Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory
polyarthritis which affects all synovial-lined diarthrodial joints;
however, the disease has a predilection for the wrist and small
joints of the hand. RA occurs in ~1% of adults and is ~2.5 times
more prevalent in females than males. A number of genetic and
environmental factors are known to affect this disease (1,2). RA
leads to progressive articular damage, joint deformities and
disability and is considered to be an autoimmune disease. A
previous study in the joints of patients with RA revealed the
presence of T cells in synovium (3). In an additional study, cluster of
differentiation (CD) 4+ cells isolated from the joints
of patients with RA and other inflammatory joint diseases were
reported to be less responsive to mitogenic stimulation in the
presence of IL-2 compared with control patients. By contrast, no
difference was found in the CD8+ cell response,
indicating that joint-resident CD4+ T cells are
potential effectors in RA (4).
However, at present, limited knowledge of target antigens relevant
to the disease process remains a major limitation for understanding
of the role of the adaptive immune response to RA (5).
Animal models have been used extensively in studies
of RA pathogenesis. Despite numerous inherent limitations, several
models have significantly contributed to the understanding of
fundamental pathological RA mechanisms and to major advances in
therapy. These models include collagen-induced arthritis (CIA)
(6), collagen antibody-induced
arthritis (7), zymosan-induced
arthritis (8), methylated BSA and
genetically manipulated or spontaneous arthritis, including
TNF-α-transgenic, K/BxN and SKG mice (9). CIA is the best known and most
extensively used model for the analysis of immunological and
pathological similarities with human RA.
CIA is an autoimmune disease of joints,
characterized by T and B cell response to autologous type II
collagen (CII). This model is reproducible in genetically
susceptible mouse strains, including DBA/1 and B10.Q by
immunization with heterologous type II collagen in complete
Freund’s adjuvant (CFA). Bovine, porcine and human collagen has
been used to reproduce this model. However, variations in response
have been identified between various strains and injection
conditions, and false positive results and reduced potency are
common as a result of minor contaminants or deglycosylated protein
(10,11). In the current study, CII was
isolated and purified from chicken sternal cartilage and observed
to successfully induce RA. The model was characterized by T helper
17 (Th17) cell infiltration.
Materials and methods
CII isolation and purification
Chicken sternal cartilage was selected as raw
material and CII was isolated according to the following protocol.
Briefly, fresh sternal cartilage was removed from the periosteum
and the calcified portion, cut into slices and conserved at −20°C.
Proteoglycans were removed using guanidine hydrochloride. The
precipitation was washed by Tris-HCl (0.05 mol/l) and acetic acid
(0.5 mol/l), then digested by 5 times the volume of pepsin (1 g/l,
pH 7.5), collecting the supernatant following centrifugal, repeated
two times; all the supernatant was collected. The supernatant was
keep pH 7.5 and chlorine sodium (NaCl) (1 mol/l), salting out
overnight at 4°C, collecting the precipitation following
centrifugal. The precipitation was dissolved with 0.1 mol/l acetic
acid and dialysis equilibrium by 0.05 mol/l Tris-HCl-0.2 mol/l NaCl
(pH 7.5), namely primary collagen. And then the CII was purified by
DE22 cellulose or DEAE-agarose.
The molecular weight of CII was identified by
SDS-PAGE and the amino acid composition was analyzed by the State
Key Laboratory of Medical Biotechnology, Nanjing University
(Nanjing, China). The absorption spectrometry was identified by
spectrophotometer (Shimadzu, Kyoto, Japan).
Mice
DBA/1 mice were purchased from Shanghai Laboratory
Animal Center, CAS (Shanghai, China) and maintained in the Animal
Center of Jiangsu University (Zhenjiang, China) in compliance with
the Guide for the Care and Use of Laboratory Animals (no. 85-23,
revised 1996). The experimental protocol was approved by the Ethics
Review Committee for Animal Experimentation of Jiangsu
University.
CIA model induction
Male DBA/1 mice (6–9 weeks) were immunized
intradermally at the base of the tail with 100 μg chicken sternal
hyaline CII dissolved in 100 μl acetic acid (0.05 mol/l) and mixed
with an equal volume of CFA (Difco Laboratories, Detroit, MI, USA).
After 3 weeks, animals were reimmunized with 100 μg CII emulsified
in incomplete Freund’s adjuvant (Difco). Mice were observed 3
times/week. After 45 days, mice were anesthetized with
pentobarbital sodium (30 mg/g body weight, i.p.) and sacrificed by
cervical dislocation. They then underwent rapid joint excision.
