis inhibitory factor. J Periodontal
Res. 36:56–63. 2001. View Article : Google Scholar
|
|
19
|
Owen TA, Aronow M, Shalhoub V, et al:
Progressive development of the rat osteoblast phenotype in vitro:
reciprocal relationships in expression of genes associated with
osteoblast proliferation and differentiation during formation of
the bone extracellular matrix. J Cell Physiol. 143:420–430. 1990.
View Article : Google Scholar
|
|
20
|
Schwartz AV, Sellmeyer DE, Vittinghoff E,
et al: Thiazolidinedione use and bone loss in older diabetic
adults. J Clin Endocrinol Metab. 91:3349–3354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin TH, Yang RS, Tang CH, Lin CP and Fu
WM: PPARgamma inhibits osteogenesis via the down-regulation of the
expression of COX-2 and iNOS in rats. Bone. 41:562–574. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Robinson JA, Harris SA, Riggs BL and
Spelsberg TC: Estrogen regulation of human osteoblastic cell
proliferation and differentiation. Endocrinology. 138:2919–2927.
1997.PubMed/NCBI
|