Introduction
The drug resistance mechanisms in bacteria, which
involve the production of inactivation enzymes, the alteration of
target protein synthesis and the alteration of membrane
permeability, thus reducing the aggregation antibiotics and
producing biofilms, have become hot research topics (1). In 1989, Stokes and Hall first put
forward the concept of integrons (2). Integrons are a hereditary unit for
gene capture and expression, situated in the bacterial plasmid,
chromosome or transposon, which have the capability of
site-specific recombination. They can also selectively capture or
remove various specific drug resistance box genes, and transfer
their drug resistance genes to different strains or different
bacterial genera through functions, such as transformation,
transduction and conjugation, a mechanism which accelerated the
spread and dissemination of bacterial drug resistance (3). New integron types are continuously
being discovered and the number of identified integron types has
increased. However, research remains focused on class I, II and III
integrons. Due to the widespread integration effect of the integron
integrase gene on bacterial drug resistance and its transitivity
among different genetic materials, multidrug-resistant bacteria are
capable of being rapidly transferred. From the related literature,
we can see that integrons are important in the dissemination of the
majority of bacterial multidrug resistance (4).
In China and the rest of the world, the most
commonly applied integron detection method is to use the specific
primer of various integrons for single PCR and examine them
separately (5). It has been
reported that integrase gene classification may be achieved by
combining restriction enzyme digestion and the PCR method (6), which uses degenerate primers through
the integron sequence of class I, II and III. Although this method
simplifies the previous method to a certain degree, the
classification results are obtained by two reactions and two
electrophoresis experiments, which is more time-consuming and uses
more materials. Therefore, it is important to improve these methods
and establish a rapid detection method. Based on the above
conditions, in this study, integrase-specific primers for class I,
II and III integrons were designed in order to establish a
multiplex PCR method that is capable of detecting the three classes
of integrons concurrently.
In recent years, although integrons have been found
in Gram-positive bacteria, the role that integrons play in
drug-resistant Staphylococcus aureus (SA) remains unclear
(7). Therefore, SA, the most
representative genus of Gram-positive bacteria, was selected to be
the subject of this study. The integron carrying conditions of
various drug-resistant SA strains, drug-resistant SA strains from
different sources, SA plasmid and genomic DNA were analysed using
this multiplex PCR method, in order to determine the role that
integrons play in mediating multidrug-resistant bacteria.
Materials and methods
Materials
Strains
All 180 SA strains were collected from clinical
specimens from January 1, 2009, to December 31, 2009, including 26
blood specimens, 86 sputum specimens (including throat swab), 15
drainage fluid specimens (including pleural fluid, ascites,
dialysate, catheter drain and synovial fluid), 3 cerebrospinal
fluid specimens, 32 urine specimens and 18 secretion specimens
(including liquor puris). The inoculation, culture and staining,
separation culture, study of biochemical events, susceptibility
tests, assessment by the Bactest microorganism analytical system
and the reported results of the specimens all strictly adhered to
the basic microorganism operating regulations. Drug-sensitive slips
were purchased from Oxoid Co. (Basingstoke, UK). The purified
strains were stored at −80°C. The quality control bacterial strain
was SA ATCC25923.
Antibacterial agents
Benzylpenicillin, cefoxitin, oxacillin,
erythromycin, clindamycin, azithromycin, bactrim, vancomycin,
linezolid, amoxicillin/clavulanic acid, piperacillin/tazobactam,
ciprofloxacin, tetracycline, rifampicin, imipenem, cefazolin,
cefuroxime, levofloxacin, gentamicin and teicoplanin were used. The
sensitivity of SA to 20 types of antibiotics was examined using the
K-B method. The susceptibility test referred to the CLSI2009
antibacterial susceptibility testing standard.
