Introduction
Benign prostatic hyperplasia (BPH) is a
non-malignant enlargement of the prostate gland, which results from
the progressive hyperplasia of stromal and glandular prostatic
cells (1). BPH causes increased
resistance to urine flow, leading to lower urinary tract symptoms
(LUTS) including urinary hesitancy, frequent urination, urgency,
thin urine flow and urinary retention (2), which are known to significantly
affect the physical and mental health of patients as well as their
quality of life. Delayed treatment results in numerous severe
complications, such as bleeding from the prostate, recurrent
infections, renal stones and even kidney failure.
Although the pathogenesis of BPH remains unclear,
the reduction of cell apoptosis, which leads to the increase in the
total number of stromal and epithelial cells, has been strongly
associated with the development of BPH (3–6).
Mitochondrial-dependent pathway is the most common apoptotic
pathway in vertebrate animal cells (7–9). The
mitochondrial membrane permeabilization, accompanied by the
collapse of electrochemical gradient across the mitochondrial
membrane, is one of the key events during cellular apoptosis
(10). This results in the release
of numerous apoptogenic proteins from the mitochondria triggering
the activation of aspartate-directed cysteine proteases (caspases)
and eventually inducing apoptosis (11–14).
Bcl-2 family proteins are key regulators of mitochondria-mediated
apoptosis, including anti-apoptotic members such as Bcl-2 and
pro-apoptotic members such as Bax (7–9).
Mitochondrial outer membrane permeabilization (MOMP) is thought to
occur through the formation of pores in the mitochondria by
pro-apoptotic Bax-like proteins (15–17),
which could be inhibited by anti-apoptotic Bcl-2-like members
(7,11,18,19).
The ratio of active anti- and pro-apoptotic Bcl-2 family members
determines the fate of cells and alteration of the ratio by
aberrant expression of these proteins impairs the normal apoptotic
program thereby contributing to various apoptosis-related diseases
including BPH (20). Therefore,
promoting cell apoptosis has been suggested as a promising strategy
for the development of anti-BPH agents.
Pharmacotherapy remains the major approach for BPH
treatment. The mainstay of pharmacotherapy includes α-adrenergic
blockers and 5α-reductase inhibitors (21,22).
α-adrenergic blockers inhibit α-adrenergic receptors, relaxing
smooth muscle in the prostate and the bladder neck, thus decreasing
the blockage of urine flow. 5α-reductase inhibitors suppress
5α-reductase, inhibiting dihydrotestosterone production and,
therefore, enlargement of the prostate. However, α-adrenergic
blockers and 5α-reductase inhibitors may have serious side-effects
such as orthostatic hypotension, decreased libido and ejaculation
or erectile dysfunction (23–27).
Due to these risks, natural products that appear to have limited
adverse effects have recently received great interest in BPH
treatment.
Qianliening capsule (QC) is a traditional Chinese
medicine formula composed of Whitmania pigra Whitman,
Rheum palmatum L., Achyranthes bidentata and Chinese Dodder.
These components together confer QC properties of heat-clearing,
detoxification, promotion of blood circulation and removal of blood
stasis and tonifying the kidney and nourishing vitality
(replenishing the kidney Qi in Chinese) (28,29).
QC has been shown to have significant therapeutic effects on BPH,
significantly improving a series of LUTS and ameliorating the
urodynamic evaluation indices in BPH patients. Previous studies on
BPH rats noted that QC was able to significantly reduce the
prostatic volume and weight and suppress prostate enlargement
(29–31). However, the precise mechanism of
its anti-BPH activity remains to be fully elucidated. In the
present study, we evaluated the therapeutic efficacy of QC in BPH
rats and investigated the underlying molecular mechanism, using a
BPH rat model.
Materials and methods
Drugs and reagents
QC was provided by the Academy of Pharmacology of
the Fujian University of Traditional Chinese Medicine (FDA approval
no. Z09104065; Minhou Shangjie, Fuzhou, Fujian, China). The drug
powder inside QC was dissolved in distilled water and stored at
4°C. Testosterone propionate injection solution (25 mg/m1) was
obtained from the Shanghai General Pharmaceutical Co., Ltd. (batch
no. H31020524; Shanghai, China). TRIzol reagent was purchased from
Invitrogen (Carlsbad, CA, USA). SuperScript™ II reverse
transcriptase was obtained from Promega (Madison, WI, USA). Bcl-2,
Bax and cleaved caspase 3 primary antibody, secondary antibody,
streptavidin-peroxidase (SP) and 3,3′-diaminobenzidine (DAB) were
purchased from Bohai Biotechnology Development Co., Ltd. (Hebei,
China). Terminal deoxynucleotidyl-transferase-mediated X-dUTP nick
end-labeling (TUNEL) In situ Cell Death Detection kit was purchased
from Roche (cat. no. 1684809; Mannheim, Germany). Additional
chemicals, unless otherwise stated, were obtained from Sigma
Chemical (St. Louis, MO, USA).
High-pressure liquid chromatography
(HPLC) analysis of QC
QC was extracted by ultrasonic-assisted extraction
(32). The standard solutions of
oleanolic acid and emodin and extracts were filtrated through
0.45-μm microporous membranes prior to being injected into the
liquid chromatography. Conditions of HPLC analysis were as follows:
the mobile phase was methanol and 0.1% phosphoric acid (70:30,
v/v), the flow rate was 1 ml/min, the injection volume was 20 μl,
the column temperature was kept at 35°C and the detection
wavelengths were set at 208 nm.
