Introduction
Fracture-induced osteonecrosis of the femoral head
(ONFH) is a severe complication following femoral neck fractures
(1). Reportedly, fracture-induced
ONFH occurs in approximately 17.5% of all surgically-treated
patients with femoral neck fractures (1–3). As
the pathogenesis of ONFH has not been elucidated clearly, there are
various treatment protocols for the disease with respective
indications, including core decompression, structural and
non-structural bone grafting, vascularized and non-vascularized
bone implantation, porous metal grafting and osteotomy, as well as
artificial joint replacement (4–6). It
remains controversial as to which method is optimal, as none of
these surgical techniques are perfect. However, it has been proven
that an early-stage ONFH achieves a better prognosis irrespective
of which type of head-preserving surgery is employed (7). Therefore, it appears critical to
confirm fracture-induced ONFH at its early stage, in an accurate
and repeatable manner.
Previously, several authors attempted radiological
and surgical methods to predict fracture-induced ONFH (8–12);
however, the shortcomings of these methods have restricted their
clinical application. Traditionally, histological examination has
been considered as the gold standard for clinical diagnosis,
including cellular and extracellular pathogenesis in particular.
Additionally, immunohistochemical methods have been widely employed
to detect cell apoptosis in modern pathology. Apoptosis may play an
important role in the pathogenesis of ONFH induced by steroids and
alcohol (13), and may be a
significant cause of bone cell death in ONFH, which is associated
with apoptosis (14). Thus,
whether apoptosis also plays a similar role in fracture-induced
ONFH remains to be determined.
In the current study, a canine model of femoral neck
fractures was made and evaluated. Within different post-fracture
intervals, the femoral head was collected for histological
examination and caspase-3 staining, which controls both cytoplasmic
and nuclear events associated with Fas-mediated apoptosis in
vivo (15). The present study
was approved by the ethics committee of Shanghai Sixth People’s
Hospital.
Materials and methods
Animals
Beagles from the Laboratory Animal Center of the
Shanghai Sixth People’s Hospital, China, were used as the canine
model and had an average age of 2.5 years and body weight of 14.5
kg (range, 13–17 kg). The animals had no history of disease and
were housed separately. Before the day of surgery, the dogs were
fasted, and penicillin sodium was injected intramuscularly. A total
of eight dogs were used in the present study.
Surgical methods and sample
acquisition
Under aseptic conditions, a 6-cm-long L-shape skin
incision was made centered to the left of the great trochanter to
expose the hip joint. The tensor fascia lata was split along the
length of its bundles, and the gluteus medius muscle was detached
partially from the great trochanter. The hip joint capsule was
opened, and electrocoagulation of soft tissue attachments at the
base of the femoral neck was performed circumferentially to
interrupt the extraosseous blood supply to the femoral head.
However, the medial and lateral circumflex arteries were not
revealed, and the ligamentum teres was kept intact. A low-speed
drill was employed to fracture the femoral neck at the narrow base.
The femoral neck fractures were left untreated to induce avascular
osteonecrosis. The maneuvers were directly visible for assurance of
anatomical reduction and reliable stabilization. Surgical
procedures were completed by one team of surgeons. Postoperatively,
the animals were housed separately and injected with penicillin
sodium for prophylactic control of infection. The right femoral
head was set for the control (N=8).
Radiological and histological
examinations
The animals underwent radiological examination two
weeks postoperatively and then monthly on three occasions. After
each radiological examination, two animals were euthanized, and the
bilateral femoral heads were collected for histological
examination. For the microscopic examination, tissue samples were
obtained from the zone of weight-bearing and the center of the
femoral head. The samples were fixed with 10% formalin for one week
and decalcified with 5 μM EDTA solution for four weeks. The
specimens were embedded in paraffin, cut into 4-μm sections,
and stained with hematoxylin and eosin (H&E).
