Introduction
Cerebrovascular disease, also known as stroke, is a
clinically common disease characterized by high morbidity,
mortality, disability and recurrence. Ischemic cerebrovascular
disease (cerebral infarction) accounts for more than 70% of
cerebrovascular disease. Two million individuals in China develop
cerebrovascular disease each year, with an incidence rate of
120/100,000 individuals. Cerebrovascular disease has a detrimental
effect on the patients’ family and society (1). Therefore, there is an urgent need to
understand the pathogenesis of this disease to improve treatment
and prevention. Currently, studies concerning stroke pathogenesis
mainly involve energy deficit, excitatory amino acid toxicity, cell
depolarization, calcium influx, inflammation and apoptosis
(2). Of these, neuronal apoptotic
mechanisms currently draw the most focus (3). Apoptosis is regulated by various
cytokines including the caspase enzyme family (4). Caspase-3 plays a key role in neuronal
apoptosis following cerebral ischemia reperfusion (5). In this study, a rat model of cerebral
ischemia reperfusion injury was established to observe the effect
of aloe polysaccharides on neuronal caspase-3 protein and mRNA
expression. This study provides mechanistic insight into the
effects of aloe polysaccharides on ischemic brain injury.
Materials and methods
Animals, drugs and reagents
Male Wistar rats (weight, 240–300 g, average
260.3±45.6) were supplied by the Experimental Animal Center of
Jiangsu Province (Nanjing City, China). The rats had free access to
food and water, but were fasted for 12 h prior to surgery. The aloe
polysaccharides were prepared by the Department of Pharmacology,
Nanjing University of Chinese Medicine (Nanjing, China). Other
supplies included nimodipine tablets (Shanghai SCOND Pharmaceutical
Co., Ltd., Shanghai, China), Ginkgo biloba tablets (Shanghai
Xingling Science and Technology Pharmaceutical Co., Ltd., Shanghai,
China), caspase-3 rabbit anti-mouse polyclonal antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), TRIzol reagent and
primer nucleotide fragments (Invitrogen, Carlsbad, CA, USA) and RNA
PCR Kit (Toyobo Co., Ltd., Osaka, Japan).
Experimental apparatus
The equipment used in the experiments includes an
automatic biological tissue hydroextractor (ZT-12P2, Xiaogan
Yaguang Medical Electronics Co., Ltd., Hubei, China), a
refrigerated investing machine (YB-6LF, Xiaogan Yaguang Medical
Electronics Co., Ltd), semi-automatic rotary microtome (HM340E,
Microm, Walldorf, Germany), an optical microscope (CX31, Olympus,
Tokyo, Japan), professional digital camera (DPIXEL200, Guangzhou
Tykor Computer Co., Ltd.), Image-Pro Plus 6.0 image analysis
software (Media Cybernetics, Bethesda, MD, USA), PCR amplification
meter (Type 5331, Eppendorf, Hamburg, Germany); DYY-III Type 613
electrophoresis apparatus, Type DYY-33B horizontal electrophoresis
tank (Beijing 61 Factory, Beijing, China) and sterilized
workstations for medical use (Suzhou Purification Equipment
Corporation, Suzhou, China).
Grouping and administration
Rats (n=80) were randomly divided into five groups
(n=16 each): aloe polysaccharide (60 mg/kg), nimodipine treatment
(21.6 mg/kg), Ginkgo biloba (135 mg/kg), model control and
sham surgery groups. The rats were administered an equal volume of
saline and received the treatments by gavage continuously for 7
days.
Establishment of model of right middle
cerebral artery occlusion
A modified method of that described by Longa et
al (6) was used to establish
right middle cerebral artery occlusion (MCAO) 30 min after the
administration of the final treatment. Following MCAO surgery (30
min after the restoration of consciousness), the neurological
deficit of the rats was scored and those with post-MCAO scores of
1–3 were included in the study. The qualified rats from the five
groups were then divided into two groups; one group was used to
measure caspase-3 protein expression in the cerebral cortex (n=6
rats per group) and the other was used to measure caspase-3 mRNA
expression (the remaining rats).