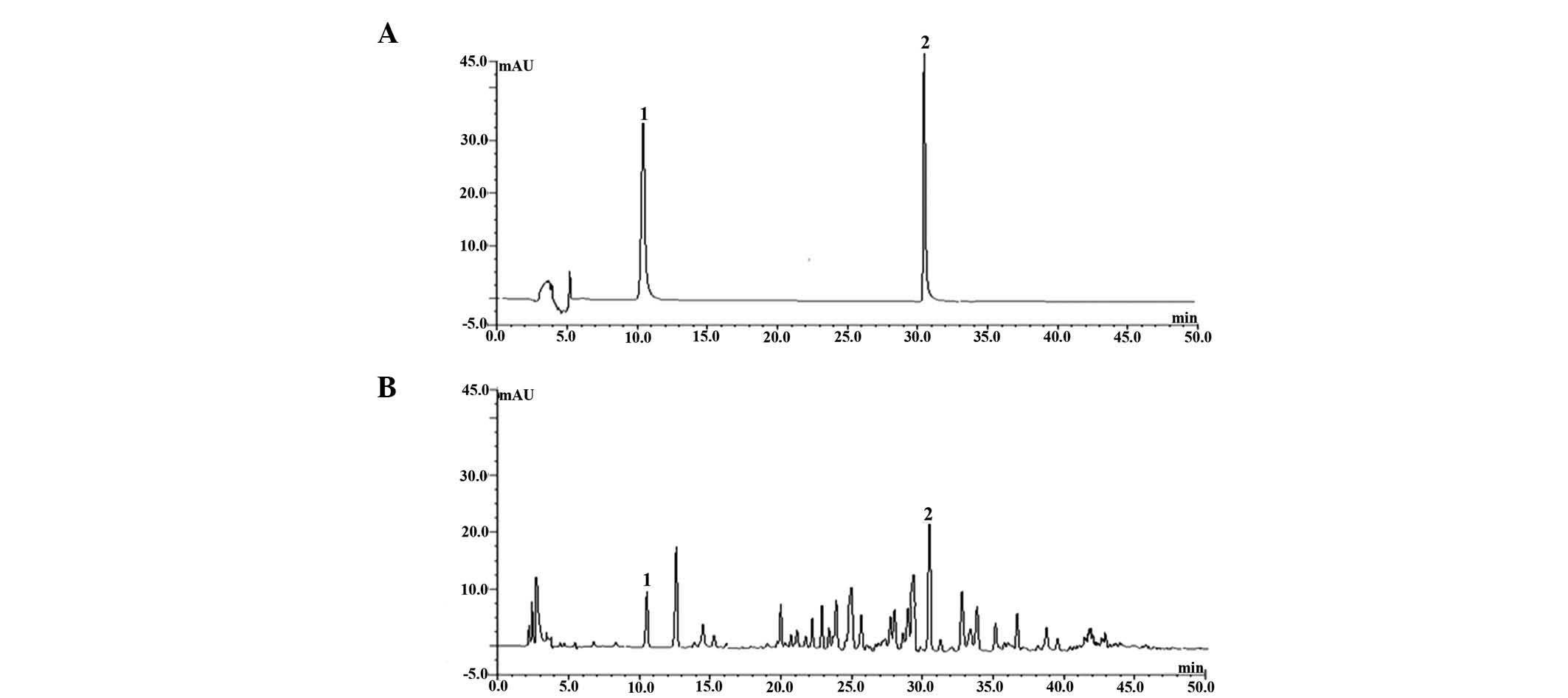
The chromatograms of sample and oleanolic acid and
emodin standard solutions are shown in Fig. 1. Oleanolic acid and emodin in QC
were baseline separated at these conditions and were identified by
the retention time with corresponding peaks compared to the
standard solution. It was found that QC was true, stable and
according to the drug requirements of China.
Animals
Fifty specific pathogen-free (SPF) grade adult male
Sprague-Dawley (SD) rats with a body weight of 200–220 g, purchased
from Shanghai Si-Lai-Ke Experimental Animal Co. Ltd. (Shanghai,
China), were housed under controlled temperature (21–23°C),
humidity and a 12-h light/dark cycle, with free access to standard
rat chow and tap water.
The animal experiments were conducted in compliance
with the International Ethical Guidelines and the National
Institutes of Health Guide concerning the Care and Use of
Laboratory Animals. The study was approved by the Animal Care and
Use Committee of Fujian University of Traditional Chinese
Medicine.
Groups and treatment
The BPH rat model was generated by subcutaneous
injection of testosterone propionate (5 mg/kg) following
castration. The scrota of 40 rats of a total 50 male SD rats were
cut open, castrated and then sutured. The remaining ten rats were
cut open and then sutured without cutting off the testicles, and
were allocated to the sham-operated group. A week after surgery,
the 40 castrated rats were randomly divided into four equal groups
(n=10). The total experimental rats were assigned into the
following five groups: sham-operated group (control), model group
(model), and three QC groups [QC-low (QC-L), QC-medium (QC-M),
QC-high (QC-H)]. The three QC groups (QC-L, QC-M and QC-H) were
intragastrically administered a dose of 2.25, 4.5 and 9 g/kg,
respectively. The injection of edible oil was subcutaneously
administered to rats of the sham-operated group and testosterone
propionate was subcutaneously administered to the castrated rats (5
mg/kg) every day. The sham-operated and model groups were
intragastrically administered normal saline (10 ml/kg) every day.
The drugs were administered daily for 28 consecutive days.
Sample collection
Twenty-four hours after the last administration,
rats were anesthetized with sodium pentobarbital [50 mg/kg
intraperitoneally (i.p.)]. Prostate was removed, weighed and the
weight index was obtained. Approximately half of the gland tissue
of each rat was fixed in 10% formalin solution for
histopathological examination and immunohistochemical (IHC)
analysis. The remaining half of the glands was plunged into liquid
nitrogen and stored at −80°C until total RNA extraction for gene
expression analysis.
Histological examination
The fixed prostatic tissue was dehydrated in graded
ethanols, embedded in paraffin, sliced in serial 5 μm sections,
deparaffinized in xylene, rehydrated in graded ethanol and then
stained with hematoxylin and eosin (H&E) for histological
observation under a light microscope.
TUNEL staining
TUNEL staining protocol was according to a Roche
protocol. Paraffin-embedded sections were dewaxed in xylene and
rehydrated in graded ethanol series to water, pemeabilized with 20
μg/ml proteinase K for 15 min at room temperature and then
incubated in TUNEL reaction mixture for 60 min at 37°C. The tissue
was then rinsed in phosphate-buffered saline (PBS) three times for
5 min and incubated in converter-peroxidase (POD) for 30 min at
37°C, rinsed in PBS three times for 5 min and color-developed with
a DAB POD substrate. The last step was to rinse and counterstain
with hematoxylin. Cells with brown-stained nuclei in five different
fields were counted under a light microscope at a magnification of
×400. Apoptotic index was calculated by multiplying the quantity
and staining intensity scores as described in the IHC analysis
section.
