Introduction
Prostate cancer is the most common form of cancer
among men in the US. In 2010, it was reported that 220,000 men were
newly diagnosed with prostate cancer and 32,050 men succumbed to
the disease (1). Almost one in six
men are likely to develop prostate cancer at some point in their
lives, with the majority of incidences occurring after the age of
50 (2). Various therapies,
including hormones, surgery, radiation and chemotherapy, have been
used for the treatment of prostate cancer. However, all of these
treatments suffer from limitations, and in the majority of cases, a
relapse of the disease occurs (3).
Therefore, new strategies for the treatment of prostate cancer are
required (4).
The Bcl-2 family of proteins, including
anti-apoptotic [Bcl-2, Bcl-xL and myeloid cell leukemia-1 (Mcl-1)]
and pro-apoptotic (Bax, Bak, Bad, Bid, Bim, Puma and Noxa) members,
regulate apoptotic processes through an intrinsic mitochondrial
apoptosis signaling pathway (5).
One of the anti-apoptotic proteins, Mcl-1, is highly expressed in a
number of cancer cell types to promote their survival (5–8).
Several studies have revealed that Mcl-1 expression correlates with
the resistance of cancer cells to chemotherapy and that the genetic
inhibition of Mcl-1 induces apoptosis in several types of cancer
(9–13). Interestingly, recent studies have
shown that the overexpression of Mcl-1 in cancer cells is inhibited
by various medicinal plants to induce apoptosis (9,14,15).
Sanguisorba officinalis L. is a valuable
medicinal plant in Korea, China and Japan, where it is used
traditionally for the treatment of inflammatory and metabolic
diseases, including diarrhea, chronic intestinal infections,
duodenal ulcers and bleeding (16). Previous studies have reported that
S. officinalis L. includes various biologically active
compounds and exhibits anticancer activity (17,18).
However, the molecular mechanism underlying this anticancer
activity has not yet been fully investigated. Therefore, the aims
of this study were to evaluate the growth-inhibitory effect of a
methanol extract of S. officinalis L. (MESO) on human
prostate cancer cells and to elucidate the signaling pathway
instrumental in mediating MESO-induced apoptosis in human prostate
cancer cells. This study provides evidence that MESO induces
apoptotic cell death to inhibit the growth of prostate cancer
cells. In addition, the downregulation of Mcl-1 expression and the
oligomerization of Bax in the mitochondrial outer membrane are
revealed to mediate the apoptotic cell death.
Materials and methods
Reagents
The antibodies to cleaved caspase 3, Mcl-1, Bax, Bak
and Bcl-xL were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Actin antibody was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
4′,6-Diamidino-2-phenylindole (DAPI) and propidium iodide (PI) were
acquired from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). MESO
was obtained from Professor K.H. Kwon (Gwangju, Korea).
Cell culture and chemical treatment
The PC3 human prostate cancer cells were provided by
Dr Hwan-Mook Kim (Korea Research Institute of Bioscience and
Biotechnology, Daejeon, Korea). The cells were cultured in RPMI
medium containing 10% fetal bovine serum (FBS) and 100 U/ml each of
penicillin and streptomycin (WelGENE Inc., Daegu, South Korea) in a
humid atmosphere of 5% CO2. Equal numbers of cells were
seeded and allowed to attach overnight. The cells were treated with
0.1% dimethyl sulfoxide (DMSO) or MESO (40, 80 and 120 μg/ml)
diluted in RPMI with 5% FBS for 48 h.
MTS assay
We used the CellTiter 96 Aqueous One Solution Cell
Proliferation Assay kit (Promega Corporation, Madison, WI, USA) for
the estimation of cell viability. The cells were seeded in 96-well
plates and incubated with various concentrations of MESO. Following
treatment with MESO for 48 h, 30 μl MTS
[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfo-phenyl)-2H-tetrazolium]
solution was added to each well and the cells were incubated for 2
h at 37°C. The MTS solution was analyzed using a microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA) at 490 and 690 nm
(background).
DAPI staining
DAPI staining was performed to determine the
morphology of the cell nuclei following treatment with MESO.
Briefly, the PC3 cells were treated with MESO or 0.1% DMSO and
harvested by trypsinization. The cells were resuspended in PBS,
deposited on poly-L-lysine-coated slides, stained with DAPI
solution (2 μg/ml) and observed under a fluorescence
microscope.
