Introduction
Breast cancer is a challenging disease for medical
science. According to the majority of previous reports from the
International Agency for Research on Cancer, in China, Singapore,
the Republic of Korea and Turkey, the 5-year age-standardized
relative survival rate ranges from 76 to 82% for breast cancer
(1). Surgery, chemotherapy,
radiotherapy and endocrine therapy are widely used to treat
patients with breast cancer (2,3).
However, these conventional treatment methods have not improved the
prognosis of patients with advanced or metastatic breast cancer.
For these reasons, novel methods are required for the treatment of
patients with breast cancer. With the development of oncobiology
and molecular technology, gene therapy represents a novel treatment
model in cancer therapy. Suicide gene therapy is a promising option
due to its high efficacy and clinically useful potential (4,5).
Suicide gene therapy is a type of cancer gene therapy which
transfers prodrug-activating enzyme genes that are found in viruses
and bacteria, but not in mammalian cells, into cancer cells via
genetic engineering, therefore expressing a number of enzymes which
catalyze non-toxic prodrugs into cytotoxic substances to confer
drug sensitivity to the cancer cells (6,7). It
has been observed that not only suicide gene-transfected cancer
cells, but also non-transfected cells, are killed during the
suicide gene therapy by means of the direct killing effect and the
so-called bystander effect (8,9).
The tissue-specific expression of suicide genes for
the selective killing of tumor cells could be realized by taking
advantage of certain tumor-specific transcription modulating
elements, including promoters and enhancers. For example, the α
fetoprotein (AFP) promoter is commonly employed in suicide gene
therapy for hepatic cancer and the erb2 promoter has been
introduced for breast cancer (10,11).
However, most of the common promoters used at present are specific
for a certain type of cancer and their usage is relatively
limited.
The endothelial cell type-specific tyrosine kinase
domain-containing receptor (KDR) is a receptor for the vascular
endothelial growth factor (VEGF). KDR is a critical regulator of
endothelial cell growth and development. It has been demonstrated
that KDR is expressed in the majority of solid cancer cells and
neogenetic vascular endothelial cells of the neoplasma, but not in
normal cells (12,13). Previous studies have revealed that
the KDR promoter has the ability to overexpress genes of interest
exclusively in tumor cells and neogenetic vascular endothelial
cells (14).
In this study, we demonstrate the activity of the
KDR promoter in breast cancer and endothelial cells and show that
the KDR promoter efficiently activates the expression of the double
suicide gene, CDglyTK, which includes the two suicide genes,
thymidine kinase (TK) and cytosine deaminase (CD) in these cells
in vivo and in vitro. The results of the present
study suggest that this system selectively reduces proliferation
and enhances apoptosis in vitro in breast cancer and
endothelial cells, and reduces tumor formation in vivo in
breast cancer.
Materials and methods
Cells and adenovirus (Ad) vector
The ECV304 human umbilical vein vascular endothelial
cells, MCF-7 breast cancer and LS174T colon carcinoma cell lines
were provided by the American Type Culture Collection (Manassas,
VA, USA). These cell lines were maintained in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum
(HyClone Laboratories Inc., Logan, UT, USA), 100 U/ml of penicillin
and 100 μg/ml of streptomycin at 37°C in 5% CO2.
Recombinant Ad vector construction
AdEasy-KDRP-CDglyTK and AdEasy-cytomegalovirus
(CMV)-CDglyTK were also constructed by homologous recombination
between an expression cosmid and the parental virus genome, as
described previously (15,16).
In vitro Ad infections
During the exponential growth phase, cells were
plated in 6-well culture plates at a density of 5×105
cells/well 24 h before the recombinant Ad infection. Immediately
prior to infection, the culture medium was aspirated and the
suspensions of Ad at various multiplicities of infection (MOI)
(from 0 to 200 MOI) were distributed over the monolayers. Following
a 24-h incubation, green fluorescent protein (GFP) expression was
observed by fluorescence microscopy.
Reverse transcription-PCR (RT-PCR)
Total cellular RNA was extracted from cells using
TRIzol reagent (Gibco BRL, Carlsbad, CA, USA) and quantified by UV
absorbance spectroscopy. The reverse transcription reaction was
performed using the RevertAid™ First-Strand cDNA Synthesis kit
(Fermentas, Vilnius, Lithuania) in a final volume of 20 μl
containing 5 μg total RNA, 0.5 μg oligo(dT) 18 primer, 10 mmol/l
deoxynucleotidetriphosphate mixture, 4 μl 5X reverse transcription
buffer, 20 unitsRiboLock™ Ribonuclease inhibitor,
diethylpyrocarbonate-treated water and 200 units RevertAid™ M-Mulv
reverse transcriptase. Following incubation at 42°C for 60 min, the
reverse transcription reaction was terminated by heating at 70°C
for 10 min. The reaction contained 2 μl of cDNA template. The newly
synthesized cDNA was amplified by PCR. The 50-μl reaction mixture
contained 2 μl of cDNA template, 2.5 units of Taq polymerase, 1.5
mmol/l MgCl2 and 0.5 μmol/l of CDglyTK primer (forward,
5′-GGGAAGCTTAGGCTAGCAATGTCGAATAACGCT-3′; reverse,
5′-GGGTCTAGATTAGTTAGCCTCCCCCATCTC-3′). The amplified PCR product
was 2.4 kb. For the β-actin primer (forward,
5′-CTTCTACAATGAGCTGCGTG-3′; reverse, 5′-TCATGAGGTAGTCAGTCAGG-3′),
the amplified PCR product was 305 bp. Amplification cycles were:
94°C for 4 min, then 30 cycles at 94°C for 35 sec, 54°C for 40 sec,
72°C for 30 sec, followed by 72°C for 10 min. Aliquots of the PCR
product were electrophoresed on 1.5% agarose gels and the PCR
fragments were visualized by UV illumination (UVP Inc., Upland, CA,
USA) and stained with ethidium bromide. The fluorescence intensity
of β-actin fragments served as the criterion for the CDglyTK
fragments.
In vitro ganciclovir
(GCV)/5-fluorocytosine (5-FC) sensitivity of cells infected with
recombinant Ad
The cells were seeded and cultured in 96-well plates
at a density of 3×103 cells in 100 μl of medium for 24
h. The culture medium was immediately removed from the wells prior
to infection and the suspensions of Ad-KDRP-CDglyTK and
Ad-CMV-CDglyTK at an MOI of 100 were placed onto the cell
monolayers. Following an incubation for an additional 24 h, the
medium containing the virus was replaced with fresh medium
containing various concentrations of 5-FC (Sigma, St. Louis, MO,
USA) and GCV (Roche Diagnostics, Mannheim, Germany) in combination.