IHC analysis
SP was employed in Bcl-2, Bax and cleaved caspase 3
IHC analysis. Sections (5 μm) were sliced, deparaffinized,
rehydrated, submerged in 1% hydrogen peroxide, epitope retrieved,
and then soaked in goat serum, followed by an overnight incubation
with primary antibodies at 4°C. Control slides were incubated with
PBS without primary antibodies. The slides were then washed in PBS
and incubated for 20 min at 37°C with biotinylated secondary
antibody. After being rinsed in PBS, the slides were exposed to
streptavidin biotin-peroxidase complex for 20 min at 37°C and again
rinsed in PBS. The slides were stained with DAB color development
solution for 6 min, then rinsed and counterstained with
hematoxylin. The IHC slides were examined with a method of
immunohistochemical score (IHS), which was calculated by combining
an estimate of the percentage of immunoreactive cells (quantity
score) with an estimate of the staining intensity (staining
intensity score), as follows: no staining, 0; 1–10%, 1; 11–50%, 2;
51–80%, 3 and 81–100%, 4. Staining intensity was rated on a scale
of 0–3, with 0, negative; 1, weak; 2, moderate and 3, strong. The
raw data were converted to the IHS by multiplying the quantity and
staining intensity scores (33).
Total RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted according to the TRIzol RNA
isolation protocol. The purified total RNA was quantified with a
spectrophotometer at wavelengths of 260 and 280 nm. The A260/A280
ratio was <1.6. One microgram of total RNA was reverse
transcribed into complementary DNA (cDNA) by using SuperScript™ II
transcriptase according to the manufacturer's instructions. The
obtained cDNA was amplied by PCR with TaqDNA polymerase (Fermentas,
Hanover, MD, USA). β-actin was used as the internal control. cDNA
transcripts were amplified using the following primer sequences:
Bcl-2: 5′-GG TGG TGG AGG AAC TCT TCA-3′ and 5′-GA GCA GCG TCT TCA
GAG ACA-3′; Bax: 5′-CC AAG AAG CTG AGC GAG TGT-3′ and 5′-TC ACG GAG
GAA GTC CAG TGT-3′; β-actin: 5′-AC TGG CAT TGT GAT GGA CTC-3′ and
5′-CA GCA CTG TGT TGG CAT AGA-3′. The PCR reaction was performed
using the following conditions: for Bcl-2, denaturation at 95°C for
45 sec, annealing at 56°C for 45 sec and extension at 72°C for 60
sec; for Bax, denaturation at 95°C for 45 sec, annealing at 53°C
for 45 sec and extension at 72°C for 60 sec; for β-actin,
denaturation at 95°C for 45 sec, annealing at 55°C for 45 sec and
extension at 72°C for 60 sec. RT-PCR products were analyzed by 1.5%
agarose gel electrophoresis. The DNA bands were examined using a
Gel Documentation System (Model Gel Doc 2000; BioRad, Hercules, CA,
USA). Transcripts were normalized to β-actin transcript levels and
the relative mRNA level was indicated as the ratio of the density
of the detected genes to β-actin at the same time point.
Statistical analysis
Data were reported as the mean ± standard deviation
(SD). Statistical analyses were performed using SPSS 16.0 by
one-way ANOVA (analysis of variance) and the Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
QC inhibits prostate growth in BPH
rats
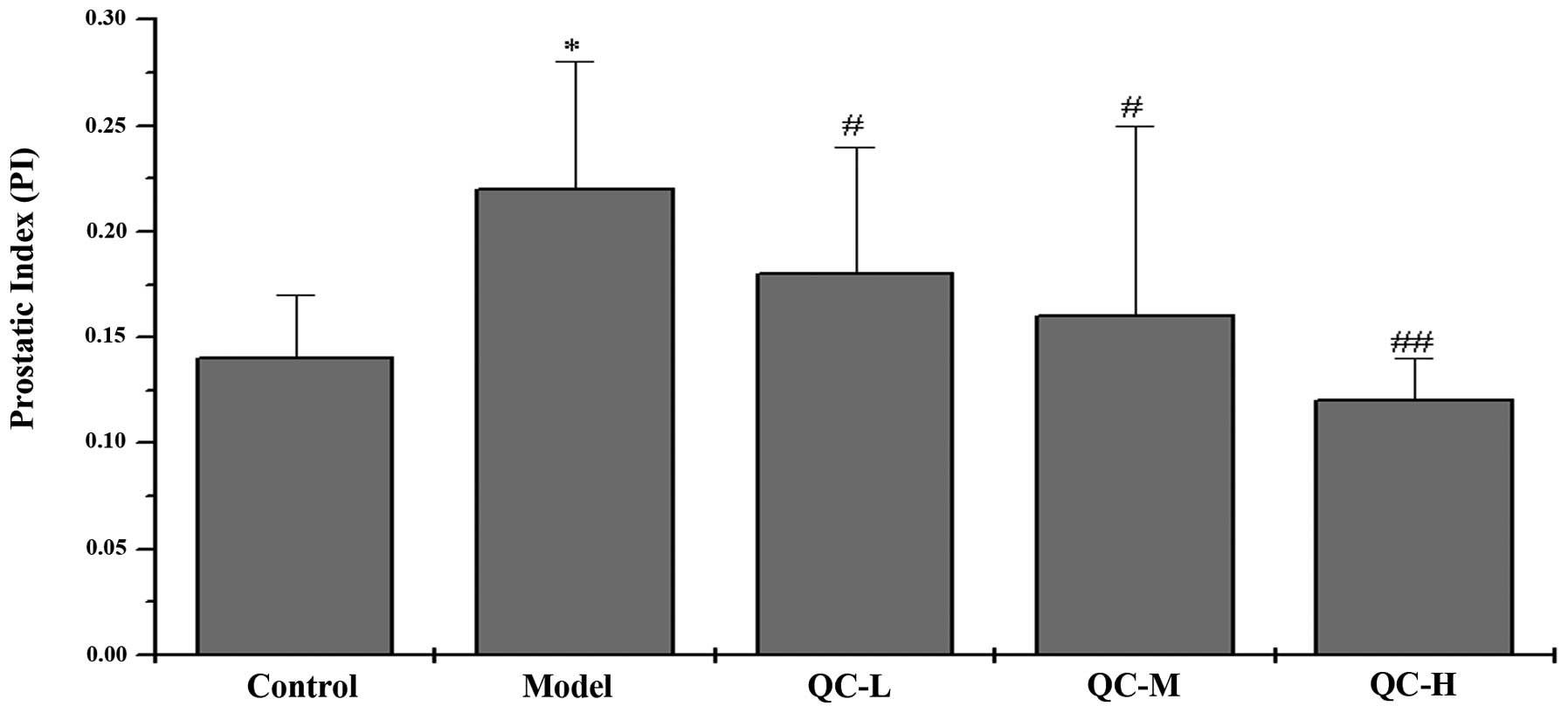
The in vivo therapeutic efficacy of QC
against BPH was investigated by evaluating its effect on prostatic
index (PI), determined by calculating the ratio of prostatic weight
to body weight. The mean PI in the model group was significantly
elevated compared with that in the control group (P<0.05),
indicating a successful model construction (Fig. 2). However, QC treatment
significantly reduced PI in BPH rats (P<0.05) in a
dose-dependent manner, demonstrating the anti-BPH efficacy of QC
in vivo.