Western blot analysis
The MESO-treated PC3 cells were harvested and
suspended in lysis buffer. Following sonication, the cell lysates
were cleared by centrifugation at 13,000 rpm for 20 min at 4°C. The
protein supernatant fractions were subjected to SDS-PAGE and then
transferred to polyvinylidene difluoride (PVDF) membranes and
blocked with 5% skimmed milk in Tris-buffered saline containing
0.1% Tween 20 (TBST). The PVDF membranes were incubated with
primary antibody in TBST overnight at 4°C. The membranes were then
washed with TBST and incubated with secondary antibody in 5%
skimmed milk in TBST for 90 min at room temperature (RT). After
washing with TBST, the membranes were developed using an enhanced
chemiluminescence detection kit (ECL, Santa Cruz
Biotechnology).
Crosslinking
To evaluate Bax oligomerization, the PC3 cells were
treated with DMSO or MESO for 48 h. The cells were harvested and
suspended in a conjugation buffer with 10 mM EDTA. The lysates were
incubated with 0.2 mM 1,6-bismaleimidohexane (BMH, Thermo Fisher
Scientific, Waltham, MA, USA) at RT for 1 h and then extracted
using lysis buffer for western blot analysis.
Statistical analysis
Data were assessed for statistical significance
using the Student’s t-test. A p-value <0.05 compared with the
vehicle control was considered to indicate a statistically
significant result.
Results
MESO decreases the growth of PC3
cells
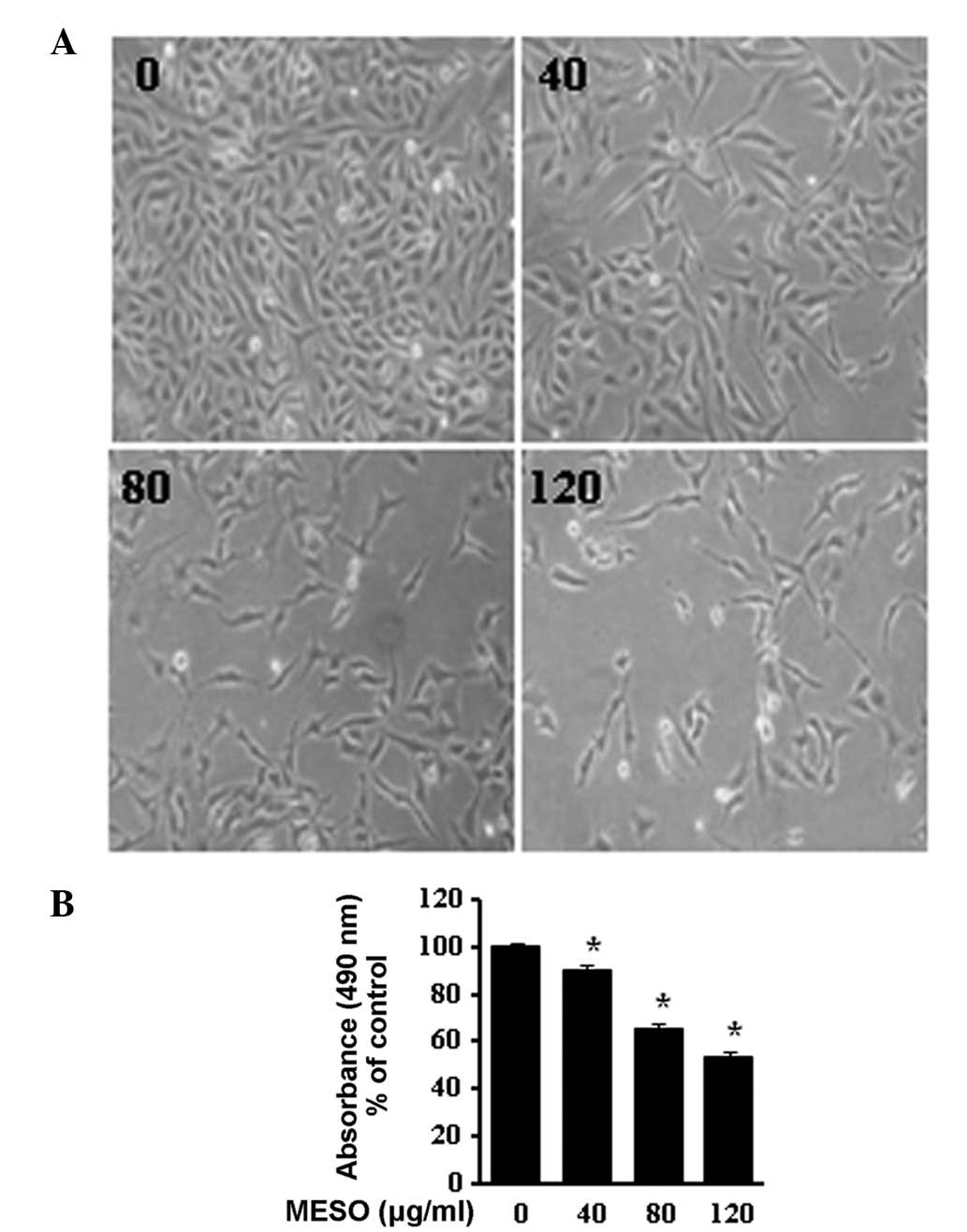
To determine the growth inhibitory effect of MESO in
PC3 cells, we first investigated the morphological changes in the
cells using optical microscopy. The images revealed that the cells
rounded up and their numbers clearly decreased in a
concentration-dependent manner (Fig.