Caspase-3 protein detection
The rats were anesthetized with an intraperitoneal
injection of 10% chloral hydrate (350 mg/kg) followed by rapid
thoracotomy to expose the heart. The right atrial appendage was cut
open, a tube was inserted from the left ventricle into the
ascending aorta and approximately 150 ml of heparin saline was
infused over 5 min. Then, 200 ml of 0.1 mol/l of phosphate buffer
containing 4% paraformaldehyde was infused slowly to allow tissue
fixation. When the infusion was complete, a cut was made from the
foramen magnum to remove the whole brain. The tissue was placed in
the same fixative at 4°C. Paraffin sections were sliced to a
thickness of 4 μm and the slices were treated using the SP method.
When the reaction was terminated, the slices were re-stained with
hematoxylin, then rinsed with clear water, stained blue and treated
with gradient ethanol dehydration, dimethylbenzene verification,
sealed with neutral resins and observed. The caspase-3
immunohistochemistry results were observed under a light
microscope. Images of the slides were captured in 10 high-power
fields and 100 cells were counted per field for a total of 1,000
cells. The average positive rate (%) was calculated as (number of
positive cells/total cells counted) × 100.
Caspase-3 mRNA detection
Chloral hydrate (10%) was intraperitoneally injected
for anesthesia. The fur on the heads of the rats was wetted with
75% ethanol and the head was removed from the neck. The scalp was
stripped and a small hole was cut in the skull. After changing
gloves, the skull was prised open with vessel pincers to expose the
brain tissue. Approximately 100 mg of cortex was cut from the
parietal lobe of the cerebral ischemic side, using a surgical blade
treated with DEPC water: a cut of 7–11 mm from the coronal plane to
frontal pole was made, the fan-shaped ischemic penumbra was