Main reagents
Tris saturated phenol was produced by Shanghai
Generay Biotech Co., Ltd. (China), protease K was produced by
Amresco Co. (Solon, OH, USA), trichloromethane was purchased from
Tianjin Northern Tianyi Chemical Agent Manufacturers (China),
drug-sensitive slips were produced by Oxoid Co. and blood agar
plates were provided by Beiruite Bio-technology (Zhengzhou) Co.,
Ltd. (China). The bacterial genomic DNA extraction kit was provided
by Beijing Tianjin Biochemical Technology Co., Ltd (China). The
high-purity 96 plasmid extraction kit was provided by the Beijing
Kangweishiji Biotech Co., Ltd. (Beijing, China). Agarose was
provided by the Shanghai Bioengineering Co., Ltd. (Shanghai,
China). Primers were synthesised by the Shanghai Bioengineering
Co., Ltd. and the sequences were as follows: Class I integron
upstream primer, 5′-CCT CCC GCA CGA TGA TC-3′ and downstream
primer, 5′-TCC ACG CAT CGT CAG GC-3′; class II integron upstream
primer, 5′-GTA GCA AAC GAG TGA CGA AAT G-3′ and downstream primer,
5′-CAC GGA TAT GCG ACA AAA AGG T-3′; class III integron upstream
primer, 5′-GCC TCC GGC AGC GAC TTT CAG-3′ and downstream primer,
5′-ACG GAT CTG CCA AAC CTG ACT-3′.
Main instruments
The PTC-1148 PCR amplification system was obtained
from Bio-Rad (Hercules, CA, USA), and DY-A electrophoretic
apparatus was from Shanghai Kangda Electronic Apparatus
Manufacturers. The Gel Logic 200 gel imaging system was obtained
from the Carestream Health Inc., (NY, USA).
Data processing
SPSS13.0 software was used for the statistical
analysis of the original data. The Chi-square test was adopted for
the rate comparison. Size of test, α=0.05.
Methods
Experimental strains for the in vitro
bacteria drug sensitivity test
The inoculation, culture and staining of specimens,
separation culture, study of biochemical events, susceptibility
tests, assessment by Bactest microorganism analytical system and
reported results all strictly adhered to the basic microorganism
operating regulations. The judgement of the results was performed
according to the CLSI standards, 2009.
Strain recovery and enrichment
The purified bacterial specimen was removed from
storage (at −80°C). The bacterial specimen was inoculated on the
blood agar culture plate using an inoculating loop, then the
culture plate was cultured in the 35°C electric-heated thermostatic
water bath for 24 h, and this operation was repeated until a single
colony appeared. The single colony was then cultured for 24 h in a
35°C electric-heated thermostatic water bath.
The extraction of SA plasmid DNA and
SA genomic DNA
The high-purity 96 plasmid extraction kit from
Beijing Kangweishiji Biotech Co., Ltd. was used for the SA plasmid
DNA extraction. The TIANamp Bacteria DNA kit from Beijing Tianjin
Biochemical Technology Co, Ltd. was used for SA genomic DNA
extraction. The kits were operated according to the manufacturer’s
instructions.
PCR amplification and product
analysis
The reaction conditions for PCR amplification were
as follows: Pre-degeneration for 4 min at 94°C, degeneration for 45
sec at 94°C, annealing for 45 sec at 55°C, elongation for 55 sec at
72°C and finally, after 30 cycles, elongation for another 8 min.
The amplification products were stored at −20°C.
In order to efficiently screen different types of
integrons, the multiplex PCR method was used for integrase gene
detection, which saved time and money. The designed primers for the
class I, II and III integrons had the following features: Similar
PCR reaction annealing temperatures, largely varying products and
electrophoresis strips which were easy to differentiate. The
laboratory procedure was as follows: Primers of the class I, II and
III integrons were set in the same reaction system by the gradient
PCR method. The six annealing temperature gradients were between
51–60°C. Finally, the annealing temperature of multiplex PCR was
set as 55°C.
After PCR amplification, 5 μl of products were added
to the well containing 2% agarose gel (ethidium bromide staining).
Products were electrophoresed for 30 min under 100 V with 0.5× TBE
liquid as the electrophoretic liquid. Strips were observed using
the Kodak gel imaging system.