Immunohistochemistry of caspase-3
expression
For immunohistochemistry, each section was de-waxed,
irradiated at 750 W in a microwave oven with 3% hydrogen peroxide
in 0.01 M sodium citrate buffer (pH 6.0) for 5 min, and
immunostained with a monoclonal anti-caspase-3 antibody (Hope,
Zhenjiang, Jiangsu, China) to detect osteogenesis and osteocyte
apoptosis in the femoral head.
Statistical analysis
The numerical data were presented as the means ±
standard deviation (SD). Fisher’s exact test was employed to
compare the percentage of caspase-3-positive cells of the necrotic
femoral head to the reference value using SPSS 17.0 software
(Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
General and radiological examination
None of the animals experienced postoperative
complications of infection. Radiologically, five dogs showed
non-union and three dogs had malunion of the femoral head. All
eight animals had fracture-induced ONFH in their left femoral heads
based on last radiological examination.
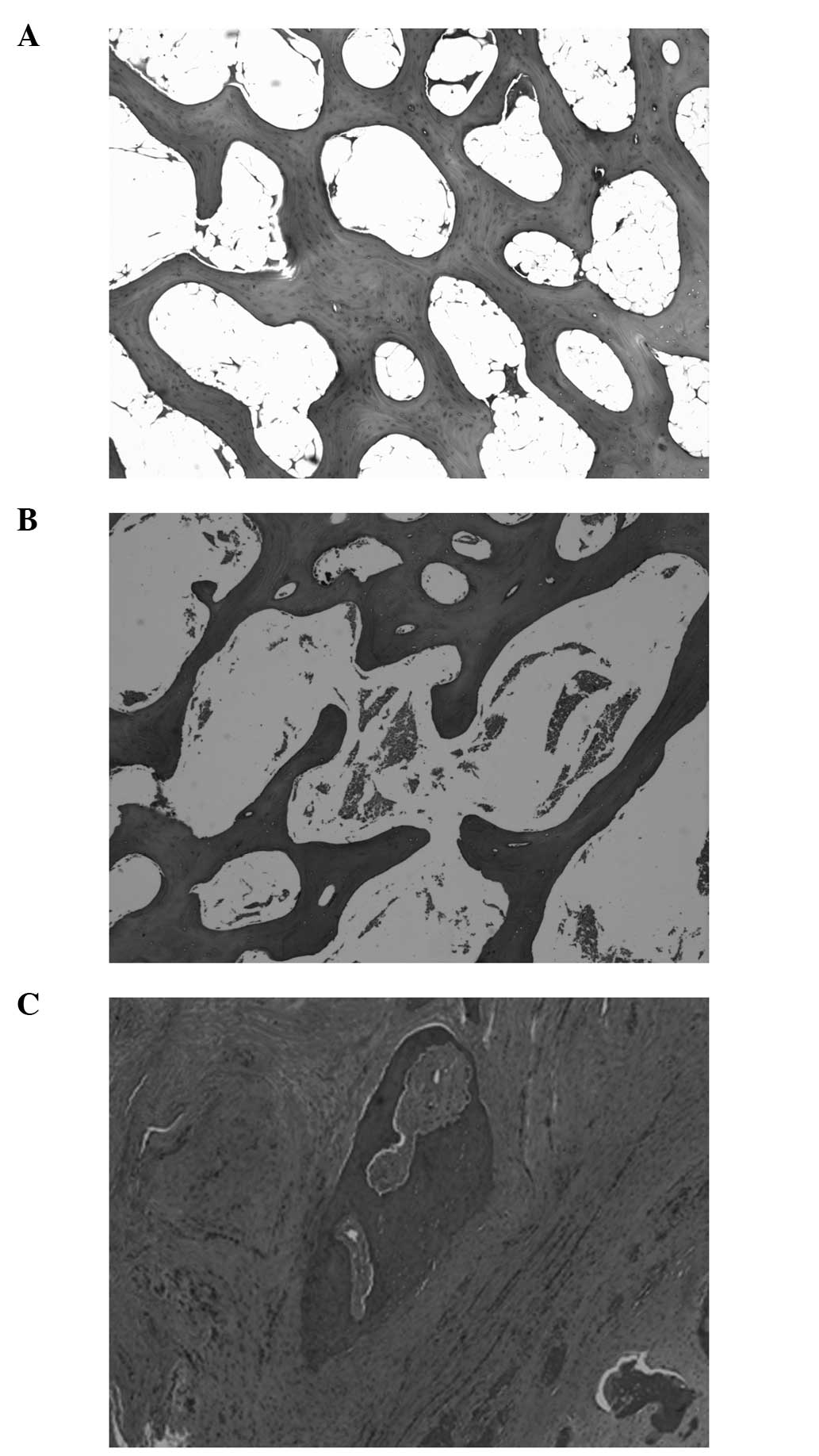
Histological examination
Morphologically, the surface of the cartilage lost
continuity for untreated femoral neck fractures. Results of the
H&E staining indicated that the untreated head developed
osteonecrosis with characteristics of an accumulation of bone
marrow cell debris, empty lacunae and/or ghost nuclei in the
lacunae, and an increase in fat cells of the bone marrow.