1A). The effect of MESO on cell viability was examined using an
MTS assay. MESO inhibited the proliferation of the PC3 cells in a
concentration-dependent manner. The ID50 value of MESO
for the PC3 cells was 120 μg/ml (Fig.
1B). These results suggest that MESO is an inhibitor of PC3
human prostate cancer cell growth.
MESO induces apoptosis through an
intrinsic signaling pathway in PC3 cells
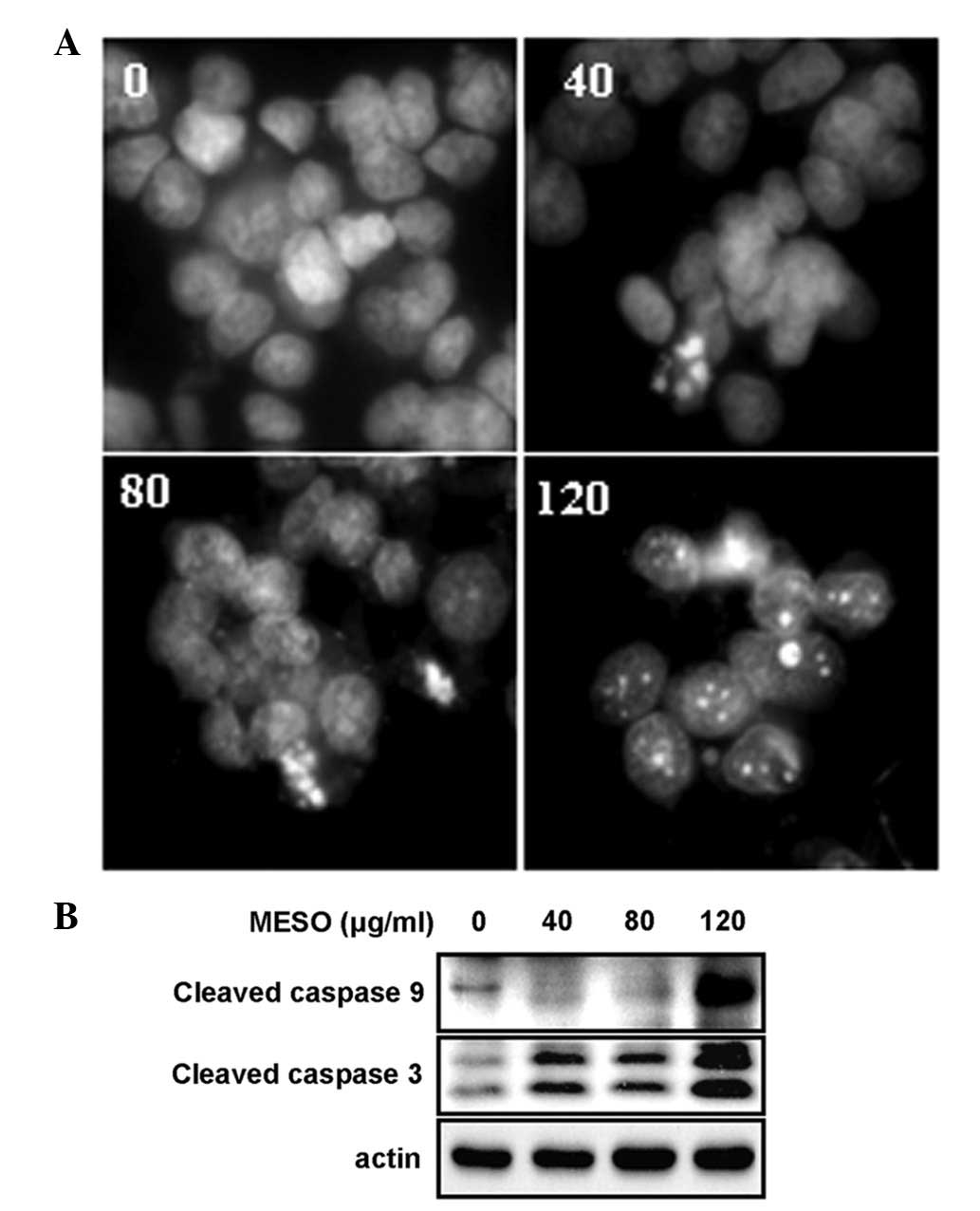
To investigate whether the MESO-induced growth
inhibition was related to an apoptotic effect, apoptotic cell death
in the MESO-treated PC3 cells was evaluated by DAPI staining and
western blot analysis using anti-caspase 9 and anti-caspase 3. The
results revealed that the treatment of the cells with MESO
increased the number of condensed and fragmented nuclei compared
with DMSO treatment (Fig. 2A). In
addition, MESO activated caspase 9 and caspase 3 (Fig. 2B). These results suggest that the