Results
Class I, II and III integrons detected by
the PCR method
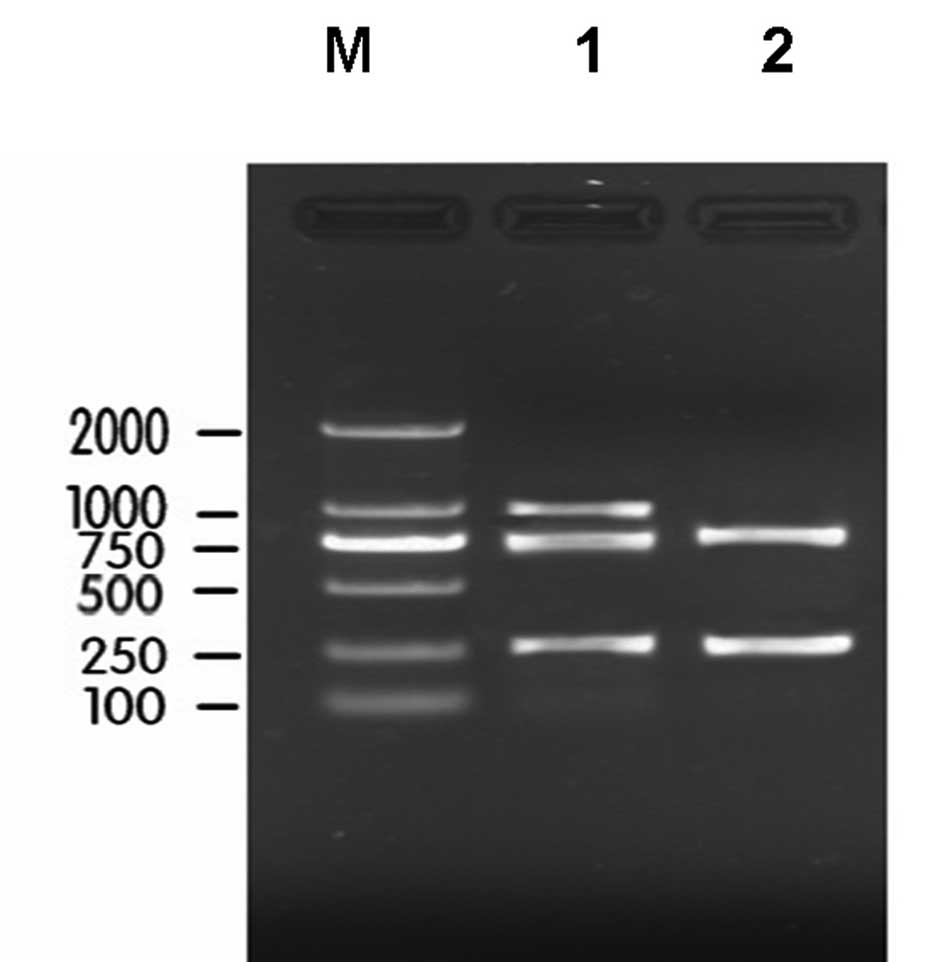
With the primers designed according to the integrase
sequence of class I, II and III integrons, the multiplex PCR method
was used for the amplification of SA plasmid DNA and SA genomic
DNA. Subsequently, 2% agar gel electrophoresis was used to observe
the amplification products. The gel electrophoresis found that
bands appeared at 280, 788 and 979 bp, and the segment length
corresponded with the anticipated results (Fig. 1).
Drug resistance analysis of SA
The difference in SA drug resistance rates to
various types of antibacterial agents was statistically significant
(P<0.05). Among the 20 detected antibiotics, the three
antibiotics with the highest drug resistance rate in SA were
benzylpenicillin (93.7%), erythromycin (81.1%) and azithromycin
(79.4%; Table I). The three
antibiotics with the lowest drug resistance rate were vancomycin
(0%), teicoplanin (2.2%) and linezolid (2.8%). SA exhibited
features of multiple drug resistance.
 | Table IDrug resistance in Staphylococcus
aureus (SA). |
Table I
Drug resistance in Staphylococcus
aureus (SA).
| Drugs | Number of resistant
strains (n=180) | Drug resistance rate
(%) |
|---|
| Cefoxitin | 95 | 52.8 |
| Benzylpenicillin | 169 | 93.7 |
| Oxacillin | 94 | 52.2 |
| Erythromycin | 146 | 81.1 |
| Azithromycin | 143 | 79.4 |
| Clindamycin | 123 | 68.3 |
| Vancomycin | 0 | 0.0 |
| Bactrim | 87 | 48.3 |
| Linezolid | 5 | 2.8 |
|
Amoxicillin/clavulanic acid | 102 | 56.7 |
|
Piperacillin/tazobactam | 99 | 55.0 |
| Ciprofloxacin | 103 | 57.2 |
| Tetracycline | 102 | 56.7 |
| Rifampicin | 75 | 41.7 |
| Imipenem | 91 | 50.6 |
| Cefazolin | 93 | 51.7 |
| Cefuroxime | 97 | 53.9 |
| Levofloxacin | 36 | 19.8 |
| Gentamicin | 94 | 52.2 |
| Teicoplanin | 4 | 2.2 |
Role of integrons in mediating drug
resistance in SA
Comparison of class I and II integron
detection in SA plasmids and genomic DNA
The positive rate of class I and II integrons in
plasmid DNA was higher than that of genomic DNA, and the positive
rate difference was statistically significant (P<0.05) (Tables II and III).