QC improves the histological damage of
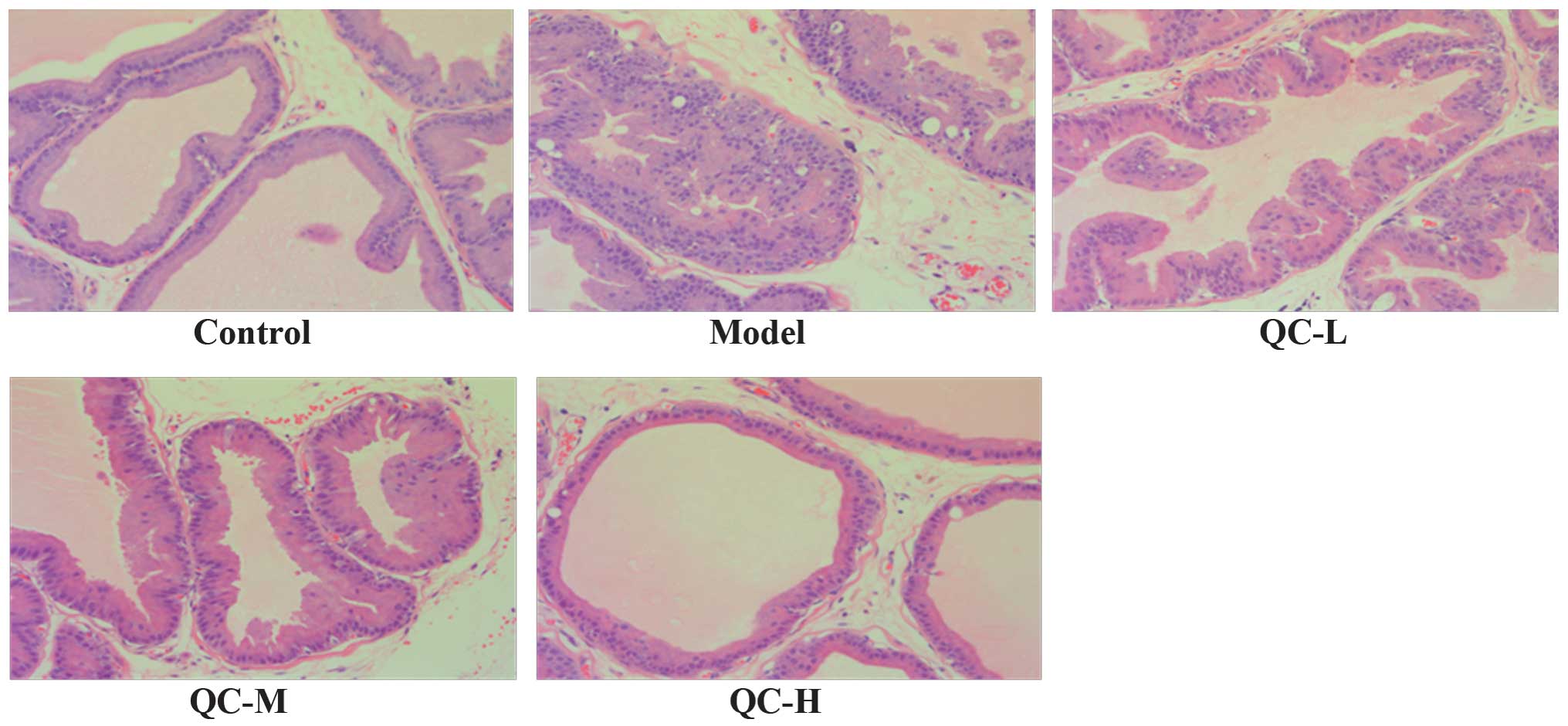
prostate tissue in BPH rats
The prostate histological changes in BPH rats were
observed via light microscopy after H&E staining. As shown in
Fig. 3, low columnar epithelial
cells in the control group were arranged as a single-layer
secretory lumen that was filled with thin acidophilic materials,
whereas in the model group the epithelial cells clearly
proliferated to develop excessive glands and cells were arranged as
multiple unorganized layers. However, the prostate
histopathological damages in BPH rats were significantly
ameliorated by QC treatment in a dose-dependent manner (Fig. 3).