growth inhibitory effect of MESO in the PC3 cells was due to
apoptotic cell death.
MESO increases the level of Bax
expression by inhibiting the Mcl-1 anti-apoptotic protein
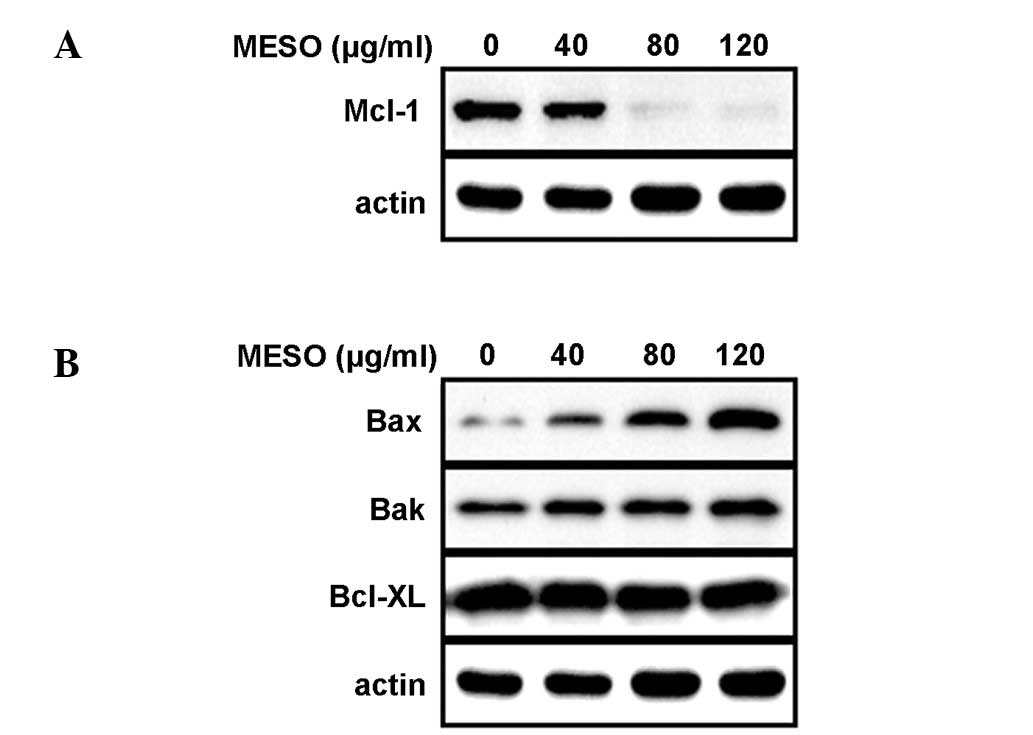
Having confirmed that MESO induced apoptosis and
thereby inhibited the growth of PC3 cells, we next investigated the
molecular mechanism underlying the MESO-induced apoptosis. When the
PC3 cells were exposed to MESO for 48 h, the expression levels of
Mcl-1 protein decreased in a concentration-dependent manner
(Fig. 3A). We also analyzed the
expression levels of the Bcl-2 family proteins that are essential
for apoptotic signaling. The results demonstrated that the
expression levels of Bax protein in the PC3 cells were increased by
MESO, whereas those of Bak and Bcl-xL proteins were not (Fig. 3B). This suggests that MESO reduces
the Mcl-1 protein levels and increases the Bax protein levels in
PC3 cells to induce apoptosis.