The cells were then cultured at 37°C in a 5% CO2
humidified atmosphere for another 48 h, and cell growth was then
assessed by the MTT assay. Cell proliferation was proportional to
the absorbance at the test wavelength (570 nm), from which the
reference wavelength (620 nm) was subtracted. The results were
expressed as the ratio between the number of viable cells in plates
containing the drugs versus the number of viable cells in the
corresponding drug-free controls.
Specimens for electron microscopy
Infected MCF7 and ECV304 cells in the exponential
phase were used and cultivated with GCV (100 mg/l) and 5-FC (2,000
mg/l) for 48 h. The cells were harvested and fixed with 25 ml/l
glutaraldehyde in 0.1 mol/l phosphate buffer (pH 7.4) for 2 h at
4°C. For transmission electron microscopy (TEM) examination, cells
corresponding to each population were collected in Haemoline
(BioChem Pharma, Allentown, PA, USA), transferred to
microcentrifuge tubes, pelleted and fixed in 1% OsO4 (in
distilled H2O). A total of 4×107 cells were
sorted to collect 2×106 cells representative of each of
the individual populations. Following dehydration through a series
of graded alcohol and propylene oxide solutions, the cells were
infiltrated with Epon (epoxy resin) and polymerized. Ultra-thin
sections were cut, recovered on Formvar-coated copper grids,
stained with uranyl acetate and lead citrate and examined using a
transmission electron microscope (JEM-1200EX, Japan Electron Optics
Laboratory, Tokyo, Japan) operated at 80 kV.
Flow cytometry analysis
We used a FACScan flow cytometer (Becton-Dickinson,
San Jose, CA, USA) equipped with a 488-nm argon ion laser. For
cell-cycle analysis, asynchronous cells (500,000 cells/ml) were
cultured in the presence (control group) or absence of GCV (40
mg/l) and 5-FC (250 mg/l) for 12 h. The cells were harvested and
washed in PBS, fixed in 70% cold ethanol for 30 min at −20°C and
washed again in PBS. The cells were then incubated for 1 h in PBS
containing 100 μg/ml RNase (Sigma) and 50 μg/ml propidium iodide
(PI; Sigma) and incubated at 4°C for 15 min. The cells were then
washed with PBS and immediately analyzed using flow cytometry.
Tumor cell xenograft
To examine the therapeutic effect of the recombinant
Ad in vivo, 4- to 6-week-old female Balb/c nu/nu athymic
mice were used as hosts for MCF7 cell xenografts;
0.5×107 MCF7 cells in 0.2 ml PBS were injected
subcutaneously into the mammary fat pad using an 18-gauge needle
and the tumors were allowed to grow for 2 weeks before randomizing
the mice by size. All tumors were at least 5 mm in diameter and the
mice were grouped according to the treatment regimen. The
recombinant Ads (1×1010 pfu) were injected into the
tumors, followed by GCV and 5-FC treatment (intraperitoneal
injection of 50 mg/kg/day and 500 mg/kg/day for 18 days,
respectively).
Antitumor effect and toxicity of
recombinant Ads and prodrugs in vivo
The perpendicular tumor diameter was measured with a
sliding caliper at 3-day intervals and tumor weight (W) was
calculated using the formula: W=(AxB2)/2 mg (where A is
the longer diameter and B is the shorter diameter) (17), then the tumor growth inhibition
rate was calculated and the growth curves of the tumors were drawn.
Moreover, the hematoxylin and eosin (H&E) staining of the tumor
tissues was performed for histological examination. In addition,
the systemic toxicities of recombinant Ad and prodrugs were
determined by examining histological changes in the heart, lung,
kidney, liver and small intestine of the nude mice following
treatment.
Microvessel density (MVD) assay
To assess tumor angiogenesis, MVD was determined by
immunohistochemical staining of CD34. The tumorous tissues were
obtained and fixed in a sufficient amount of 10% neutral-buffered
formaldehyde for 24 h. The tumorous tissues were embedded in
paraffin and 4-μm sections were made. After blocking the endogenous
peroxidase reaction with 3% H2O2 and
following sodium citrate antigen retrieval (0.01 mmol/l, pH 6.0),
rabbit anti-human CD34 monoclonal antibody (1:150 dilution) was
allowed to react on the serial sections and incubated overnight at
4°C. Biotin-labeled IgG was added to the sections and incubated at
37°C for 30 min. SP complex was added and then diaminobenzidine was
used as a substrate for horseradish peroxidase in the developmental
step. MVD was assessed according to the report by Weidner et
al (18). A single
CD34-positive cell, clusters of endothelial cells clearly separated
from adjacent microvessels and other connective tissue elements
were considered to be vessels. The stained sections were screened
at ×100 magnification under a light microscope to identify the five
regions of the section with the highest vascular density. The
vessels were counted in the five regions at ×200 magnification, and
the average number of microvessels was recorded. The MVD of each
tumorous tissue was expressed as the mean number of microvessels
counted in five high power fields. The microvessels were counted by
two observers and the mean value was used for analysis (19).
Statistical analysis
All data are presented as the means ± standard
deviation (SD). The data were evaluated by one-way ANOVA followed
by the least significant difference (LSD) test as a post-hoc test.
P<0.05 was considered to indicate a statistically significant
result.