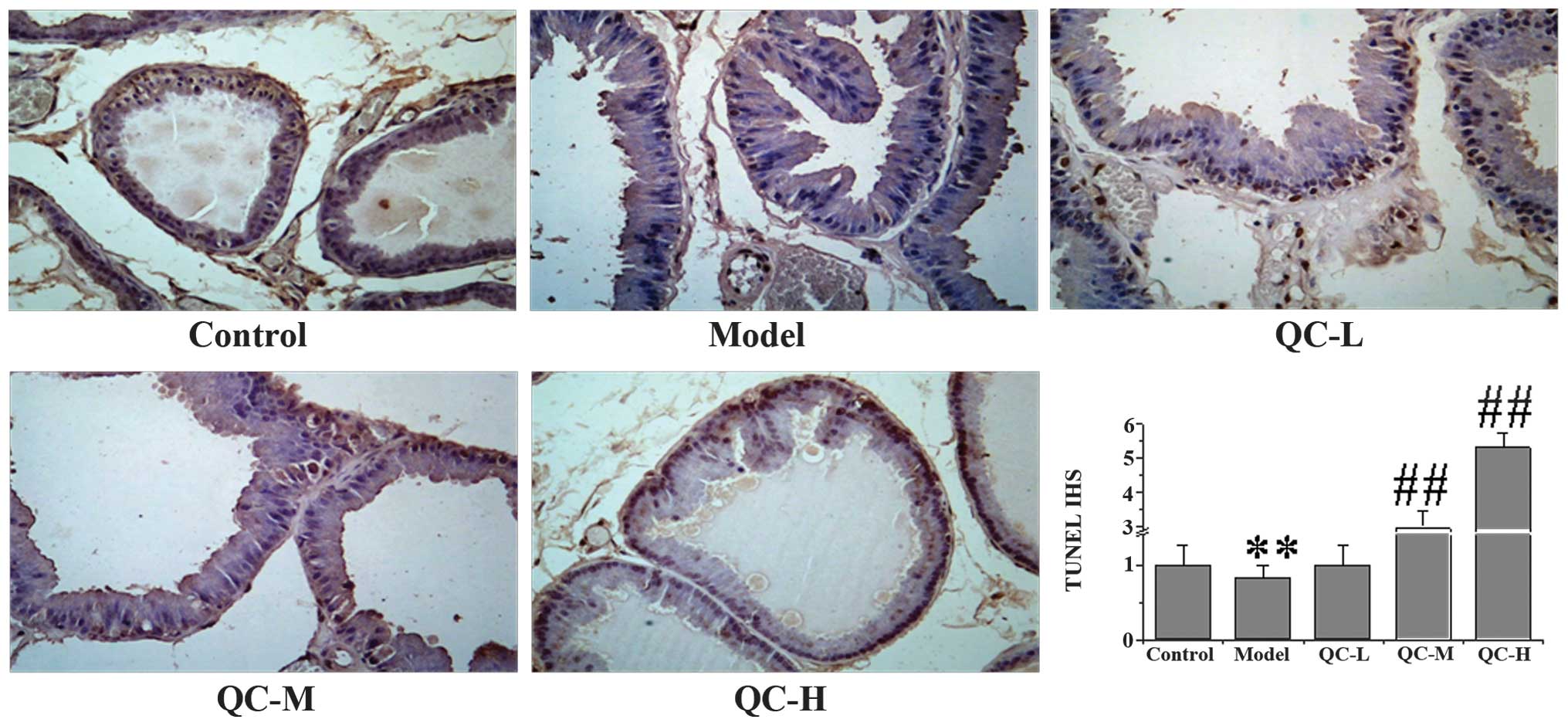
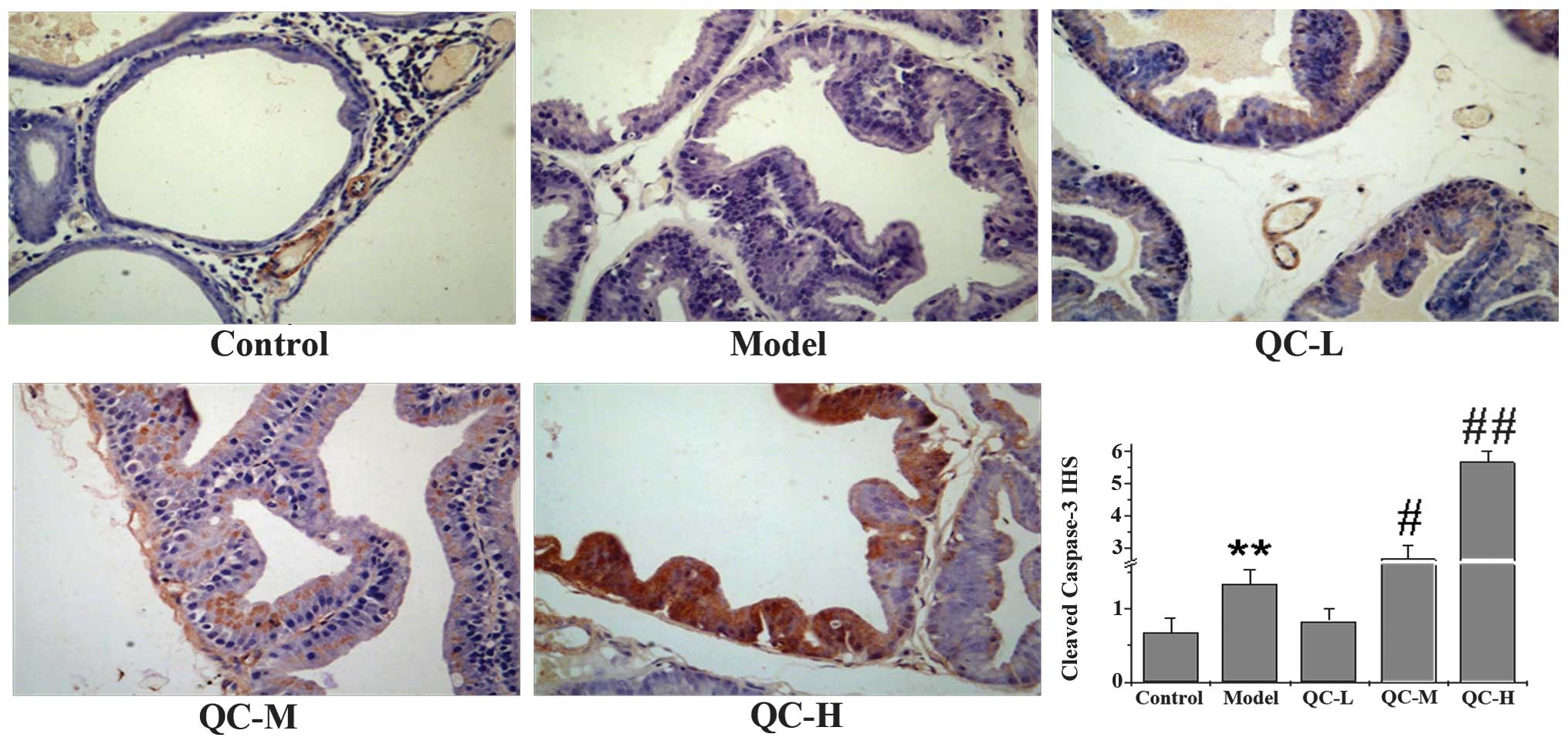
QC induces apoptosis in BPH rats
To determine whether the inhibitory effect of QC on
prostate growth was due to cell apoptosis, we examined its effect
on cell apoptosis in BPH rats via immunohistochemical staining for
TUNEL. Data in Fig. 4 show that QC
treatment increased the proportion of TUNEL-positive cells in a
dose-dependent manner, compared with the control and model groups,
demonstrating a pro-apoptotic activity of QC in vivo. To
further evaluate these results, we performed IHS to evaluate the
effect of QC on the cleavage of caspase 3, a critical event during
apoptosis. As shown in Fig. 5, QC
treatment increased the level of cleaved caspase 3 in prostate
tissues of BPH rats in a dose-dependent manner. Taken together, it
is suggested that the QC-mediated inhibition of prostate growth is
accompanied by its pro-apoptotic activity.