MESO increases Bax oligomerization in the
mitochondrial outer membrane
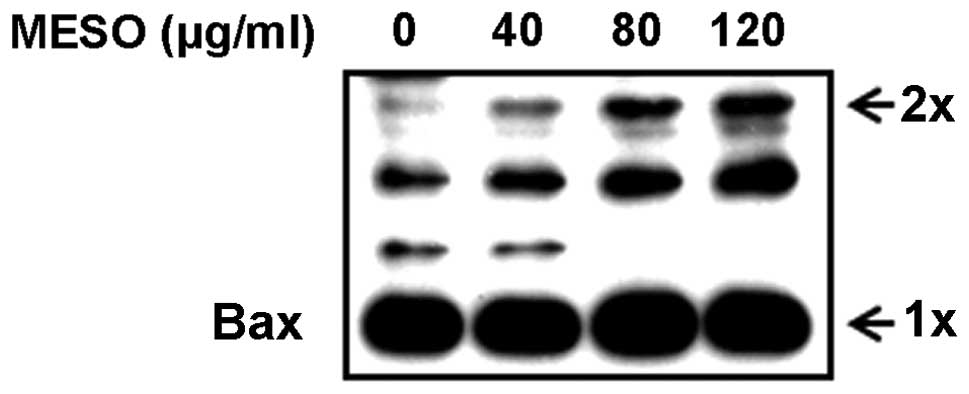
According to previous studies, when apoptotic
signals are received, BH3-only proteins competitively bind to the
hydrophobic groove of the anti-apoptotic proteins and displace Bax,
which mediates structural reorganization, leading to mitochondrial
targeting and homo-oligomerization (19). Therefore, we examined whether MESO
affected Bax oligomerization in the PC3 cells. The results revealed
that MESO increased Bax oligomerization in a
concentration-dependent manner (Fig.
4). This suggests that MESO promotes Bax oligomerization in the
mitochondrial outer membranes of the PC3 cells.
Discussion
Several studies have revealed that certain naturally
occurring medicinal plants inhibit the growth of various cancers
(20–22). Specifically, it has been reported
that the use of medicinal plants among prostate cancer patients is
extremely popular (23,24). One of these plants, Sanguisorba
officinalis L., has been effectively used for the treatment of
inflammation and metabolic diseases, as well as cancer (24,25).
Its ethanol extract exhibits anticancer activity by inhibiting
nitric oxide (NO) and prostaglandin E2 through suppression of the
NF-κB and AP-1 activation signaling cascades. However, the
anticancer activity of a methanol extract in PC3 human prostate
cancer cells has not yet been investigated. Therefore, we evaluated
the effects of MESO on the growth of PC3 cells and the mechanisms
underlying these effects.
Initially, we investigated the effects of MESO on
cell morphology and viability using light microscopic observation
and MTS assays. The exposure of the PC3 cells to various
concentrations of MESO clearly caused a concentration-dependent
inhibition of cell growth and cell detachment, suggesting that MESO
inhibited the proliferation of the prostate cancer cells by
affecting cell viability. We then investigated the apoptotic
effects of MESO in the PC3 cells and found that MESO induced
apoptosis, as evidenced by the concentration-dependent appearance
of nuclear condensation and fragmentation and increased amounts of
cleaved poly(ADP-ribose) polymerase. Mammalian cell apoptosis is
initiated by intrinsic or extrinsic pathways (20). The intrinsically mediated pathway
is known as the mitochondria-initiated pathway and in this pathway
cytochrome c is released from the mitochondria, which converts
procaspase 9 into active caspase 9. Activated caspase 9 then
cleaves and activates downstream caspases, including caspases 3, 6
and 7 (19). We sought to
determine whether the MESO-induced apoptosis was intrinsic or
extrinsic. The results revealed that the levels of the cleaved
forms of caspase 9 and caspase 3 were increased by MESO, indicating
that MESO induced apoptosis through the intrinsic signaling pathway
to inhibit the growth of PC3 cells.