 | Table IIComparison of class I integron
detection in Staphylococcus aureus (SA) plasmid and genomic
DNA. |
Table II
Comparison of class I integron
detection in Staphylococcus aureus (SA) plasmid and genomic
DNA.
| Class I
integrons | |
|---|
|
| |
|---|
| DNA resources | Positive strains | Negative strains | Positive rate
(%) |
|---|
| Genomic DNA | 71 | 109 | 39.44 |
| Plasmid DNA | 92 | 88 | 51.11 |
| χ2 | | 4.94 | |
| P-value | | 0.026 | |
 | Table IIIComparison of class II integron
detection in Staphylo- coccus aureus (SA) plasmid and
genomic DNA. |
Table III
Comparison of class II integron
detection in Staphylo- coccus aureus (SA) plasmid and
genomic DNA.
| Class II
integrons | |
|---|
|
| |
|---|
| DNA resources | Positive strains | Negative strains | Positive rate
(%) |
|---|
| Genomic DNA | 10 | 170 | 5.88 |
| Plasmid DNA | 23 | 157 | 12.78 |
| χ2 | | 5.64 | |
| P-value | | 0.018 | |
Class I and II integron detection in
the SA plasmids with different drug resistance
According to the drug selection standards for SA
(CLSI 2009) and the different antibiotic-resistant strains of SA,
the strains were divided into the following three groups: Group A,
single drug resistance strains; group B and C were multidrug
resistance strains with different degrees of resistance. The
difference between class I integron detection rates in these groups
was statistically significant (P<0.01). The class I integron
detection rate in group C was higher than that in group A (Table IV). The difference in the class II
integron detection rate was not statistically significant
(P<0.05). Class I, II and III integrons were not detected in the
quality-control strain, ATCC25923.
 | Table IVClass I and II integron detection in
Staphylococcus aureus (SA) plasmid DNA with different SA
drug resistance. |
Table IV
Class I and II integron detection in
Staphylococcus aureus (SA) plasmid DNA with different SA
drug resistance.
| Drug-resistant
type | Number of resistant
strains | Positive rate of
class I integron (%) | Positive rate of
class II integron (%) |
|---|
| A | 80 | 43.8 | 8.8 |
| B | 62 | 59.7 | 16.1 |
| C | 25 | 80.0 | 24.0 |
| χ2 | | 9.28 | 4.19 |
| P-value | | <0.001 | 0.15 |
Class I and II integron detection in
the SA plasmid DNA obtained from different specimens
The specimens were divided into six groups according
to their source (sputum, blood, cerebrospinal fluid, drainage
fluid, excretion and urine). The detection rate of class I
integrons in the plasmid DNA from the six sources was statistically
different (P<0.01). Multiple comparison analyses were then
performed on the samples and the difference was found to be
statistically significant (P<0.05). The detection rate of class
I integrons was the highest in the plasmid DNA from the urine
specimen, followed by the sputum specimen, while the detection rate
of class I integrons was the lowest in the plasmid DNA from the
blood specimen. The detection rate of class II integrons in the
plasmid DNA obtained from the six types of specimens was not
statistically different (P>0.05) (Table V and VI).
 | Table VDetection of class I integrons in the
Staphylococcus aureus (SA) plasmid DNA from different
specimen sources. |
Table V
Detection of class I integrons in the
Staphylococcus aureus (SA) plasmid DNA from different
specimen sources.