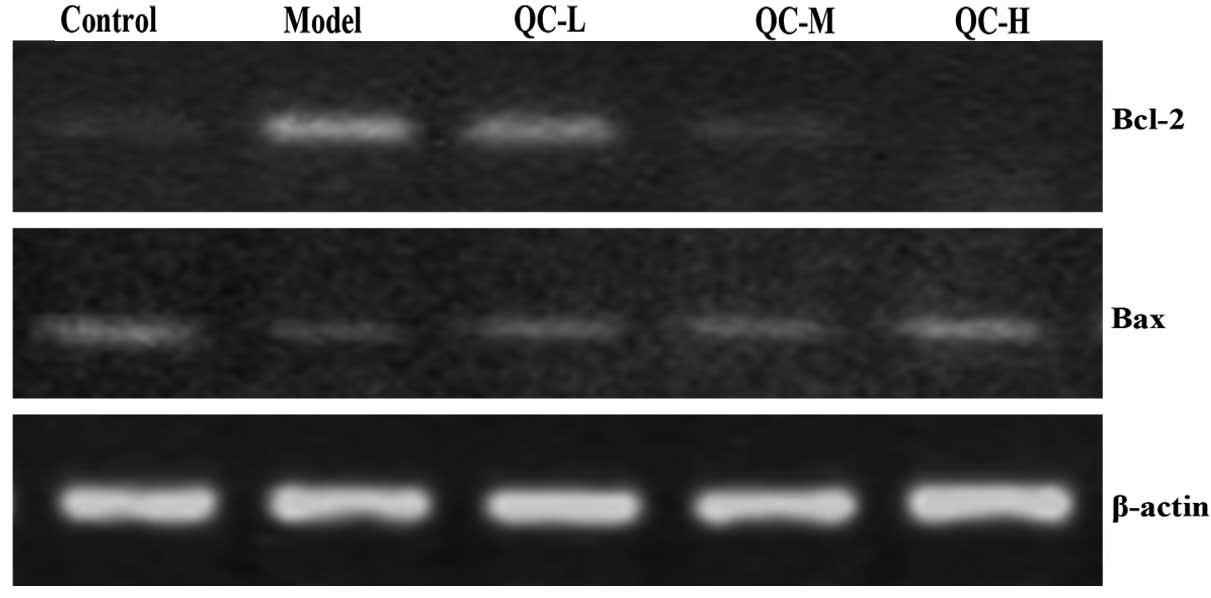
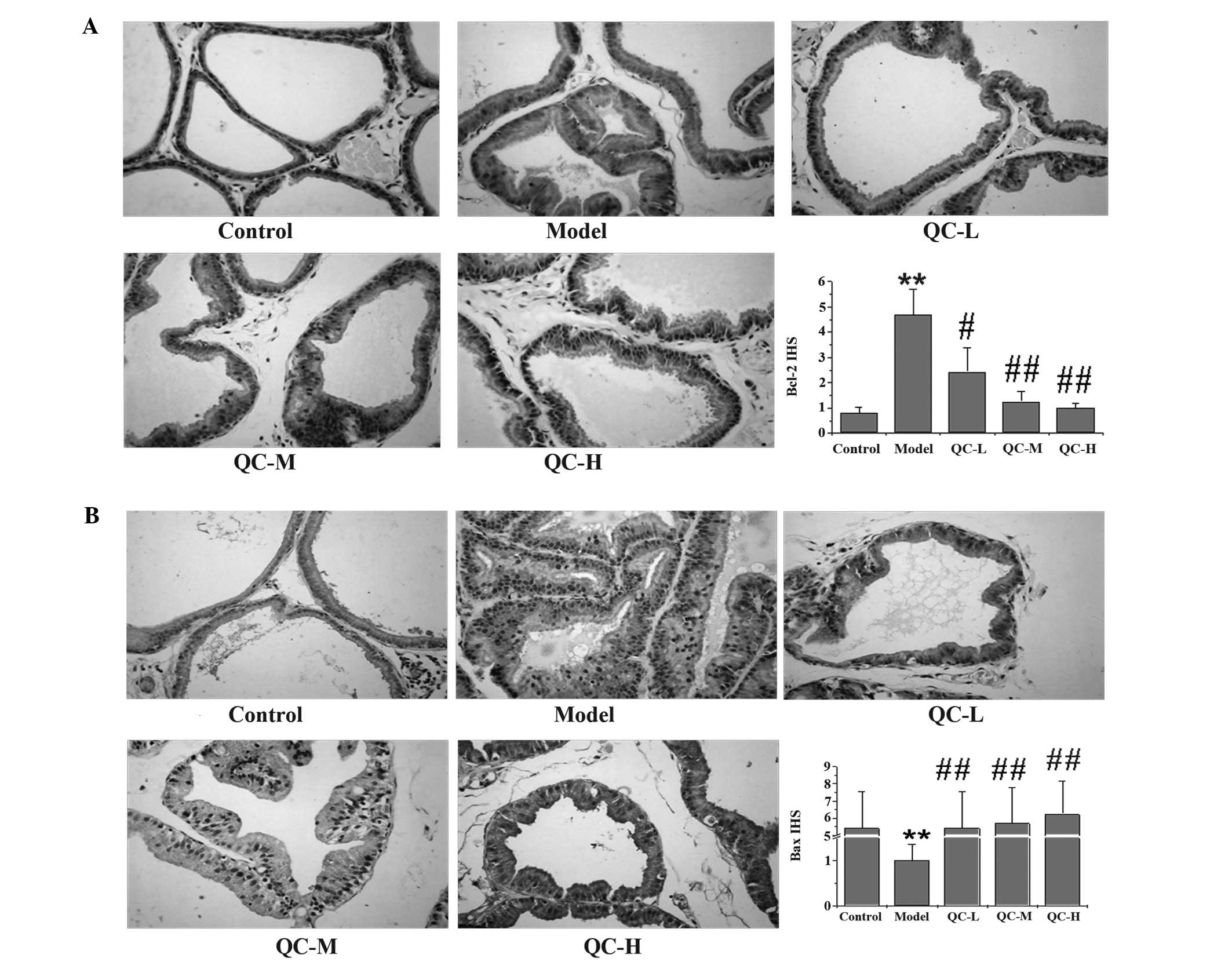
QC increases the pro-apoptotic Bax/Bcl-2
ratio in BPH rats
To further explore the mechanism of the
pro-apoptotic activity of QC, we examined its effect on Bcl-2 and
Bax expression using RT-PCR and IHS. Data from the RT-PCR assay
indicated that QC significantly reduced the mRNA expression of
anti-apoptotic Bcl-2 in BPH rats, while the expression of
pro-apoptotic Bax was significantly increased following QC
treatment (Fig. 6). Similarly,
results of IHS showed that the protein expression pattern of Bcl-2
and Bax were similar to their respective protein levels (Fig. 7). These data demonstrate that QC
promotes prostate cell apoptosis in vivo by increasing the
pro-apoptotic Bax/anti-apoptotic Bcl-2 ratio.
Discussion
Treatment options for BPH include surgery and
pharmacotherapy. Although surgery is more efficient for elderly
patients and those with severe heart, lung and kidney dysfunction,
pharmacotherapy remains the most common modality of choice. The two
main drugs for the management of BPH are α-adrenergic blockers and
5α-reductase inhibitors, both with their own side-effects.
Therefore, herbal remedies are often preferred for the management
of BPH since they usually have fewer negative effects and exhibit
therapeutic efficacy. As a traditional Chinese medicine formula
that has been used in clinical practice, QC has been shown to be
effective in the treatment of BPH. However, the mechanism of its
anti-BPH activity still remains to be elucidated. Therefore, before
QC is further developed in an anti-BPH agent, the precise mechanism
mediating its biological activities should be investigated.
In the present study, it was found that QC inhibited
prostate growth in vivo, using a BPH rat model. In addition,
by using TUNEL assay we demonstrated that the inhibitory role of QC
in BPH was due to its pro-apoptotic activity. The
mitochondrial-dependent pathway is the most common apoptotic
pathway in vertebrate animal cells, which is highly regulated by