Proliferation of fibrous tissue was triggered for pathological
reparation as well (Fig.
1A-C).
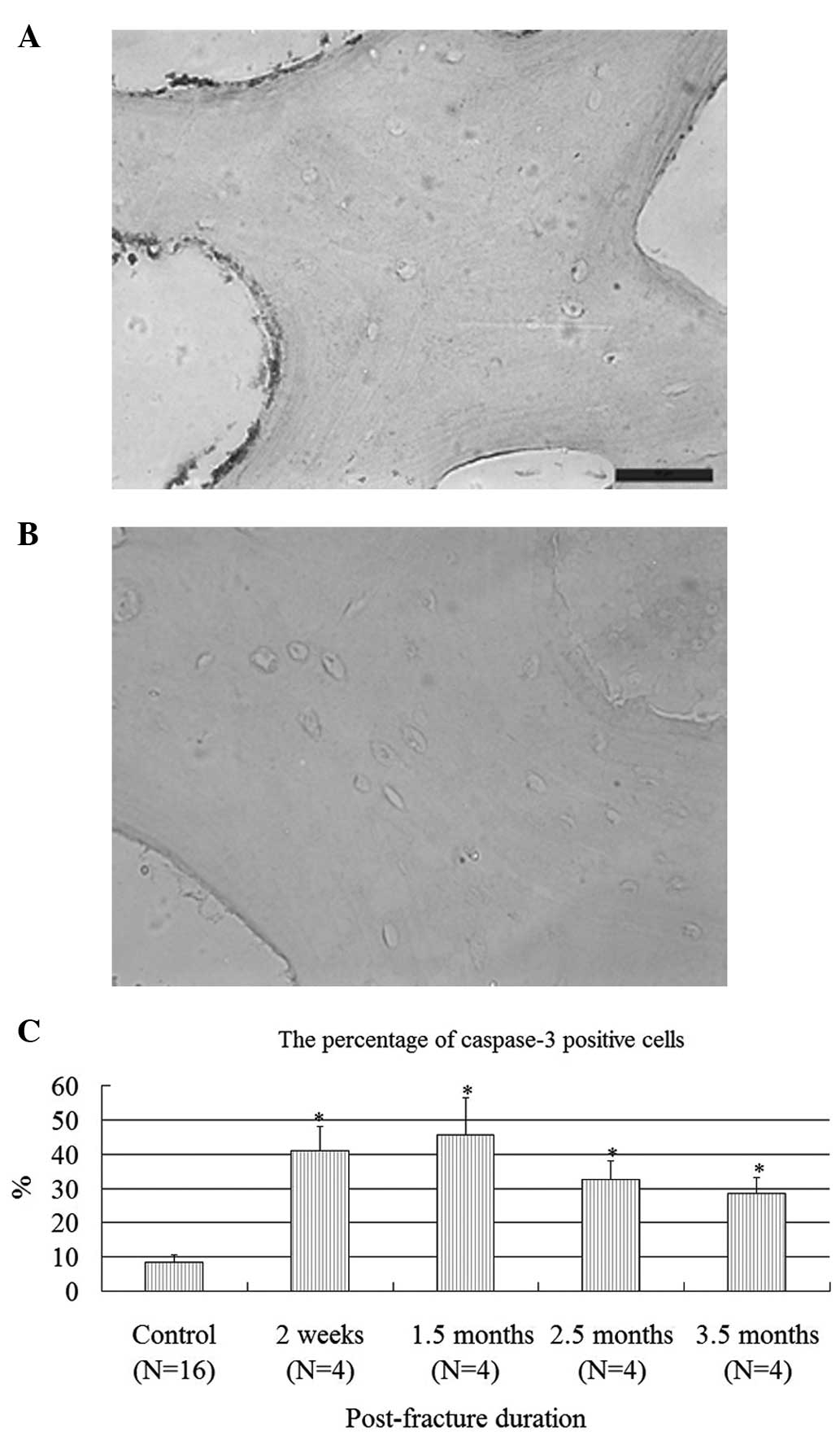
For the immunohistochemical staining of caspase-3,
10 fields of positive and total cells were randomly counted by two
authors, and the percentage was used for statistical analysis.
There were eight normal femoral heads, which were set as a
reference value. There were 8.5±2.2% positive cells in the normal
head; however, there were 41, 45.5, 32.5 and 28.5% positive cells
in the osteonecrotic head two weeks, 1.5, 2.5 and 3.5 months
following femoral neck fractures, respectively. It was found that
there were more caspase-3-positive cells in untreated and
osteonecrotic femoral head as early as two weeks to 3.5 months
following femoral neck fractures, which showed a statistical
difference in comparison to the reference value (Fig. 2A-C).
Discussion
Fracture-induced ONFH is a complication that occurs
following femoral neck fractures, due to its unsatisfactory
prognosis with current treatment protocols. If the femoral head
were to experience biomechanical collapse, the results would be
poor, and artificial joint replacement would become inevitable to
restore a functional hip. Based on up-to-date clinical reports,
pre-collapse ONFH is therefore likely to achieve much better
results in comparison with collapsed osteonecrosis when the head is
to be preserved (16). Therefore,
it is of great significance to make an early diagnosis of ONFH, to
facilitate early treatment accordingly. As indicated previously,
although there have been various methods to predict
fracture-induced ONFH, none of these techniques have been applied