| Strain source | Multidrug resistant
rate (%) | Class I
integrons | Positive rate
(%) |
|---|
|
|---|
| Positive
strains | Negative
strains |
|---|
| Sputum | 67.9 | 52 | 34 | 60.47 |
| Blood | 28.3 | 4 | 22 | 15.38 |
| Drain | 42.9 | 7 | 8 | 46.67 |
| Excretion | 39.7 | 5 | 13 | 27.78 |
| Cerebrospinal
fluid | 66.7 | 1 | 2 | 33.33 |
| Urine | 79.4 | 21 | 11 | 65.63 |
| Total | | 90 | 90 | - |
 | Table VIDetection of class II integrons in
the Staphylococcus aureus (SA) plasmid DNA from different
specimen sources. |
Table VI
Detection of class II integrons in
the Staphylococcus aureus (SA) plasmid DNA from different
specimen sources.
| Strain source | Multidrug resistant
rate (%) | Class II
integrons | Positive rate
(%) |
|---|
|
|---|
| Positive
strains | Negative
strains |
|---|
| Sputum | 67.9 | 11 | 75 | 12.79 |
| Blood | 28.3 | 1 | 25 | 3.85 |
| Drain | 42.9 | 2 | 13 | 13.33 |
| Excretion | 39.7 | 1 | 17 | 5.56 |
| Cerebrospinal
fluid | 66.7 | 0 | 3 | 0 |
| Urine | 79.4 | 8 | 24 | 25.0 |
| Total | | 23 | 157 | - |
Discussion
The collected specimens underwent a susceptibility
test to 20 types of antibiotics. The results indicated that SA had
different degrees of drug resistance to 19 types of antibiotics,
with the exception of vancomycin. The top three antibiotics to
which SA had the highest drug resistance rate were benzylpenicillin
(93.7%), erythromycin (81.1%) and azithromycin (79.4%). SA showed
multidrug resistance features. This indicates that the phenomena of
drug resistance in SA is currently a serious one.
The bacterial drug resistance situation is becoming
increasingly serious, as the production speed of antibacterial
agents is unable to catch up with the bacterial drug resistance
production speed. Soon after the application of a neotype
antibacterial agent, the bacteria that are capable of resisting
this type of agent would appear. The generation and transmission
mechanisms of the drug resistance genes have become a hot research
topic in order to control the spread of drug-resistant bacteria.
Integron, the fluid element of genetic transmission, has drawn
great attention in mediating bacterial drug resistance (8).
At present, nine classes of integrons may be
retrieved from GenBank. However, only the first four classes have
been confirmed. Among them, class I integrons have been found more
frequently in various types of bacteria, particularly Gram-negative
bacteria, which may be observed in a number of studies (9,10).
The classification of different integrons is mainly based on
differences in the gene structure of integrases (11). The typical integron structure
includes 5′ and 3′ conservative regions and a variable gene cluster
in the middle. The 5′ conservative end consists of an encoding
integrase gene, intI, a recombination site, attl, and a gene
segment in the promoter sequence area, which is the basic structure
of all integrons. Although several genes carry promoter sequences,
the majority of box genes have no promoter sequence; therefore, the
5′ promoter sequence plays an important role in promoting the
transcription of its downstream box genes (12). Not all integrons have the 3′
conservative fragment structure. Most class I integrons have a 3′
conservative end, which is comprised of the qacE1 gene which
encodes drug resistance in bacteria, the sun gene which encodes
drug resistance against sulfamido and an open reading frame of
unknown function (4,13). There are a few class I integrons
that have no 3′ conservative fragments or lack a typical 3′
conservative sequence (14). Class
II integrons are generally situated in Tn7, whose integrase gene,
intI2, is internally separated by the terminal codons, becoming the
defective gene. The 3′ end of class II integrons is constituted by
the gene participating in the Th7 transposition mechanism, which is
different from the 3′ conservative end of class I integrons
(15). There are only a few
reports on class III integrons with the first case found in
Serratia marcescens(16).
Another case was found in Klebsiella pneumoniae(15) with two promoter sequences carrying
two types of drug resistance box genes, laGEs and blaox/aaIb. Class
IV integrons, the so-called super integrons, mainly exist in the
Vibrio cholerae, according to previous reports (13,17).
Class IV integrons carry tens to hundreds of box genes and have
several virulence genes, with the exception of a drug resistance
gene.
Comparatively, since SA has not been extensively
studied, it was selected as the research subject in our study. Only
one case with class III integrons was detected; the carrying
conditions of class I and II integrons in SA were mainly analysed.