Bcl-2 family members. During apoptosis, the pro-apoptotic Bax
translocates to the mitochondria and integrates into the outer
mitochondrial membrane, where it induces MOMP, resulting in the
release of cytochrome c and, subsequently, in the destruction of
cells. By contrast, the anti-apoptotic protein Bcl-2 prevents this
process by preserving mitochondrial integrity. The ratio Bcl-2/Bax
is important for determining the fate of cells and an increased
Bcl-2/Bax ratio by aberrant expression of the proteins is commonly
found in BPH. In this study, we found that QC treatment enhanced
Bax and reduced Bcl-2 expression in prostatic tissues of BPH
rats.
Caspases, represented by a family of cysteine
proteases, are the key proteins that modulate the apoptotic
response. Caspase 3 is a critical executioner of apoptosis, as it
is either partially or completely responsible for the proteolytic
cleavage of many key proteins. Similar to other caspases, caspase 3
exists as inactive proenzymes until it is cleaved by an initiator
caspase after apoptotic signaling events have occurred. In this
study, we found that QC treatment significantly induced the
cleavage activation of caspase 3 in BPH rats in a dose-dependent
manner.
To the best of our knowledge, this study has
demonstrated for the first time that QC inhibited prostate growth
in vivo by promoting apoptosis of prostatic cells, which was
mediated by the regulation of Bcl-2 family members. These findings
suggest that QC is a potential novel therapeutic agent for BPH
treatment.
Acknowledgements
This study was supported by the Nature Science
Foundation of China (nos. 81072927 and 81173433), and the Natural
Science Foundation of Fujian Province of China (nos. 2010J01199 and
2009J01169).
Abbreviations:
|
QC
|
Qianliening capsule
|
|
BPH
|
benign prostatic hyperplasia
|
|
PI
|
prostatic index
|
|
SD
|
Sprague-Dawley
|
|
IHC
|
immunohistochemistry
|
|
SP
|
streptavidin-peroxidase
|
References
|
1
|
Berry SJ, Coffey DS, Walsh PC and Ewing
LL: The development of human benign prostatic hyperplasia with age.