in the clinic and further reports regarding such methods are
non-existent. In their study, Cho et al found that
intraoperative bleeding from the cannulated screws used for the
fixation of intracapsular femoral neck fractures could be employed
as a predictive method for subsequent ONFH (10). However, evidently, the method may
be affected by extra-screw bleeding, and postoperative vascular
restoration was neglected. Moreover, for certain intracapsular neck
fractures, the optimal implant may not be cannulated screws, which
has restricted its broad application (17). Watanabe et al measured the
intramedullary oxygen tension of the proximal femur and evaluated
its usefulness in monitoring for the prediction of fracture-induced
ONFH (9). Although the method was
simple and less invasive, it may be affected by various subjective
factors, and consecutive monitoring was not be realized.
Non-invasive methods, including magnetic resonance imaging (MRI),
bone scintigraphy and positron emission tomography (PET), have been
advocated for the prediction of ONFH induced by various etiologies.
However, clinical application of these methods is limited due to a
lack of instruments, the non-quantitative results, as well as the
use of radioactive materials. Risk factors for fracture-induced
ONFH have been indicated, including primary fracture displacement,
the quality of reduction and internal fixation, and operative
methods; however, the intrinsic relationship of these factors to
the occurrence of ONFH is unclear (18–20).
Although the pathogenesis of ONFH is poorly
understood, it has been found that cell apoptosis plays an
important role in the initiation and progression of ONFH induced by
alcohol and steroids (13).