According to the known data, the integrase gene, intI1, of class I
integrons encodes 337 amino acids (18), and the integrase gene, intI2, of
class II integrons encodes 325 amino acids, which share 46%
homology with intI1 (19); the
integrase gene intI3 of class III integrons encodes 320 amino
acids, having 61% homology with intI1 (20). In view of the homology that the
integrases of the three classes of integrons have (21), the multiplex PCR method was used to
amplify these three types of integrases. Primers for these three
types of integrases, consistent with the multiplex PCR
amplification conditions, were designed based on their shared
sequences. The designed primers of class I, II and III integrons
have similar annealing temperatures, significantly different
product sizes and the electrophoresis bands are easily
differentiated. The experimental results showed that the designed
integrase primers of class I, II and III integrons are capable of
effectively detecting and differentiating the class I, II and III
integrons using the multiplex PCR method. Integrons, which exist in
the genomic DNA and plasmid DNA, can be disseminated between the
same and different bacterial genera through transposons,
integrating bacteriophages and conjugal plasmids. These
experimental results indicated that the positive rate of class I
and II integrons in SA plasmid DNA was higher than that in the SA
genomic DNA. The reason for this may be that the drug resistance
gene of class II integrons in plasmids can easily take part in
horizontal transfer.
At present, the majority of box genes found in class
I integrons are drug resistance box genes, whose encoding product
enables bacteria to resist almost all the antibiotics applied in
the clinic. One integron may carry several box genes. The
production of bacterial multidrug resistance is closely related to
the integrons. Integrons are commonly observed in Gram-negative
bacteria (22). The experimental
subject in this study was SA, a Gram-negative bacteria. Research in
China on SA is comparatively rare. The experimental results
indicated that the class I integron detection rate was highest in
the strains resistant to most types of antibiotics, which was
identical to the findings of Madiyarov et al(23). This indicated that the detection
rate of class I integrons positively correlated with the SA drug
resistance. This finding proposes a new direction for the research
of bacterial multi-drug resistance mechanisms. The drug resistance
gene may be transferred between different integrons through
integrating or removing the box gene under the influence of
integrase, and disseminated through the movement of plasmids and
transposons (13). Therefore, the
fast experimental detection of integrons may be regarded as a
useful tool for the monitoring of bacterial drug resistance.
In this experiment, after comparing the class I and
II integron detection conditions of SA specimens from sputum,
blood, cerebrospinal fluid, drain fluid, excretion and urine, the
class I integron detection rate in the plasmid DNA from the urine
specimen was the highest, followed by plasmid DNA from the sputum
specimen. The reason for the comparatively higher amount of
integrons in SA obtained from the urine specimens (24) may be that infection found in the
urine had already been serious when bacteria invaded the urine, and
the environment that drug-resistant SA was in had positive
selection. The class I integron positive rate of SA from the urine
specimen was higher than that from other specimens, which indicated
that the SA drug resistant environment had a certain influence on
the carrying rate of integrons.
In conclusion, multiplex PCR is an effective method
to detect class I, II and III integrons, which has the advantage of
saving time and resources. The carrying rate of class I and II
integrons of in plasmid DNA was higher than that in genomic DNA in
drug-resistant SA, which indicated that the plasmid is the main
carrier for transferring integron. The more drug-resistant the SA,
the higher the carrying rate of class I integron, which indicated
that integron mediates the SA multidrug resistance. SA plasmid
extracted from the specimen with a high rate of drug resistance had
a high class I integron carrying rate, which indicated that the SA
environment had a certain influence on the carriage of
integrons.
Acknowledgements
This study was funded by the Henan scientific and
technological project (No. 112102310155). We are extremely thankful
for the theoretical and technological instruction and generous help
of teachers in bacterium room, laboratory department and PCR room
of Zhengzhou University.
References
|
1
|
Wright GD and Thompson PR: Aminoglycoside
phosphotransferases: proteins, structure, and mechanism. Frontiers
Biosci. 4:D9–D21. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stokes HW and Hall RM: A novel family of
potentially mobile DNA elements encoding site-specific
gene-integration functions: integrons. Mol Microbiol. 3:1669–1683.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nield BS, Holmes AJ, Gillings MR, et al:
Recovery of new integron classes from environmental DNA. FEMS
Microbiol Lett. 195:59–65. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nemergut DR, Martin AP and Schmidt SK:
Integron diversity in heavy-metal-contaminated mine tailings and
inferences about integron evolution. Appl Environ Microbiol.