Results
Ad-mediated gene transfer efficiency and
CDglyTK production in various cell lines
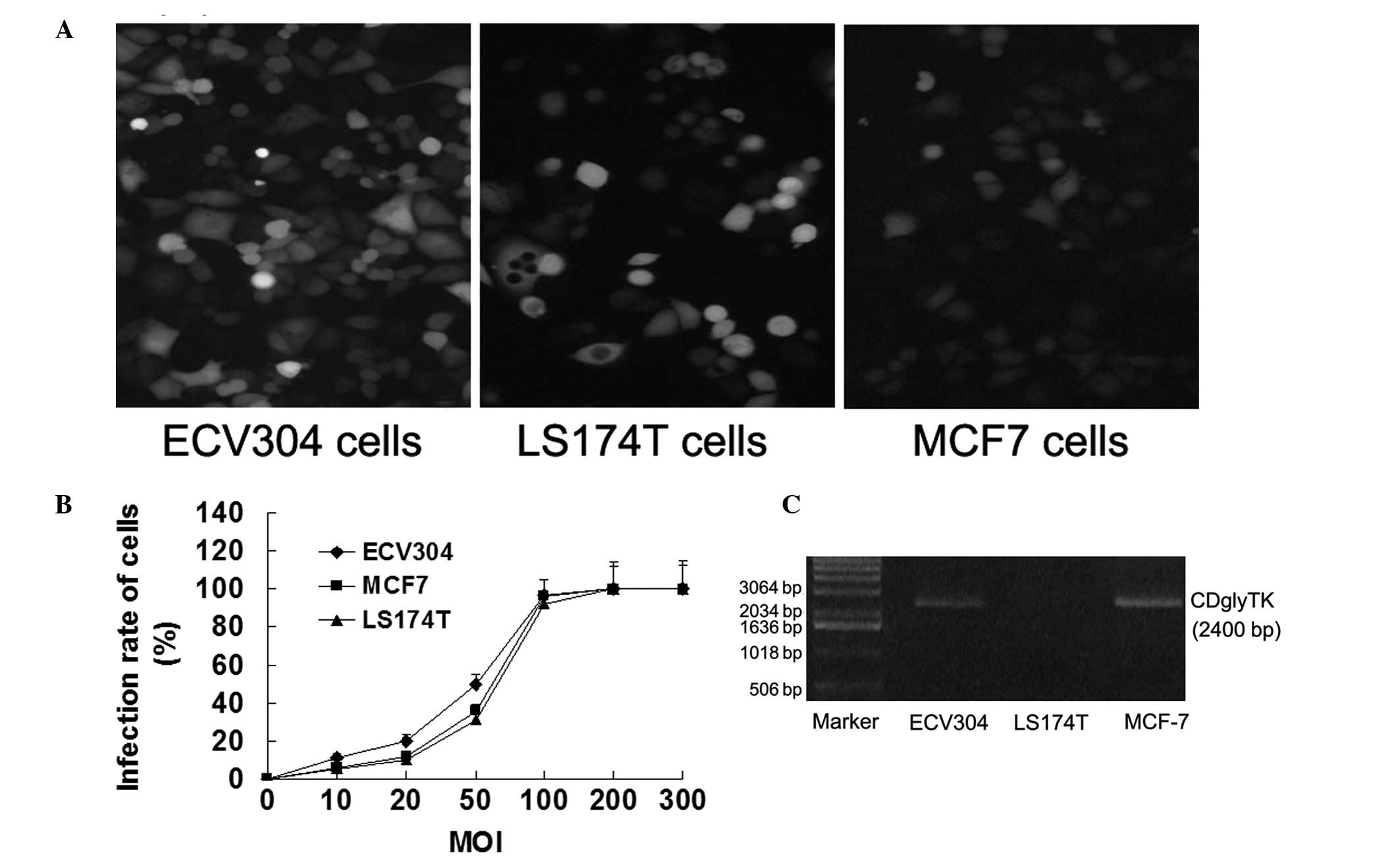
To examine the Ad-mediated gene transfer
efficiencies, MCF7 breast cancer, ECV304 human vascular endothelial
and LS174T human colon carcinoma cell lines were transfected with
Ad-KDRP-CDglyTK and Ad-CMV-CDglyTK at various MOI. When the MOI was
10, only a few cells expressed GFP. When the MOI was 100, 95% of
the cells expressed GFP. When the MOI was 200, almost all the cells
expressed GFP (Fig. 1A and B).
CDglyTK mRNA expression was detected in all the transfected cells
with the exception of in the LS174T cells transfected with
Ad-KDRP-CDglyTK (Fig. 1C).
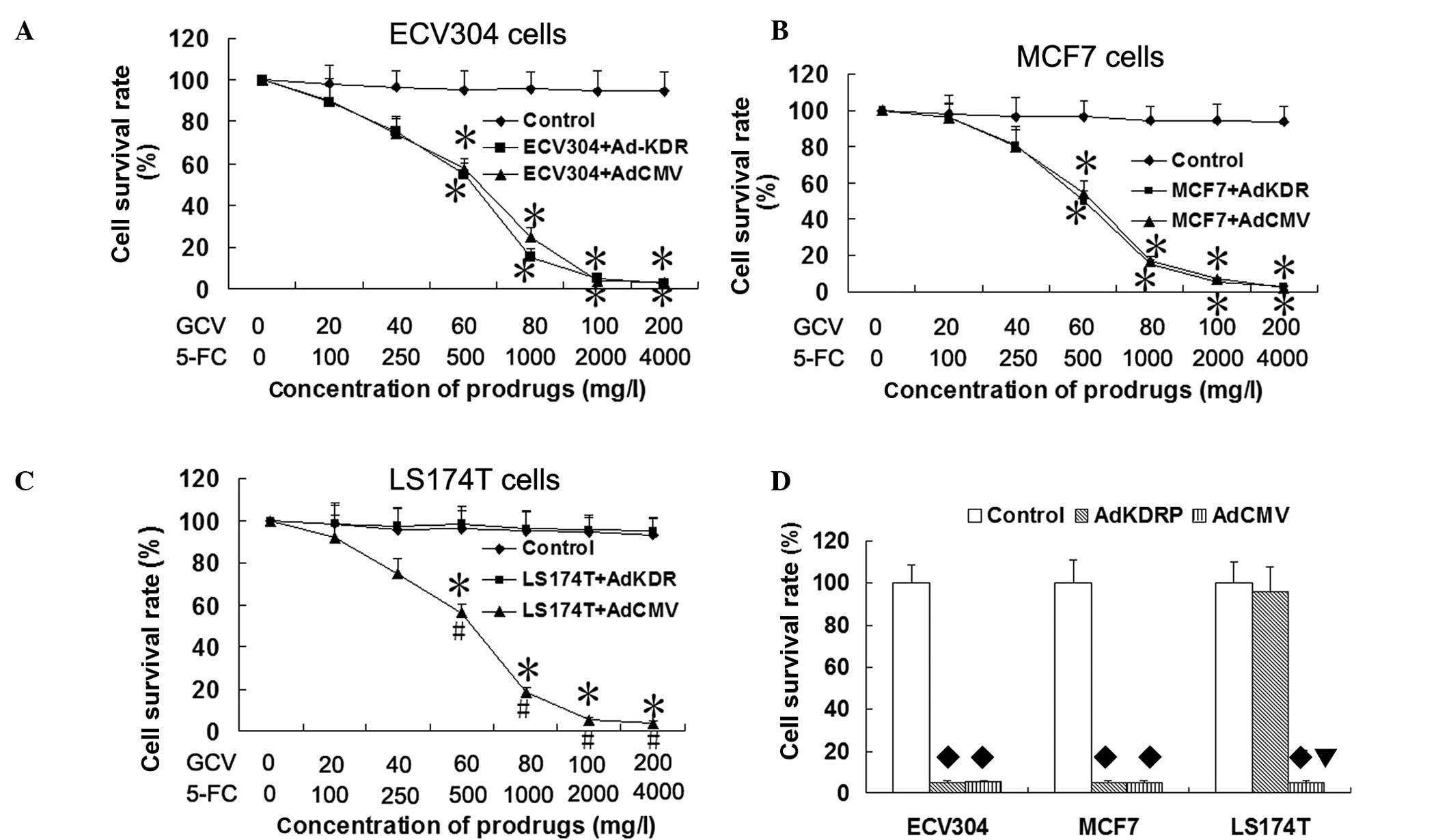
Specificity and efficiency of
Ad-KDRP-CDglyTK in KDR-expressing cells
To analyze the specificity and efficiency of the KDR
promoter induced by CDglyTK gene expression, KDR-expressing cells
(MCF7 and ECV304) and KDR-deficient cells (LS174T) were infected
with Ad-KDRP-CDglyTK or Ad-CMV-CDglyTK at an MOI of 100 and were
exposed to various concentrations of the prodrugs GCV/5-FC for 48
h. As shown in Fig. 2, compared
with the control group, the cell survival rate of the ECV304 and
MCF7 cells infected with Ad-CMV-CDglyTK and Ad-KDRP-CDglyTK and
LS174T cells infected with Ad-CMV-CDglyTK were significantly
decreased following exposure to the prodrugs GCV and 5-FC (all