separated, and 1/3 of the distance from longitudinal fissure to
brain cortex on the lateral brain was removed. The cortex was
placed in cold saline to remove the blood and absorb water. The
brain tissue was placed in numbered freezing tubes and then into
liquid nitrogen and stored in an ultra-low temperature refrigerator
at −80°C. The reverse transcription-polymerase chain reaction
(RT-PCR) was used to detect caspase-3 mRNA expression.
Statistical analysis
SPSS 13.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis and data were presented
as the mean ± standard deviation. Single-factor analysis of
variance was applied to compare the average positive rates of
caspase-3 protein and the caspase-3 mRNA/ β-actin ratio among the
groups followed by inter-group, pairwise comparison using the SNK
method. The above hypothesis test is two-sided with a test level α
of 0.05. P-values of <0.05 were considered to indicate a
statistically significant result.
Results
Caspase-3 protein expression among the
groups
The immunohistochemistry results showed that
caspase-3-positive cells exhibited brown particles in the cytoplasm
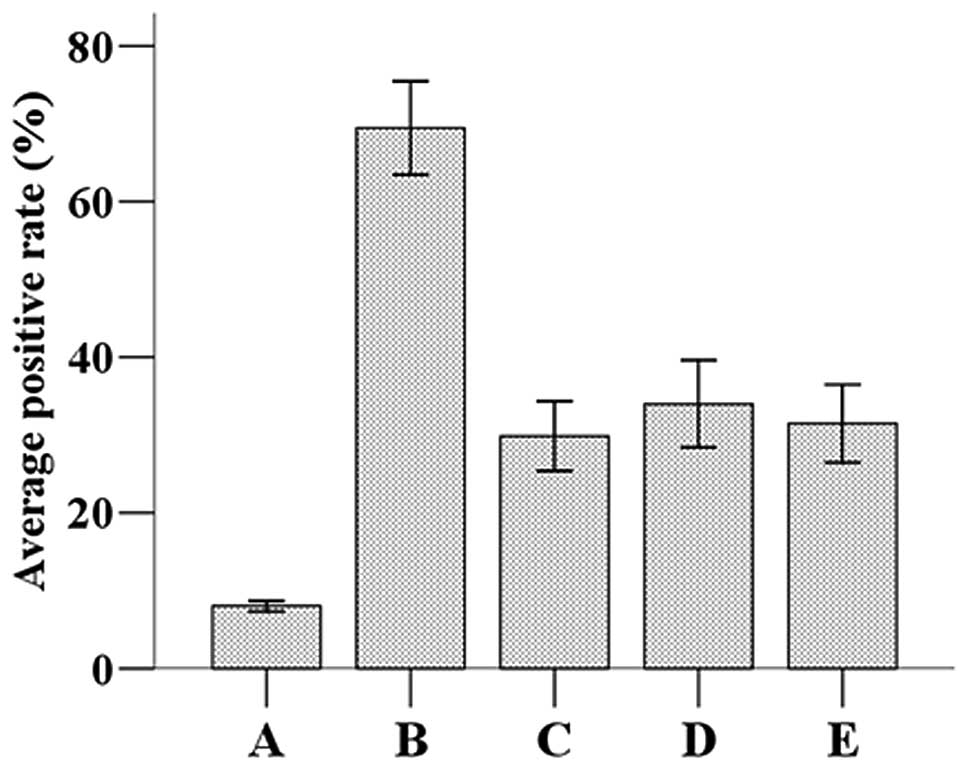
and/or nuclei. Caspase-3 protein expression and the average
positive rates among the groups are shown in Fig. 1. The statistical analysis revealed
that the average positive rate of caspase-3 protein in the model
group (69.453±5.747) was significantly higher compared with the
sham surgery group (8.039±0.653; P<0.05). The average positive
rates of caspase-3 protein in the nimodipine (29.839±4.294),
Ginkgo biloba (33.970±5.328) and the aloe polysaccharide
groups (31.479±4.763) were significantly lower than those in the
model group, but higher than those in the sham group (P<0.05).
However, no significant difference was found in the positive rates
of caspase-3 protein among the nimodipine, Ginkgo biloba and
aloe polysaccharide groups (P>0.05).