Apoptosis, also known as programmed cell death, is triggered by
cellular stress, such as serum deprivation and hypoxia resulting
from femoral neck fractures. Sen et al used a core biopsy
from the supra-lateral femoral head of patients with unreduced hip
dislocation or fracture dislocation and found dead osseous
fragments and necrotic osteocytes. Those authors concluded that
core biopsy biology as well as marrow-aspirate volume and
morphology were capable of predicting the development of subsequent
ONFH following trauma (11).
During the development of ischemic ONFH, oxygen-regulated protein
150 and haemoxygenase 1 may play an important role in the mechanism
of cell apoptosis (21).
Similarly, apoptosis of osteocytes was abundantly found in ONFH
induced by corticosteroid and hydrogen peroxide (22–25).
Caspase-3 controls both cytoplasmic and nuclear events associated
with Fas-mediated apoptosis in vivo (15), and previously, the activation of
caspase-3 has been indicated in the initiation of ONFH (20). Histological methods, strengthened
by modern immunological means, are traditionally considered as the
gold standard for an early diagnosis of this complex disease.
As indicated by results of the present study,
caspase-3 is activated as early as two weeks following femoral neck
fractures, and is closely correlated with the occurrence of induced
ONFH. Therefore, we speculated that caspase-3 may be employed as a
predictor for fracture-induced ONFH. As biopsy could be achieved
intraoperatively or could be CT-guided postoperatively, and the
immunohistochemical staining of caspase-3 is a relatively simple
procedure, we believe that monitoring of the caspase-3 expression
in the femoral head is practical for clinical application. However,
there were limitations to our study. More methods shoule be
employed to detect apoptosis in addition to morphological
observation and caspase-3 staining, such as the terminal
deoxynucleotidyl transferase-mediated dUTP nick end-labeling
(TUNEL) assay. An in vitro experiment is required to reflect
the intrinsic mechanisms of fracture-induced apoptosis. Moreover,
in order for caspase-3 to be used in clinical practice, more
studies are required to observe the differences of biological and
biomechanical properties between humans and the canine model.
Acknowledgements
The current research was financially supported by a
Creative Research Scholarship for Ph.D. Candidates of Shanghai Jiao
Tong University School of Medicine (No. BXJ2011038).
References
|
1
|
Bachiller F, Caballer AP and Portal LF:
Avascular necrosis of the femoral head after femoral neck fracture.
Clin Orthop Relat Res. 399:87–109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davidovitch RI, Jordan CJ, Egol KA and
Vrahas MS: Challenges in the treatment of femoral neck fractures in
the nonelderly adult. J Trauma. 68:236–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ly TV and Swiontkowski MF: Management of
femoral neck fractures in young adults. Indian J Orthop. 42:3–12.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nikolopoulos KE, Papadakis SA, Kateros KT,
Themistocleous GS, Vlamis JA, Papagelopoulos PJ and Nikiforidis PA:
Long-term outcome of patients with avascular necrosis, after
internal fixation of femoral neck fractures. Injury. 34:525–528.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haidukewych GJ: Salvage of failed
treatment of femoral neck fractures. Instr Course Lect. 58:83–90.
2009.PubMed/NCBI
|
|
6
|
Beris AE, Payatakes AH, Kostopoulos VK, et
al: Non-union of femoral neck fractures with osteonecrosis of the
femoral head: treatment with combined free vascularized fibular
grafting and subtrochanteric valgus osteotomy. Orthop Clin North
Am. 35:335–243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Assouline-Dayan Y, Chang C, Greenspan A,
Shoenfeld Y and Gershwin ME: Pathogenesis and natural history of
osteonecrosis. Seminars Arthritis Rheum. 32:94–124. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lausten GS, Hesse B, Thygesen V and Fogh
J: Prediction of late complications of femoral neck fractures by
scintigraph. Int Orthop. 16:260–264. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe Y, Terashima Y, Takenaka N,
Kobayashi M and Matsushita T: Prediction of avascular necrosis of
the femoral head by measuring intramedullary oxygen tension after
femoral neck fracture. J Orthop Trauma. 21:456–461. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho MR, Lee SW, Shin DK, Kim SK, Kim SY,
Ko SB and Kwun KW: A predictive method for subsequent avascular
necrosis of the femoral head (AVNFH) by observation of bleeding
from the cannulated screw used for fixation of intracapsular
femoral neck fractures. J Orthop Trauma. 21:158–164. 2007.