P<0.05). The survival rates of the sensitive cells decreased
gradually with the increase in the prodrug concentration. Compared
with the control group, the LS174T cells infected with
Ad-KDRP-CDglyTK were insensitive to the two prodrugs (P>0.05).
It was verified that the specific and high-performance killing
effect of Ad-KDRP-CDglyTK was present in the KDR-expressing
cells.
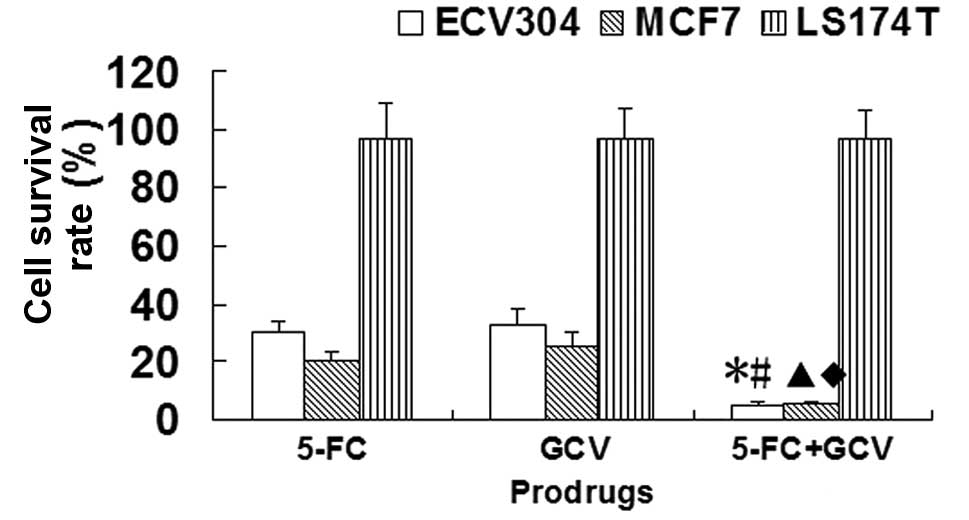
Sensitivity of CDglyTK-expressing cells
to different prodrugs
Compared with the 250 mg/l 5-FC group or 40 mg/l GCV
group, the survival rates of the ECV304 cells infected with
Ad-KDRP-CDglyTK were significantly decreased by treatment with a
combination of GCV and 5-FC (P<0.05). A similar result was
observed in the MCF7 cells infected with Ad-KDRP-CDglyTK. However,
this was not observed in the LS174T cells infected with
Ad-KDRP-CDglyTK (Fig. 3). This
demonstrates that the effect of the double suicide gene was much
stronger than that of each single suicide gene.
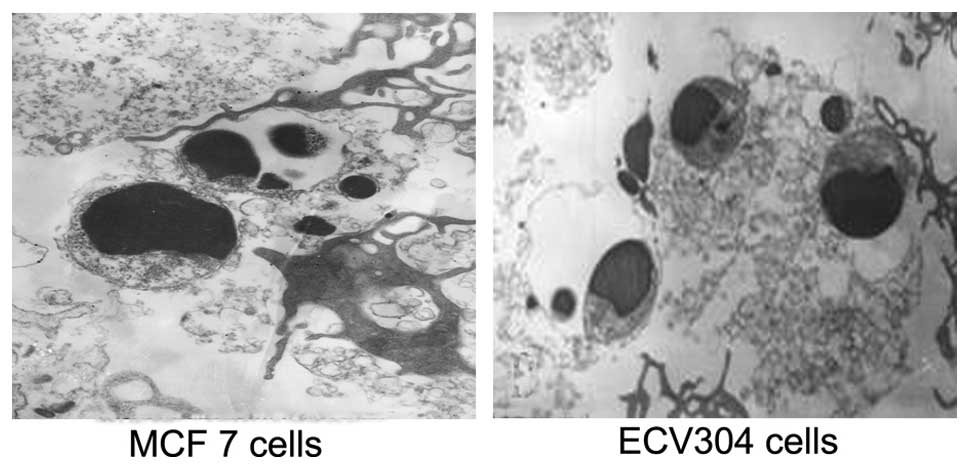
Ultrastructural features of apoptotic
MCF7 and ECV304 cells
After exposure to GCV and 5-FC for 48 h, the
ultrastructure of the cells transfected with KDRP-CDglyTK was
observed using TEM. Cell shrinkage, chromatin gathering along the
nuclear membrane, cell budding, chromatin condensation, chromatin
fragmentation and apoptotic bodies were observed in some infected
cells and the other cells exhibited necrosis (Fig. 4).