70:1160–1168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antunes P, Machado J and Peixe L:
Charaeterization of antimicrobial resistance and class 1 and 2
integrons in Salmonella enteric isolates from different sources in
Portugal. J Antimicrob Chemother. 58:297–304. 2006.PubMed/NCBI
|
|
6
|
Li XH, Shi L, Yang WQ, et al: Construction
and application of degenerate primers PCR assay to screen class 1,
2 and 3 integrase (intI). Chin J Microbiol Immunol. 25:156–160.
2005.
|
|
7
|
Nield BS, Willows RD, Torda AE, et al: New
enzymes from environmental cassette arrays: functional attributes
of a phosphotransferase and an RNA-methyl -transferase. Protein
Sci. 13:1651–1659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yanhong MA and Wei QI: Research progress
of integron in Gram-positive bacteria. World Notes Antibiot.
30:131–134. 2009.
|
|
9
|
Goldstein C, Lee MD, Sanchez S, et al:
Incidence of class 1 and 2 integrases in clinical and commensal
bacteria from livestock, companion animals, and exotics. Antimicrob
Agents Chemother. 45:723–726. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Phongpaichit S, Wuttananupan K and
Samasanti W: Class 1 integrons and multidrug resistance among
Escherichia coli isolates from human stools. Southeast Asian
J Trop Med Public Health. 39:279–287. 2008.PubMed/NCBI
|
|
11
|
Mevelec MN, Bout D, Desolme B, et al:
Evaluation of protective effect of DNA vaccination with genes
encoding antigens GRA4 and SAG1 associated with GM-CSF plasmid,
against acute, chronical and congenital toxoplasmosis in mice.
Vaccine. 23:4489–4499. 2005. View Article : Google Scholar
|
|
12
|
Mazel D, Dychinco B, Webb VA, et al: A
distinctive class of integron in the Vibrio cholerae genome.
Science. 280:605–608. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heidelberg JF, Eisen JA, Nelson WC, et al:
DNA sequence of both chromosomes of the cholera pathogen Vibrio
cholerae. Nature. 406:477–483. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rowe-Magnus DA, Guerout AM, Ploncard P,
Dychinco B, Davies J and Mazel D: The evolutionary history of
chromosomal super-integrons provides an ancestry for multiresistant
integrons. Proc Natl Acad Sci USA. 98:652–657. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Correia M, Boavida F, Grosso F, et al:
Molecular characterization of a new class 3 integron in
Klebsiella pneumoniae. Antimicrob Agents Chemother.
47:2838–2843. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao S, McDennott PE, White DG, et al:
Characterization of multidrug resistant Salmonella recovered from
diseased animals. Vet Microbiol. 56:268–272. 2007.
|
|
17
|
Rowe-Magnus DA, Guérout AM and Mazel D:
Super-integrons. Res Microbiol. 150:641–651. 1999. View Article : Google Scholar
|
|
18
|
Arakawa Y, Murakami M, Suzuki K, et al: A
novel integron-like element carrying the metallo-beta-lactamase
gene blaIMP. Antimicrob Agents Chemother. 39:1612–1615. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nandi S, Maurer JJ, Hofacre C and Summers
AO: Gram-positive bacteria are a major reservoir of class 1
antibiotic resistance integrons in poultry litter. Proc Natl Acad
Sci USA. 101:7118–7122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramírez MS, Vargas LJ, Cagnoni V, Tokumoto
M and Centrón D: Class 2 integron with a novel cassette array in a
Burkholderia cenocepacia isolate. Antimicrob Agents Chemother.
49:4418–4420. 2005.PubMed/NCBI
|
|
21
|
Hansson K, Sundstrom L, Pelletier A and
Roy PH: IntI2 integron integrase in Tn7. J Bacteriol.
184:1712–1721. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Messier N and Roy PH: Integron integrases
possess a unique additional domain necessary for activity. J
Bacteriol. 183:6699–6706. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Madiyarov RS, Bektemirov AM, Ibadova GA,
et al: Antimicrobial resistance patterns and prevalence of class 1
and 2 integrons in Shigella flexneri and Shigella sonnei isolated
in Uzbekistan. Gut Pathog. 2:182010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen J, Wang C, Wang J and Yu F:
Antimicrobial-resistant profile of Staphylococcus aureus
from different origins. Chin J Nosocomiol. 19:571–573. 2009.
|