Caspase-3 mRNA expression among the
groups
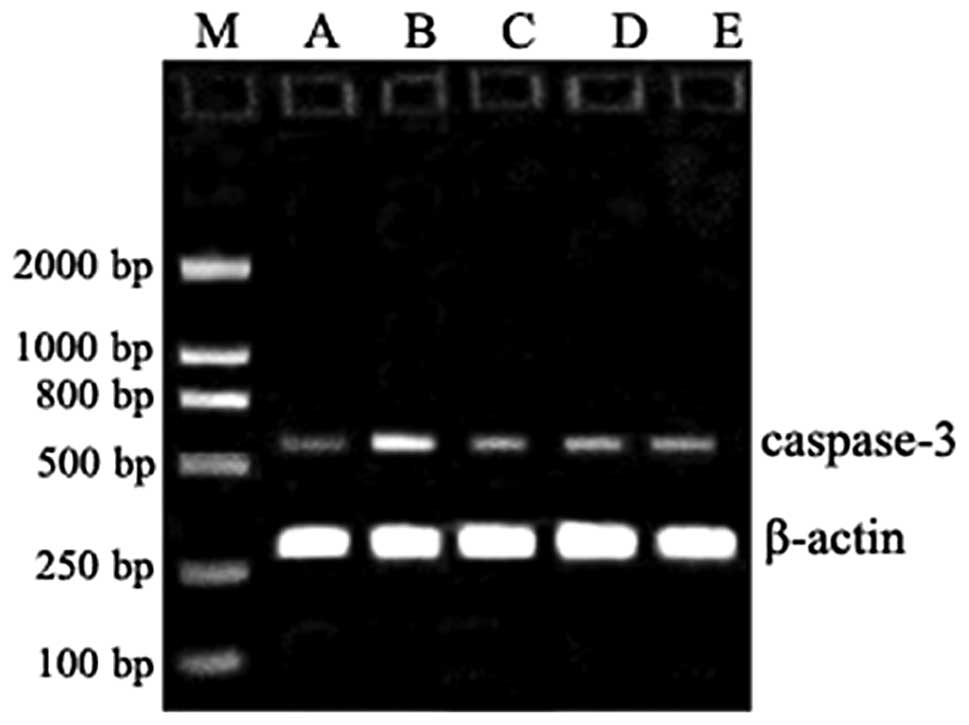
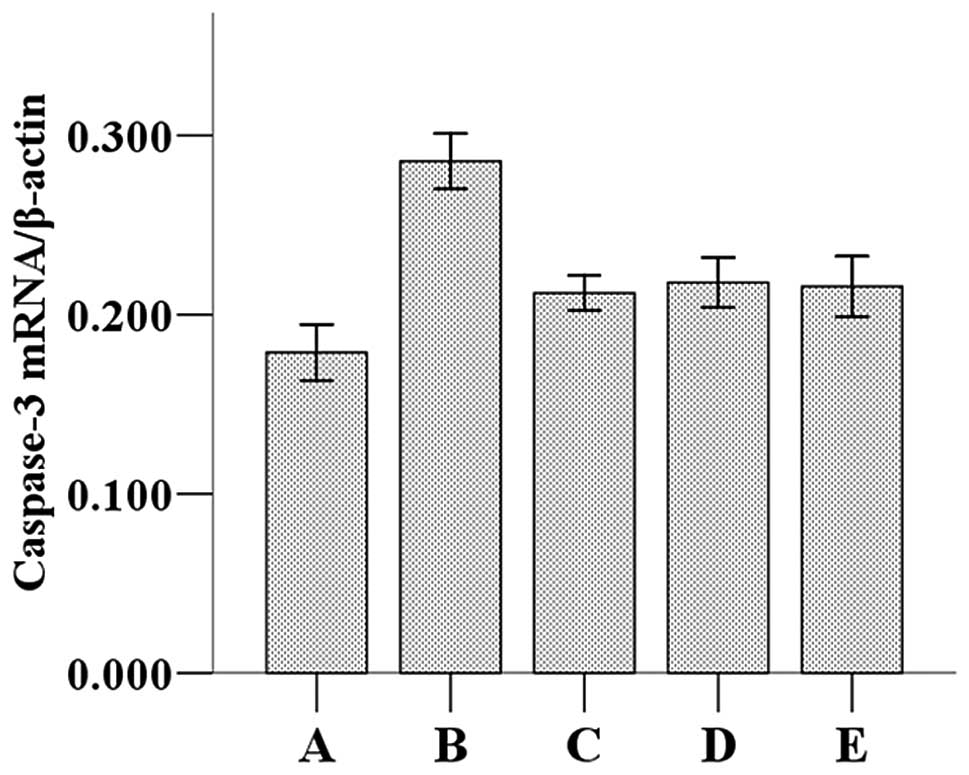
The caspase-3 mRNA expression and mRNA/β-actin
values in the groups are shown in Figs. 2 and 3. The statistical analysis revealed that
the caspase-3/β-actin ratio (0.286±0.020) was significantly higher
compared with the sham group (0.179±0.022, P<0.05). The
caspase-3/β-actin values in the nimodipine (0.212±0.012), Ginkgo
biloba (0.218±0.017) and the aloe polysaccharide (0.216±0.022)
groups were significantly lower than those in the model group but
higher than those in the sham group (P<0.05). However, no
significant difference was observed between the caspase-3/β-actin
ratio among the nimodipine, Ginkgo biloba and the aloe
polysaccharide groups (P>0.05).
Discussion
During ischemic brain damage, a series of cell
metabolism abnormalities may be induced due to interrupted blood,
oxygen and glucose supply. This energy depletion eventually leads
to neuronal death or apoptosis (7). Previous studies have revealed that
neuronal apoptosis plays a key role in the pathological process of
cerebral ischemia-reperfusion injury (7). In the process of apoptosis, proteins
that have crucial functions are affected by specific caspase
enzymes (8). Caspases belong to a
highly conserved cysteine protease family that triggers a cascade
of proteolysis. All caspases decompose at substrate-specific
aspartic acid residues. Upstream caspases successively activate the
downstream apoptosis-promoting executive caspases (9). In the caspase cascade, caspase-8, as
an initiating protease, activates a series of proteases, including
caspase-9 and -7, and finally activates caspase-3, which is an
executor of apoptosis. Caspase-3 is able to incise itself and
affect key proteins during apoptosis execution, including the DNA
breaks that are typical of apoptosis (10).