Effect of prodrugs on cell cycle of MCF7
and ECV304 cells transfected with KDRP-CDglyTK
The MCF7 and ECV304 cells transfected with
KDRP-CDglyTK were treated with GCV (40 mg/l) and 5-FC (250 mg/l)
for 12 h, the cell cycle was tested by flow cytometry. As shown in
Table I, the percentages of cells
in the G1 and G2 phase were significantly decreased and the
percentage of cells in the S phase was significantly increased
compared with the control group following treatment with GCV (40
mg/l) and 5-FC (250 mg/l) for 12 h in MCF7 cells transfected with
KDRP-CDglyTK (P<0.05). Similar results were observed in ECV304
cells infected with KDRP-CDglyTK (P<0.05; Table II). These results suggest that the
cell cycle was arrested at the S phase following exposure to GCV
(40 mg/l) and 5-FC (250 mg/l) for 12 h in MCF7 and ECV304 cells
transfected with KDRP-CDglyTK.
 | Table IEffect of prodrugs on the cell cycle
in MCF7 cells transfected with KDRP-CDglyTK. |
Table I
Effect of prodrugs on the cell cycle
in MCF7 cells transfected with KDRP-CDglyTK.
| Groups | G1 phase (%) | S phase (%) | G2 phase (%) |
|---|
| Control | 54.50±4.27 | 33.90±2.96 | 11.58±0.75 |
| Transgene | 42.43±3.65a | 57.20±5.14a | 0.43±0.06a |
 | Table IIEffect of prodrugs on the cell cycle
in ECV304 cells transfected with KDRP-CDglyTK. |
Table II
Effect of prodrugs on the cell cycle
in ECV304 cells transfected with KDRP-CDglyTK.
| Groups | G1 phase (%) | S phase (%) | G2 phase (%) |
|---|
| Control | 48.80±2.65 | 34.83±1.74 | 16.48±1.12 |
| Transgene | 37.22±2.01a | 62.60±4.37a | 0.20±0.08a |
Antitumor effect of the recombinant Ads
containing fusion suicide gene in vivo
MCF7 human breast tumor cells were injected into the
corresponding syngeneic nude mice by subcutaneous injection. Tumors
with a mean diameter of 50 mm were established 12 days after the
MCF7 cells were injected. The recombinant Ads (1×1010
pfu) were injected intratumorally, followed by GCV (50 mg/kg/day)
and 5-FC (500 mg/kg/day) treatment by intraperitoneal injection for
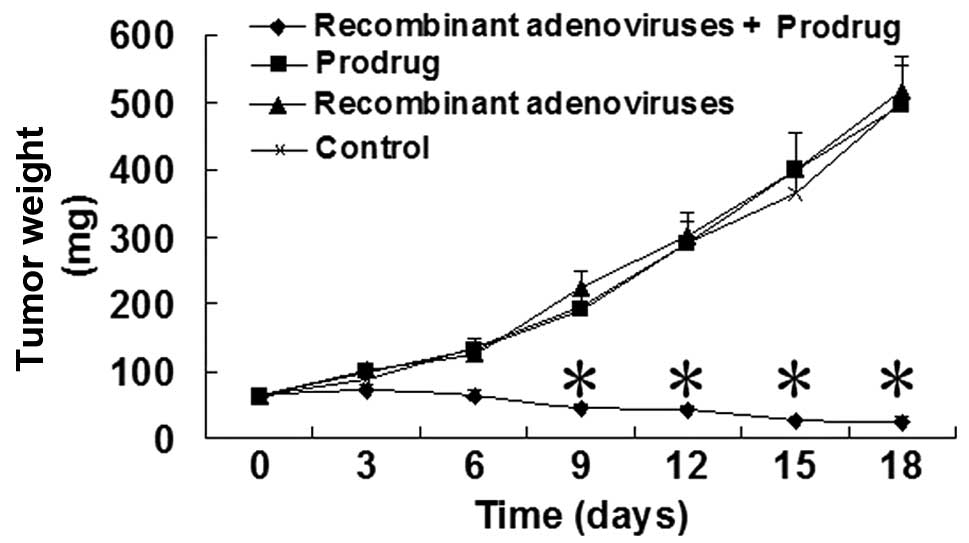
18 days. As shown in Fig. 5, the
tumor weights from the nude mice were significantly decreased in
the recombinant Ad + prodrug group compared with the control group
at 9, 12, 15 and 18 days after the recombinant Ads were injected.
The tumor weights were significantly different between the prodrug,
recombinant Ad and control groups.