J Urol. 132:474–479. 1984.PubMed/NCBI
|
|
2
|
Djavan B: Lower urinary tract
symptoms/benign prostatic hyperplasia: fast control of the
patient's quality of life. Urology. 62:6–14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang MD, Zhao YN and An LW: B-cell
lymphoma/leukemia-2 and benign prostatic hyperplasia. Zhonghua Nan
Ke Xue. 15:452–454. 2009.(In Chinese).
|
|
4
|
Kyprianou N, Tu H and Jacobs SC: Apoptotic
versus proliferative activities in human benign prostatic
hyperplasia. Hum Pathol. 27:668–675. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Claus S, Berges R, Senge T and Schulze H:
Cell kinetic in epithelium and stroma of benign prostatic
hyperplasia. J Urol. 158:217–221. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roehrborn CG: Pathology of benign
prostatic hyperplasia. Int J Impot Res. 20:S11–S18. 2008.
View Article : Google Scholar
|
|
7
|
Gross A, McDonnell JM and Korsmeyer SJ:
Bcl-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reed JC: Mechanisms of apoptosis. Am J
Pathol. 157:1415–1430. 2000. View Article : Google Scholar
|
|
9
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mäntymaa P, Siitonen T, Guttorm T, Säily
M, Kinnula V, Savolainen ER and Koistinen P: Induction of
mitochondrial manganese superoxide dismutase confers resistance to
apoptosis in acute myeloblastic leukaemia cells exposed to
etoposide. Br J Haematol. 108:574–581. 2000.
|
|
11
|
Yang J, Liu X, Bhalla K, et al: Prevention
of apoptosis by Bcl-2: release of cytochrome c from mitochondria
blocked. Science. 275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: a
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jürgensmeier JM, Xie Z, Deveraux Q,
Ellerby L, Bredesen D and Reed JC: Bax directly induces release of
cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA.
95:4997–5002. 1998.PubMed/NCBI
|
|
14
|
Antonsson B, Montessuit S, Lauper S, Eskes
R and Martinou JC: Bax oligomerization is required for
channel-forming activity in liposomes and to trigger cytochrome c
release from mitochondria. Biochem J. 345:271–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsu YT, Wolter K and Youle RJ:
Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during
apoptosis. Proc Natl Acad Sci USA. 94:3668–3672. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wolter KG, Hsu YT, Smith CL, Nechushtan A,
Xi XG and Youle RJ: Movement of Bax from the cytosol to
mitochondria during apoptosis. J Cell Biol. 139:1281–1292. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei MC, Lindsten T, Mootha VK, et al:
tBid, a membrane-targeted death ligand, oligomerizes Bak to release
cytochrome c. Genes Dev. 14:2060–2071. 2000.PubMed/NCBI
|
|
18
|
Thomenius MJ, Wang NS, Reineks EZ, Wang Z
and Distelhorst CW: Bcl-2 on the endoplasmic reticulum regulates
Bax activity by binding to BH3-only proteins. J Biol Chem.
278:6243–6250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Antonsson B, Conti F, Ciavatta A, et al:
Inhibition of Bax channel-forming activity by Bcl-2. Science.
277:370–372. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roehrborn CG, Nuckolls JG, Wei JT and
Steers W: BPH Registry and Patient Survey Steering Committee. The
benign prostatic hyperplasia registry and patient survey: study
design, methods and patient baseline characteristics. BJU Int.
100:813–819. 2007. View Article : Google Scholar
|
|
22
|
Black L, Naslund MJ, Gilbert TD Jr, Davis
EA and Ollendorf DA: An examination of treatment patterns and costs
of care among patients with benign prostatic hyperplasia. Am J
Manag Care. 12:S99–S110. 2006.PubMed/NCBI
|
|
23
|
MacDonald R and Wilt TJ: Alfuzosin for
treatment of lower urinary tract symptoms compatible with benign
prostatic hyperplasia: A systematic review of efficacy and adverse
effects. Urology. 66:780–788. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roehrborn CG: Efficacy and safety of
once-daily alfuzosin in the treatment of lower urinary tract
symptoms and clinical benign prostatic hyperplasia: a randomized,
placebo-controlled trial. Urology. 58:953–959. 2001. View Article : Google Scholar
|
|
25
|
Djavan B and Marberger M: A meta-analysis
on the efficacy and tolerability of alpha1-adrenoceptor antagonists
in patients with lower urinary tract symptoms suggestive of benign
prostatic obstruction. Eur Urol. 36:1–13. 1999. View Article : Google Scholar
|
|
26
|
Gormley GJ, Stoner E, Bruskewitz RC, et
al: The effect of finasteride in men with benign prostatic
hyperplasia. N Engl J Med. 327:1185–1191. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roehrborn CG, Boyle P, Nickel JC, et al:
Efficacy and safety of a dual inhibitor of 5-alpha-reductase types
1 and 2 (dutasteride) in men with benign prostatic hyperplasia.
Urology. 60:434–441. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou JH, Lin JM, Xu W, Zhong XY, Xie JD
and Hong ZF: Effects of Qianliening capsule on the expression of
IL-10 and TNF-α in benign prostatic hyperplasia. Chin Archives Trad
Chin Med. 28:2657–2569. 2010.PubMed/NCBI
|
|
29
|
Zhou JH, Hong ZF, Lin JM, Zhao JY and Zhou
HT: Effect of Qianliening granule on experimental hyperplasia of
prostate. J Fujian Univ Trad Chin Med. 18:45–47. 2008.
|
|
30
|
Lin JM, Zhou JH, Zhong XY, et al: Effects
of Qianliening capsule on the expression of EGF and EGFR in BPH
Rats. Fujian J Trad Chin Med. 41:45–47. 2010.
|
|
31
|
Zhou HT, Lin JM, Zhao JY, Zhou JH and Hong
ZF: Inhibition effects of Qianliening granule on IL-1β and its mRNA
expression in model rats. J Fujian Univ Trad Chin Med. 20:21–24.
2010.
|
|
32
|
Huang W, Li M, Lin ZZ, Li ZM, Lai XP and
Su ZR: HPLC determination of emodin and oleanolic acid in
Yigankangfuling capsules. Chin J Pharm Anal. 28:1728–1731.
2008.
|
|
33
|
Soslow RA, Dannenberg AJ, Rush D, Woerner
BM, Khan KN, Masferrer J and Koki AT: Cox-2 is expressed in human
pulmonary, colonic, and mammary tumors. Cancer. 89:2637–2645. 2000.
View Article : Google Scholar : PubMed/NCBI
|