Numerous apoptosis-related genes, including
pro-apoptotic genes (Bax and Bak) and anti-apoptotic genes (Bcl-xL,
Bcl-2 and Mcl-1), play significant roles in the apoptotic signaling
pathway. The expression of Mcl-1 protects cancer cells from the
apoptotic signaling pathway (26).
Several studies have reported that prostate carcinogenesis is
induced by the involvement of overexpressed Mcl-1 genes, and that
downregulation of the Mcl-1 gene leads to apoptosis in prostate
cancer cells (27,28). This suggests that Mcl-1 is a good
molecular target for the treatment of prostate cancer. Since the
mechanism by which MESO exerts its apoptotic effects is unclear, we
investigated the effects of MESO on the expression of the Mcl-1
protein in PC3 cells. We found that MESO decreased the expression
levels of the Mcl-1 protein. The Mcl-1 protein is primarily
localized in the outer mitochondrial membrane and promotes cell
survival by suppressing cytochrome c release from the mitochondria
via hetero-dimerization with, and neutralization of, effector
pro-apoptotic Bcl-2 family members, including Bax and Bak (5,29,30).
However, when apoptotic signals are received, activator BH3-only
proteins (Bim, PUMA and tBid) bind and activate Bax and/or Bak
directly if they are not bound and neutralized by Bcl-2-like
proteins, including Mcl-1 (5,31–36).
Thus, we investigated the effects of MESO on Bax, Bak and Bcl-XL.
The data demonstrated that MESO increased the Bax protein levels in
PC3 cells whereas the levels of Bak and Bcl-xL proteins were
unchanged. These findings indicated that MESO may regulate Bax
protein levels as a downstream molecule of the Mcl-1 protein.
The permeabilization of the mitochondrial outer
membrane is initiated by changes in the expression of Bcl-2 family
proteins. The structural reorganization of Bax from its inactive
conformation leads to mitochondrial targeting and
homo-oligomerization (19).
Oligomerization releases cytochrome c from the mitochondrial
intermembrane space into the cytosol, where it binds to Apaf-1 and
coordinates the formation of the Apaf-1/caspase 9 apoptosome
(37–39). Bax activation is mediated by
structural reorganization and leads to mitochondrial targeting and
homo-oligomerization (19).
Several studies have reported that various anticancer drugs derived
from plant extracts induce apoptosis in cancer cells that is
accompanied by Bax oligomerization and the release of cytochrome c
from the mitochondria into the cytosol (40–42).
Therefore, we sought to confirm the occurrence of Bax
oligomerization in the PC-3 cells. In our study, we revealed that
MESO induced Bax oligomerization in the PC3 cells in a
concentration-dependent manner. These findings suggest that the
MESO-induced Bax oligomerization promotes apoptosis through an
intrinsic mitochondria-initiated apoptosis signaling pathway in the
PC3 human prostate cancer cells.
In conclusion, we demonstrated that MESO has a
growth inhibitory effect on PC3 cells and induces apoptosis via an
intrinsic apoptotic pathway. We also provided evidence that the
apoptotic effect of MESO is caused by the modulation of Mcl-1 and
Bax protein levels, leading to the oligomerization of Bax in the
mitochondrial outer membranes. Therefore, we suggest that MESO is a
meaningful medicinal plant extract and a drug candidate for the
treatment of prostate cancer.
Acknowledgements
This study is supported by National Research
Foundation of Korea (NRF) funded by the Ministry of Education,
Science, and Technology [20111-0019173].
References
|
1
|
Dayyani F, Gallick GE, Logothetis CJ and
Corn PG: Novel therapies for metastatic castrate-resistant prostate
cancer (Review). J Natl Cancer Inst. 103:1665–1675. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eisermann K, Tandon S, Bazarov A, Brett A,
Fraizer G and Piontkivska H: Evolutionary conservation of zinc
finger transcription factor binding sites in promoters of genes
co-expressed with WT1 in prostate cancer. BMC Genomics. 9:3372008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaur M and Agarwal R: Transcription
factors: molecular targets for prostate cancer intervention by
phytochemicals (Review). Curr Cancer Drug Targets. 7:355–367. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bardia A, Platz EA, Yegnasubramanian S, De
Marzo AM and Nelson WG: Anti-inflammatory drugs, antioxidants, and
prostate cancer prevention (Review). Curr Opin Pharmacol.