Effect of recombinant Ads containing
fusion suicide gene on the histology and MVD of human breast tumors
in nude mice
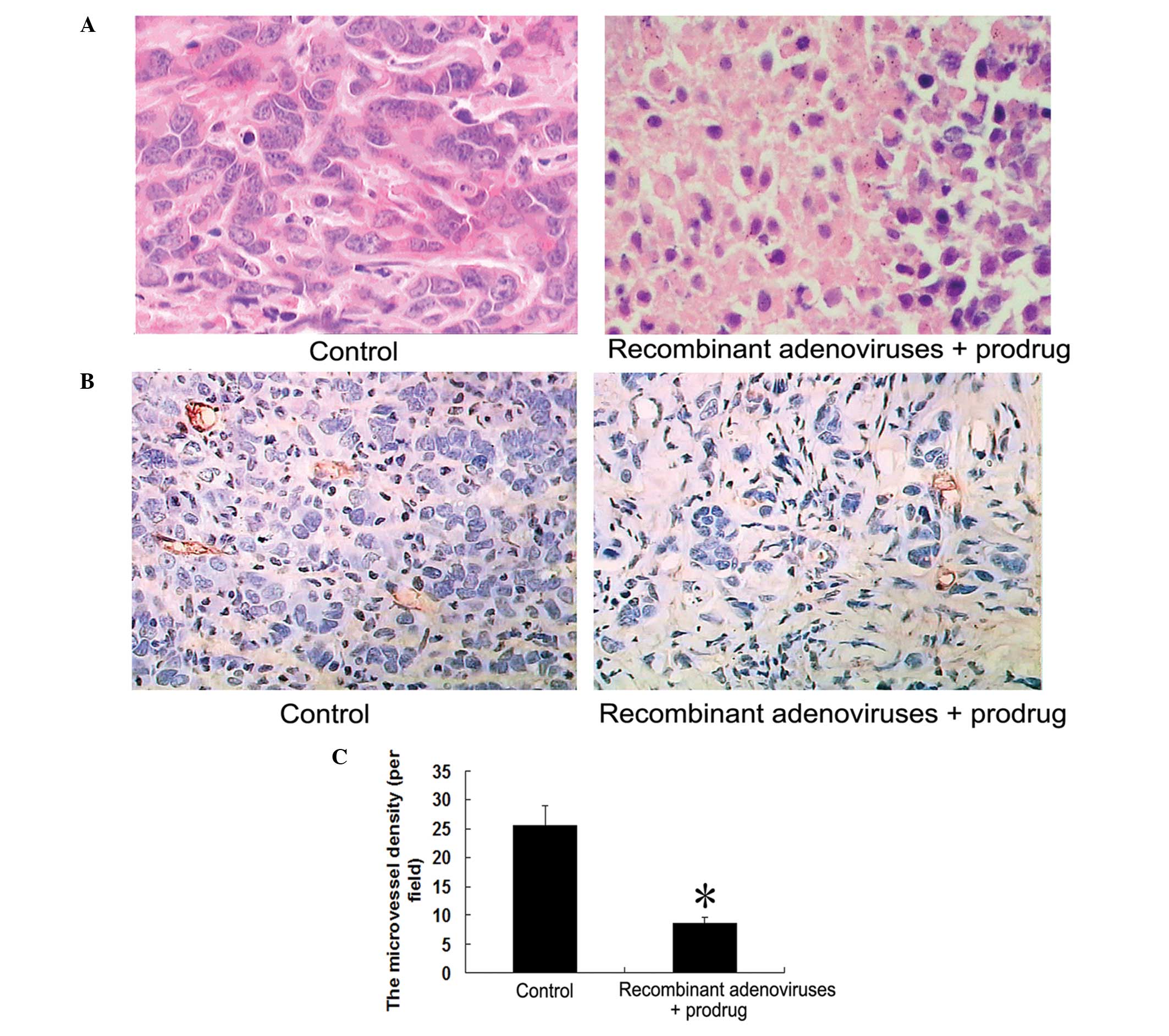
The tumors were excised and analyzed by H&E
staining. H&E sections showed lamellar necrosis of tumor cells
and leukocyte infiltration in the recombinant Ad + prodrug group
(Fig. 6A).
The MVD of the tumor tissue was assessed using
immunohistochemistry with CD34 monoclonal antibody. Compared with
the control group, the MVD of the tumor tissue was significantly
decreased in the recombinant Ad + prodrug group (P<0.05;
Fig. 6B and C).
Histology of heart, liver, lung, kidney
and small intestine
To observe the systemic toxicity of the recombinant
Ads and prodrugs, the liver, heart, lung, kidney and small
intestine of the nude mice transplanted with human breast tumors,
injected with the recombinant Ads and treated with GCV and 5-FC
were examined by H&E staining. There was no abnormal histology
in the heart, liver, lung, kidney and small intestine of the nude
mice transplanted with human breast tumors and injected with the
recombinant Ads and treated with GCV and 5-FC (Fig. 7).
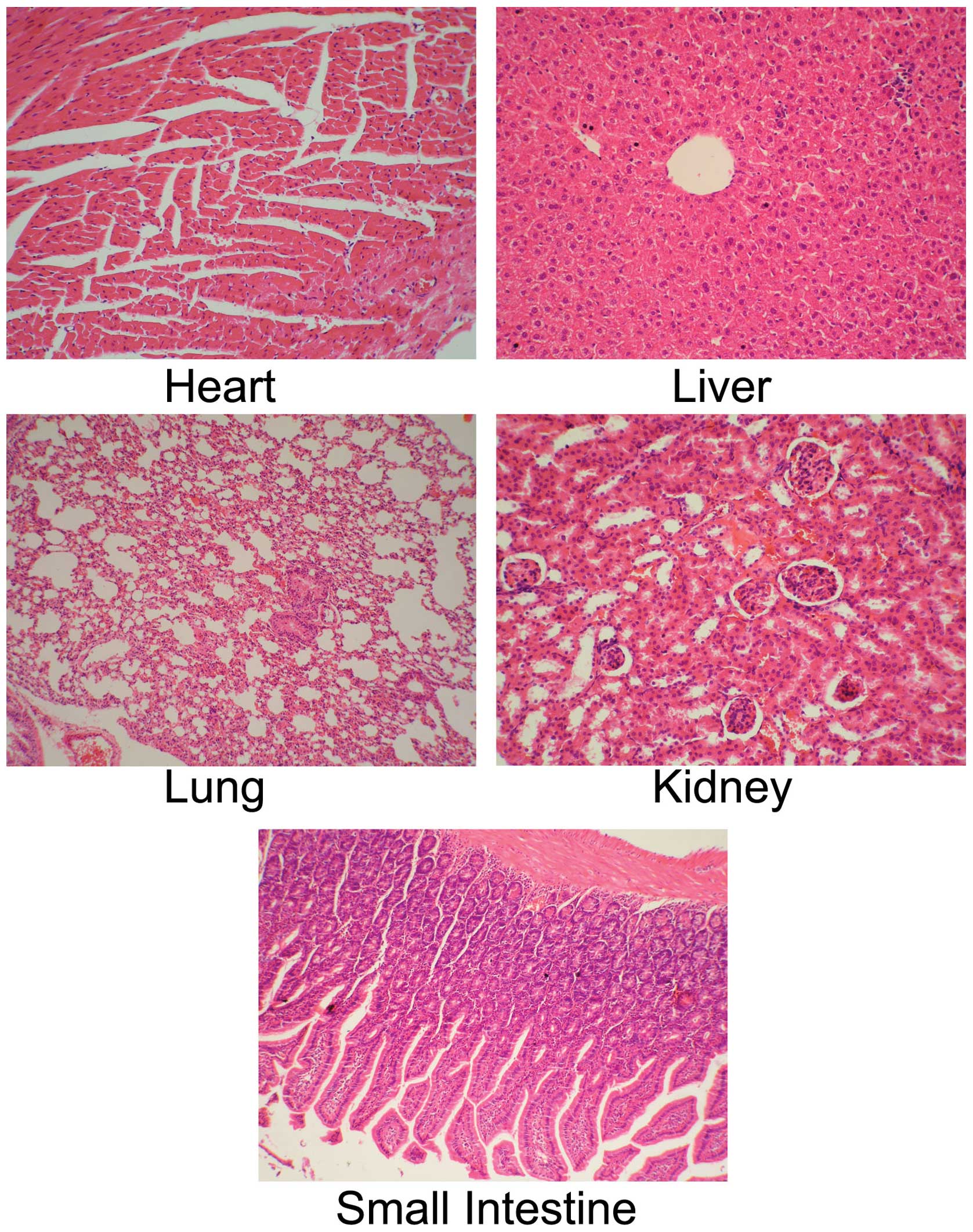 | Figure 7Histology of heart, liver, lung,
kidney and small intestine in nude mice transplanted with human
breast tumors and injected with the recombinant Ad and treated with
GCV and 5-FC. Human breast tumor cells MCF7 were injected into the
nude mice by subcutaneous injection. The tumors, of 50 mm mean
diameter, were established 12 days after MCF7 cells were injected.