In the normal mouse brain, caspase-3 is expressed at
low levels (11). Krupinski et
al found that following cerebral ischemic injury, caspase-3
peaks at 12–24 h in the zone of infarction and the surrounding
penumbra in rats (12). According
to a study by Harrison et al, compared with the ipsilateral
cortex in the control and sham groups and the contralateral cortex
of the MCAO rats, the caspase-3 mRNA expression in the ipsilateral
cortex (the side with damage) in MCAO rats significantly increased
at 6, 12 and 24 h, suggesting the involvement of caspase-3 in
infarction (13). The results of
the present study reveal that caspase-3 protein and mRNA expression
levels in an ischemia-reperfusion injury model in rats were
significantly higher compared with the sham group. This finding
indicates that caspase-3 is involved in the pathological process of
cerebral ischemia-reperfusion injury.
While the caspase-3 protein and mRNA expression
levels in rats treated with aloe polysaccharides were lower
compared with those in the sham group, they were not significantly
different compared with rats treated with nimodipine and Ginkgo
biloba. This observation indicates that aloe polysaccharides
are able to downregulate caspase-3 expression and have a preventive
effect on neuronal apoptosis following ischemic brain injury. The
mechanism by which this occurs may be associated with the
prevention of the activation of the caspase cascade by regulating
cellular proteins or cytokine secretion. It may also be associated
with directing the inhibition of caspase-3 expression and reduced
brain neuronal apoptosis. Other relevant anti-ischemic mechanisms
require further study and confirmation.
In conclusion, caspase-3 is important during
neuronal apoptosis and its downregulation reduces neuronal
apoptosis and cerebral ischemia-reperfusion injury. Aloe
polysaccharides are able to inhibit caspase-3 expression, limit
apoptosis and provide neuroprotection.
References
|
1
|
Caplan LR, Searls DE and Hon FK:
Cerebrovascular disease. Med Clin North Am. 93:353–369. 2009.
View Article : Google Scholar
|
|
2
|
Siegel C, Turtzo C and McCullough LD: Sex
differences in cerebral ischemia: possible molecular mechanisms. J
Neurosci Res. 88:2765–2774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:331–339.
2009. View Article : Google Scholar
|
|
4
|
Kumar S and Dorstyn L: Analysing caspase
activation and caspase activity in apoptotic cells. Methods Mol
Biol. 559:3–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li M, Li H, Li C, et al: Alpha fetoprotein
is a novel protein-binding partner for caspase-3 and blocks the
apoptotic signaling pathway in human hepatoma cells. Int J Cancer.
124:2845–2854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Longa EZ, Weinstein PR, Carlson S, et al:
Reversible middle cerebral artery occlusion without craniectomy in
rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakka VP, Gusain A, Mehta SL, et al:
Molecular mechanisms of apoptosis in cerebral ischemia: multiple
neuroprotective opportunities. Mol Neurobiol. 37:7–38. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho BB and Toledo-Pereyra LH:
Caspase-independent programmed cell death following ischemic
stroke. J Invest Surg. 21:141–147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar S: Caspase function in programmed
cell death. Cell Death Differ. 14:32–43. 2007. View Article : Google Scholar
|
|
10
|
Walsh JG, Cullen SP, Sheridan C, et al:
Executioner caspase-3 and caspase-7 are functionally distinct
proteases. Proc Natl Acad Sci USA. 105:12815–12819. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimohama S, Tanino H and Fujimoto S:
Differential expression of rat brain caspase family proteins during
development and aging. Biochem Biophys Res Commun. 289:1063–1066.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krupinski J, Lopez E, Marti E, et al:
Expression of caspases and their substrates in the rat model of
focal cerebral ischemia. Neurobiol Dis. 7:332–342. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harrison DC, Davis RP, Bond BC, et al:
Caspase mRNA expression in a rat model of focal cerebral ischemia.
Brain Res Mol Brain Res. 89:133–146. 2001. View Article : Google Scholar : PubMed/NCBI
|