9:419–426. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akgul C: Mcl-1 is a potential therapeutic
target in multiple types of cancer (Review). Cell Mol Life Sci.
66:1326–1336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang H, Shao H, Yu C and Hou J: Mcl-1
downregulation by YM155 contributes to its synergistic anti-tumor
activities with ABT-263. Biochem Pharmacol. 82:1066–1072. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Z, Yang H, Wu G, Li Z, Song T and Li
XQ: Probing the difference between BH3 groove of Mcl-1 and Bcl-2
protein: Implications for dual inhibitors design. Eur J Med Chem.
46:3909–3916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Warr MR and Shore GC: Unique biology of
Mcl-1: therapeutic opportunities in cancer (Review). Curr Mol Med.
8:138–147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi H, Chen MC, Pham H, et al:
Baicalein, a component of Scutellaria baicalensis, induces
apoptosis by Mcl-1 down-regulation in human pancreatic cancer
cells. Biochim Biophys Acta. 1813:1465–1474. 2011.
|
|
10
|
Chen W, Bai L, Wang X, Xu S, Belinsky SA
and Lin Y: Acquired activation of the Akt/cyclooxygenase-2/Mcl-1
pathway renders lung cancer cells resistant to apoptosis. Mol
Pharmacol. 77:416–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei SH, Dong K, Lin F, et al: Inducing
apoptosis and enhancing chemosensitivity to gemcitabine via RNA
interference targeting Mcl-1 gene in pancreatic carcinoma cell.
Cancer Chemother Pharmacol. 62:1055–1064. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schulze-Bergkamen H, Fleischer B,
Schuchmann M, et al: Suppression of Mcl-1 via RNA interference
sensitizes human hepatocellular carcinoma cells towards apoptosis
induction. BMC Cancer. 6:2322006. View Article : Google Scholar
|
|
13
|
Guoan X, Hanning W, Kaiyun C and Hao L:
Adenovirus-mediated siRNA targeting Mcl-1 gene increases
radiosensitivity of pancreatic carcinoma cells in vitro and in
vivo. Surgery. 147:553–561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bredholt T, Dimba EA, Hagland HR, et al:
Camptothecin and khat (Catha edulis Forsk.) induced distinct
cell death phenotypes involving modulation of c-FLIPL, Mcl-1,
procaspase-8 and mitochondrial function in acute myeloid leukemia
cell lines. Mol Cancer. 8:1012009.PubMed/NCBI
|
|
15
|
Kim YI, Park SW, Choi IH, Lee JH, Woo HJ
and Kim Y: Effect of Orostachys japonicus on cell growth and
apoptosis in human hepatic stellate cell line LX2. Am J Chin Med.
39:601–613. 2011.
|
|
16
|
Lee NH, Lee MY, Lee JA, et al:
Anti-asthmatic effect of Sanguisorba officinalis L. and
potential role of heme oxygenase-1 in an ovalbumin-induced murine
asthma model. Int J Mol Med. 26:201–208. 2010.
|
|
17
|
Cho JY, Yoo ES, Cha BC, Park HJ, Rhee MH
and Han YN: The inhibitory effect of triterpenoid glycosides
originating from Sanguisorba officinalis on tissue factor
activity and the production of TNF-alpha. Planta Med. 72:1279–1284.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goun EA, Petrichenko VM, Solodnikov SU, et
al: Anticancer and antithrombin activity of Russian plants. J
Ethnopharmacol. 81:337–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma Y, Zhang A, Shi Z, et al: A
mitochondria-mediated apoptotic pathway induced by deoxynivalenol
in human colon cancer cells. Toxicol In Vitro. 26:414–420. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Zhang F, Yang L, et al: Ursolic
acid inhibits proliferation and induces apoptosis of cancer cells
in vitro and in vivo. J Biomed Biotechnol. 2011:4193432011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen XR, Lu R, Dan HX, et al: Honokiol: a
promising small molecular weight natural agent for the growth
inhibition of oral squamous cell carcinoma cells. Int J Oral Sci.