The recombinant Ad (1×1010 pfu) were injected into the
tumor, followed by GCV (50 mg/kg/day) and 5-FC (500 mg/kg/day)
treatment by intraperitoneal injection for 18 days. The heart,
liver, lung, kidney and small intestine of nude mice were excised
and analyzed by hematoxylin and eosin (H&E) staining. The
original magnification was ×400. Ad, adenovirus; GVC, ganciclovir;
5-FC, 5-fluorocytosine. |
Discussion
The KDR gene is specifically expressed in certain
tumor and vascular endothelial cells; therefore the KDR promoter
has been used to express target genes in certain tumors due to its
tumor-specific expression (20–22).
In the present study, we report a potential treatment modality for
breast cancer. This strategy involves the use of the tumor and
tumor blood vessel double targeting tissue-specific promoter, KDR,
incorporated into recombinant Ad vectors, to target the expression
of the CDglyTK fusion gene transcriptionally to breast cancer cells
and neogenetic vascular endothelial cells. The Ad-mediated and KDR
promoter-driven CD/TK double suicide gene system was successfully
established and transfected into the MCF7 human breast cancer and
the ECV304 endothelial cell lines, which expressed endogenous KDR,
and the colon carcinoma cell line, LS174T, which did not express
endogenous KDR. The CD/TK gene was expressed in MCF7 and ECV304
cells, but was not expressed in LS174T cells. These results
revealed that the CDglyTK gene was stably expressed in the cells
with a higher level of endogenous KDR.
The results of this study suggest that the double
suicide genes were functionally activated in the three cell lines
(FCF-7, ECV304 and LS174T cells) infected with Ad-CMV-CDglyTK.
However, in the cells infected with Ad-KDRP-CDglyTK, the double
suicide genes were functionally activated only in the MCF7 and
ECV304 cells which expressed endogenous KDR in vitro.
Therefore, treatment with 5-FC, GCV and 5-FC + GCV did not
influence the cell survival rate in LS174T and LS174T-CDglyTK
cells. These results show that the transgenic CDglyTK double
suicide genes are not expressed in the LS174T cells due to the
inactivity of the KDR promoter. The cell survival rate was
significantly decreased by treatment with 5-FC, GC or 5-FC + GCV in
the MCF7 and ECV304 cells transfected with the CDglyTK double
suicide genes. The results of the present study demonstrate that
the tumor-targeted expression of CDglyTK driven by the CMV promoter
has a high performance but does not have specificity and that the
tumor-targeted expression of CDglyTK driven by the KDR promoter has
a high specificity and performance.
A previous study revealed that the killing
efficiency of the combined suicide gene system is higher compared
with any single system in human lung cancer cells (14). Rogulski et al demonstrated
that neuroglioma cells transfected with the CDglyTK double suicide
gene are easily inhibited and that double suicide gene therapy
augments the antitumor activity of a replication-competent lytic Ad
via enhanced cytotoxicity and radiosensitization (23). Qiu et al reported that the
recombinant plasmid, pCEA-TK/CD, containing a carcinoembryonic
antigen (CEA) promoter and the double suicide genes, TK and CD,
decreased the half maximal inhibitory concentration of the prodrugs
and increased apoptosis and cyclomorphosis in the presence of the
prodrugs, 5-FC and GCV, in lung cancer cells (24). The TK/GCV and CD/5-FC suicide gene
therapy induced cell death via the mitochondrial pathway triggered
by the modulation of Bcl-2 proteins in glioma cells (25). Our study indicates that the effect
of double suicide genes is much stronger than that of individual
suicide genes. The combined treatment of 5-FC and GCV resulted in a
lower cell survival rate than the single prodrug treatment, which
suggests that the killing effect of the CDglyTK double suicide
genes combined with prodrug treatment was enhanced. The results of
the present study also showed that the CDglyTK-transduced MCF7 and
ECV304 cells were arrested at the S phase following treatment with
the prodrugs. Moreover, apoptosis and necrosis of cells were
exhibited in the CDglyTK-transduced MCF7 and ECV304 cells.
In order to observe the antitumor effect of
Ad-KDRP-CDglyTK in vivo, breast cancer nude mouse models
from human MCF7 were established. The results from these models
showed that the tumors from the breast cancer cells were
significantly suppressed and that the MVD of tumors was decreased
by the systemic treatment of the prodrugs, 5-FC and GCV, in the
breast cancer nude mouse models with the CDglyTK gene. Therefore,
Ad-KDRP-CDglyTK has both tumor cell-targeting and tumor vessel
blood-targeting effects. Abnormal histology of liver, heart, lung,
kidney and small intestine was not observed in the breast cancer
nude mouse models with the CDglyTK gene. These results suggest that
the recombinant Ads and prodrugs do not exert systemic toxicity to
nude mice.
In conclusion, our study suggests that the KDR
promoter is capable of regulating a double suicide gene system in
human breast cancer cells and vascular endothelial cells, thus
providing evidence for the development of a gene therapy approach
to treating breast cancer. Our research indicates that the
expression of CDglyTK genes under the control of the KDR promotor
represents a new strategy for the effective gene therapy of breast
cancer.