View Article : Google Scholar
|
|
11
|
Sen RK, Tripathy SK, Gill SS, Verma N,
Singh P and Radotra BD: Prediction of posttraumatic femoral head
osteonecrosis by quantitative intraosseous aspirate and core biopsy
analysis: a prospective study. Acta Orthop Belg. 76:486–492.
2010.PubMed/NCBI
|
|
12
|
Ehlinger M, Moser T, Adam P, Bierry G,
Gangi A, de Mathelin M and Bonnomet F: Early prediction of femoral
head avascular necrosis following neck fracture. Orthop Traumatol
Surg Res. 97:79–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Youm YS, Lee SY and Lee SH: Apoptosis in
the osteonecrosis of the femoral head. Clin Orthop Surg. 2:250–255.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calder JD, Buttery L, Revell PA, Pearse M
and Polak JM: Apoptosis - a significant cause of bone cell death in
osteonecrosis of the femoral head. J Bone Joint Surg Br.
86:1209–1213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng TS, Schlosser SF, Dao T, Hingorani
R, Crispe IN, Boyer JL and Flavell RA: Caspase-3 controls both
cytoplasmic and nuclear events associated with Fas-mediated
apoptosis in vivo. Proc Natl Acad Sci USA. 95:13618–13623.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Petrigliano FA and Lieberman JR:
Osteonecrosis of the hip: novel approach to evaluation and
treatment. Clin Ortho Relat Res. 465:53–62. 2007.
|
|
17
|
Bhandari M, Tornetta P, Hanson B and
Swiontkowski MF: Optimal internal fixation for femoral neck
fractures: multiple screws or sliding hip screws? J Orthop Trauma.
23:403–407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duckworth AD, Bennet SJ, Aderinto J and
Keating JF: Fixation of intracapsular fractures of the femoral neck
in young patients: risk factors for failure. J Bone Joint Surg Br.
93:811–816. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song KS: Displaced fracture of the femoral
neck in children: open versus closed reduction. J Bone Joint Surg
Br. 92:1148–1151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kakar S, Tornetta P III, Schemitsch EH, et
al: Technical considerations in the operative management of femoral
neck fractures in elderly patients: a multinational survey. J
Trauma. 63:641–646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sato M, Sugano N, Ohzono K, Nomura S,
Kitamura Y, Tsukamoto Y and Ogawa S: Apoptosis and expression of
stress protein (ORP150, HO1) during development of ischemic
osteonecrosis in the rat. J Bone Joint Surg Br. 83:751–759. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weinstein RS, Nicholas RW and Manolagas
SC: Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis
of the hip. J Clin Endocrinol Metab. 85:2907–2912. 2000.PubMed/NCBI
|
|
23
|
Kikuyama A, Fukuda K, Mori S, Okada M,
Yamaguchi H and Hamanishi C: Hydrogen peroxide induces apoptosis of
osteocytes: involvement of calcium ion and caspase activity. Calcif
Tissue Int. 71:243–248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zalavras C, Shah S, Birnbaum MJ and
Frenkel B: Role of apoptosis in glucocorticoid-induced osteoporosis
and osteonecrosis. Crit Rev Eukaryot Gene Expr. 13:221–235. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kabata T, Kubo T, Matsumoto T, Nishino M,
Tomita K, Katsuda S, Horii T, Uto N and Kitajima I: Apoptotic cell
death in steroid induced osteonecrosis: an experimental study in
rabbits. J Rheumatol. 27:2166–2171. 2002.PubMed/NCBI
|