3:34–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Deng L, Zhong H, Jiang X and Chen
J: Natural plant extract tubeimoside I promotes apoptosis-mediated
cell death in cultured human hepatoma (HepG2) cells. Biol Pharm
Bull. 34:831–838. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chun JY, Tummala R, Nadiminty N, et al:
Andrographolide, a herbal medicine, inhibits interleukin-6
expression and suppresses prostate cancer cell growth. Genes
Cancer. 1:868–876. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin YH, Chen KK and Chiu JH:
Coprescription of Chinese herbal medicine and western medications
among prostate cancer patients: a population-based study in Taiwan.
Evid Based Complement Alternat Med. 2012:1470152012.PubMed/NCBI
|
|
25
|
Wang Z, Loo WT, Wang N, Chow LW, Wang D,
Han F, Zheng X and Chen JP: Effect of Sanguisorba
officinalis L. on breast cancer growth and angiogenesis. Expert
Opin Ther Targets. 16(Suppl 1): S79–S89. 2012.
|
|
26
|
Quinn BA, Dash R, Azab B, et al: Targeting
Mcl-1 for the therapy of cancer (Review). Expert Opin Investig
Drugs. 20:1397–1411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dash R, Azab B, Quinn BA, et al:
Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1
sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity.
Proc Natl Acad Sci USA. 108:8785–8790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Senft D, Berking C, Graf SA, Kammerbauer
C, Ruzicka T and Besch R: Selective induction of cell death in
melanoma cell lines through targeting of Mcl-1 and A1. PLoS One.
7:e308212012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimazu T, Degenhardt K, Nur-E-Kamal A, et
al: NBK/BIK antagonizes MCL-1 and BCL-XL and activates BAK-mediated
apoptosis in response to protein synthesis inhibition. Genes Dev.
21:929–941. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dewson G and Kluck RM: Mechanisms by which
Bak and Bax permeabilise mitochondria during apoptosis. J Cell Sci.
122:2801–2808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim H, Rafiuddin-Shah M, Tu HC, et al:
Hierarchical regulation of mitochondrion-dependent apoptosis by
BCL-2 subfamilies. Nat Cell Biol. 8:1348–1358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clohessy JG, Zhuang J, de Boer J,
Gil-Gómez G and Brady HJ: Mcl-1 interacts with truncated Bid and
inhibits its induction of cytochrome c release and its role in
receptor-mediated apoptosis. J Biol Chem. 281:5750–5759. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: mechanism and therapeutic potential (Review). Curr Opin
Immunol. 19:488–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mitchell C, Yacoub A, Hossein H, et al:
Inhibition of MCL-1 in breast cancer cells promotes cell death in
vitro and in vivo. Cancer Biol Ther. 10:903–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ménoret E, Gomez-Bougie P, Surget S, et
al: Mcl-1(128–350) fragment induces apoptosis through direct
interaction with Bax. FEBS Lett. 584:487–492. 2010.
|
|
36
|
Jiang CC, Wroblewski D, Yang F, Hersey P
and Zhang XD: Human melanoma cells under endoplasmic reticulum
stress are more susceptible to apoptosis induced by the BH3 mimetic
obatoclax. Neoplasia. 11:945–955. 2009.PubMed/NCBI
|
|
37
|
van Delft MF and Huang DC: How the Bcl-2
family of proteins interact to regulate apoptosis (Review). Cell
Res. 16:203–213. 2006.PubMed/NCBI
|
|
38
|
Adams JM: Ways of dying: multiple pathways
to apoptosis. Genes Dev. 17:2481–2495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death (Review). Science.
305:626–629. 2004. View Article : Google Scholar
|
|
40
|
Chu R, Upreti M, Ding WX, Yin XM and
Chambers TC: Regulation of Bax by c-Jun NH2-terminal kinase and
Bcl-xL in vinblastine-induced apoptosis. Biochem Pharmacol.
78:241–248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qanungo S, Das M, Haldar S and Basu A:
Epigallocatechin-3-gallate induces mitochondrial membrane
depolarization and caspase-dependent apoptosis in pancreatic cancer
cells. Carcinogenesis. 26:958–967. 2005. View Article : Google Scholar
|
|
42
|
Zhao L, He F, Liu H, et al: Natural
diterpenoid compound elevates expression of Bim protein, which
interacts with antiapoptotic protein Bcl-2, converting it to
proapoptotic Bax-like molecule. J Biol Chem. 287:1054–1065. 2012.
View Article : Google Scholar : PubMed/NCBI
|