Acknowledgements
This study was supported by a grant from the
National High Technology Research and Development Program of China
(2001AA217171), Natural Science Foundation of Guangdong Province
(No. 013072) and the Program of Health Bureau of Xiamen City
(WSZ0609).
References
|
1
|
Sankaranarayanan R, Swaminathan R, Jayant
K and Brenner H: An overview of cancer survival in Africa, Asia,
the Caribbean and Central America: the case for investment in
cancer health services. IARC Sci Publ. 162:257–291. 2011.PubMed/NCBI
|
|
2
|
Gebbia V, Boussen H and Valerio MR: Oral
metronomic cyclophosphamide with and without methotrexate as
palliative treatment for patients with metastatic breast carcinoma.
Anticancer Res. 32:529–536. 2012.PubMed/NCBI
|
|
3
|
Mustacchi G, Cazzaniga ME, Pronzato P, et
al: Breast cancer in elderly women: a different reality? Results
from the NORA study. Ann Oncol. 18:991–996. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cocconi G, Caminiti C, Zaninetta G, et al:
National survey of medical choices in caring for terminally ill
patients in Italy, a cross-sectional study. Tumori. 96:122–130.
2010.PubMed/NCBI
|
|
5
|
Chatterjee SJ and Pandey S:
Chemo-resistant melanoma sensitized by tamoxifen to low dose
curcumin treatment through induction of apoptosis and autophagy.
Cancer Biol Ther. 11:216–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim KY, Kim SU, Leung PC, et al: Influence
of the prodrugs 5-fluorocytosine and CPT-11 on ovarian cancer cells
using genetically engineered stem cells: tumor-tropic potential and
inhibition of ovarian cancer cell growth. Cancer Sci. 101:955–962.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tai CK, Wang W, Lai YH, et al: Enhanced
efficiency of prodrug activation therapy by tumor-selective
replicating retrovirus vectors armed with the Escherichia
coli purine nucleoside phosphorylase gene. Cancer Gene Ther.
17:614–623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kucerova L, Matuskova M, Hlubinova K, et
al: Bystander cytotoxicity in human medullary thyroid carcinoma
cells mediated by fusion yeast cytosine deaminase and
5-fluorocytosine. Cancer Lett. 311:101–112. 2011. View Article : Google Scholar
|
|
9
|
Cottin S, Gould PV, Cantin L, et al: Gap
junctions in human glioblastomas: implications for suicide gene
therapy. Cancer Gene Ther. 18:674–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vernimmen D, Gueders M, Pisvin S, et al:
Different mechanisms are implicated in ERBB2 gene overexpression in
breast and in other cancers. Br J Cancer. 89:899–906. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomizawa M, Yu L, Wada A, et al: A
promoter region of the midkine gene that is frequently expressed in
human hepatocellular carcinoma can activate a suicide gene as
effectively as the alpha-fetoprotein promoter. Br J Cancer.
89:1086–1090. 2003. View Article : Google Scholar
|
|
12
|
Beierle EA, Dai W, Langham MR Jr, et al:
Expression of VEGF receptors in cocultured neuroblastoma cells. J
Surg Res. 119:56–65. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siemann DW and Shi W: Efficacy of combined
antiangiogenic and vascular disrupting agents in treatment of solid
tumors. Int J Radiat Oncol Biol Phys. 60:1233–1240. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma J, Li M, Mei L, et al: Double suicide
genes driven by kinase domain insert containing receptor promoter
selectively kill human lung cancer cells. Genet Vaccines Ther.
9:1–6. 2011.PubMed/NCBI
|
|
15
|
Yu Z, Wang H, Zhang L, et al: Both
p53-PUMA/NOXA-Bax-mitochondrion and p53-p21cip1 pathways are
involved in the CDglyTK-mediated tumor cell suppression. Biochem
Biophys Res Commun. 386:607–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang D, Yang J, Lu F, et al: A new
membrane re-anchored protein originating from GPC3 against hepatoma
cells HepG2. Mol Med Report. 4:1067–1073. 2011.PubMed/NCBI
|
|
17
|
Huber BE, Austin EA, Good SS, et al: In
vivo antitumor activity of 5-fluorocytosine on human colorectal
carcinoma cells genetically modified to express cytosine deaminase.
Cancer Res. 53:4619–4626. 1993.
|
|
18
|
Weidner N, Folkman J, Pozza F, et al:
Tumor angiogenesis: a new significant and independent prognostic
indicator in early-stage breast carcinoma. J Natl Cancer Inst.
84:1875–1887. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mauro LV, Bellido M, Morandi A, et al:
Association between mast cells of different phenotypes and
angiogenesis in colorectal cancer. Mol Med Report. 1:895–902.
2008.PubMed/NCBI
|
|
20
|
Oh JY, Park MY, Kim DR, et al: Combination
gene therapy of lung cancer with conditionally replicating
adenovirus and adenovirus-herpes simplex virus thymidine kinase.
Int J Mol Med. 25:369–376. 2010.PubMed/NCBI
|
|
21
|
Ge YL, Zhang JY, Zhang X, et al:
Chemically modified siRNA directed against the KDR gene inhibits
the proliferation of breast cancer cells. Mol Med Report.
2:121–127. 2009.PubMed/NCBI
|
|
22
|
Szary J, Kalita K, Przybyszewska M, et al:
KDR promoter can transcriptionally target cytosine deaminase
suicide gene to cancer cells of nonendothelial origin. Anticancer
Res. 21:3471–3475. 2001.PubMed/NCBI
|
|
23
|
Rogulski KR, Wing MS, Paielli DL, et al:
Double suicide gene therapy augments the antitumor activity of a
replication-competent lytic adenovirus through enhanced
cytotoxicity and radiosensitization. Hum Gene Ther. 11:67–76. 2000.
View Article : Google Scholar
|
|
24
|
Qiu Y, Peng GL, Liu QC, et al: Selective
killing of lung cancer cells using carcinoembryonic antigen
promoter and double suicide genes, thymidine kinase and cytosine
deaminase (pCEA-TK/CD). Cancer Lett. 316:31–38. 2012. View Article : Google Scholar
|
|
25
|
Fischer U, Steffens S, Frank S, et al:
Mechanisms of thymidine kinase/ganciclovir and cytosine
deaminase/5-fluorocytosine suicide gene therapy-induced cell death
in glioma cells. Oncogene. 24:1231–1243. 2005. View Article : Google Scholar : PubMed/NCBI
|