Mice were inspected for the development of CIA and
inflammation of the four paws was graded between 0 and 4: 0, paws
with no swelling; 1, paws with swelling of finger joints or focal
redness; 2, paws with mild swelling of wrist or ankle joints; 3,
paws with severe swelling of the entire paw; and 4, paws with
deformity or ankylosis. Each paw was graded and the four scores
were totaled (12).
Histological analysis
Knee joints from the mice were removed and fixed in
10% formalin. After 4 days, joints were placed in 5% formic acid
for decalcification. Tissue sections were stained with hematoxylin
and eosin.
Immunofluorescence
Immunofluorescence staining of paraffin-embedded
mouse joints was performed as described in a previous study
(13). Following
deparaffinization, rehydration and antigen unmasking, samples were
immersed in blocking buffer for 60 min. Then, primary antibodies
against CD4 and interleukin (IL)-17 (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) were applied for 2 h at room
temperature. Following washing, fluorescein isothiocyanate and
phycoerythrin labeled secondary antibodies were added for 1 h.
Sections were viewed under a fluorescence microscope (Olympus,
Tokyo, Japan) and then analyzed using Image J software.
Macrophages cells isolates and
treatment
Macrophages isolated from DBA/1 mice were cultured
at 1×106 cells/well in RPMI-1640 medium (Invitrogen Life
Technologies, Carlsbad, CA, USA) supplemented with 20% fetal bovine
serum and 1% streptomycin/penicillin and then stimulated with 0.5
μg/ml CII for 30 min, 1 h, 90 min and 2 h for mRNA analysis or
treated for 1, 2 and 3 days for cytokine analysis.
For blockade of toll-like receptor 2 (TLR2), 10
μg/ml anti-TLR2 antibody (Santa Cruz Biotechnology) was added prior
to 1 h treatment and then stimulated with 0.5 μg/ml CII for 3 days.
The supernatant was collected and used for IL-1β, transforming
growth factor (TGF)-β and IL-6 assays.
Cytokine measurement
Mouse serum and cell culture supernatant were
collected and stored at −80°C until use. Serum levels of TGF-β and
IL-6 and -17 were detected using ELISA kits (Bender MedSystems
GmbH, Vienna, Austria), according to the manufacturer’s
instructions.
Real-time quantitative polymerase chain
reaction (RT-qPCR)
TLR2, 4, 5, 7, 8 and 9 and Dectin 1 and 2 mRNA
levels were assessed by RT-qPCR as described previously (14). Briefly, total RNA was isolated from
cells using TRIzol reagent (Invitrogen Life Technologies) according
to the manufacturer’s instructions and reverse transcribed into
first-strand cDNA using the Moloney murine leukemia virus reverse
transcriptase system. Following cDNA synthesis, real-time PCR was
performed with iQ SYBR-Green Supermix (Bio-Rad Laboratories,
Hercules, CA, USA), using a 7500 Fast Real-Time PCR System (Applied
Biosystems, Carlsbad, CA, USA) with β-actin as an internal control.
Primer sequences are presented in Table I. Quantification of gene expression
was calculated relative to β-actin.
 | Table IPrimer sequences used for RT-qPCR. |
Table I
Primer sequences used for RT-qPCR.
| Gene | Sequence(5′-3′) | Amplicon length |
|---|
| TLR2 |
CATGGGCCCCAGGTCCTT
CAACTCTCTCAACGGCCA | 269 |
| TLR4 |
CTGAGCAGCCGCTCTGGC
AGCCCCAGGTGAGCTGTA | 307 |
| TLR7 |
GGGTGTGTGATGGCCGCT
AAAGGGCCCGAACCAG | 279 |
| TLR8 |
TGGCTTCTTCTCCGAAGC
AGGTGGTGAACCAGAGCA | 366 |
| TLR9 |
ACGCAGCGCCCAAACTCC
GCGGTCTTCCAACAGGCG | 301 |
| Dectin1 |
TGGGTTAGTGAGCCTCAT
CACCCAGCACTGCAGCAA | 278 |
| Dectin2 |
TGTGGGGATGCTTGCCCA
TGGGTTCATGGGGGTGCC | 285 |
| IFN-γ |
TATTCGGTAACTGACTTG
AATCACATAGCCTTGC | 378 |
| IL-17 |
TCTCCAAAGGAAGCCTGA
CAAGACTGAACACCGACT | 231 |
| β-actin |
CACGAAACTACCTTCAACT
CATACTCCTGCTTGCTGA | 265 |
Results
Characteristics of CII isolated and
purified from chicken sternal cartilage
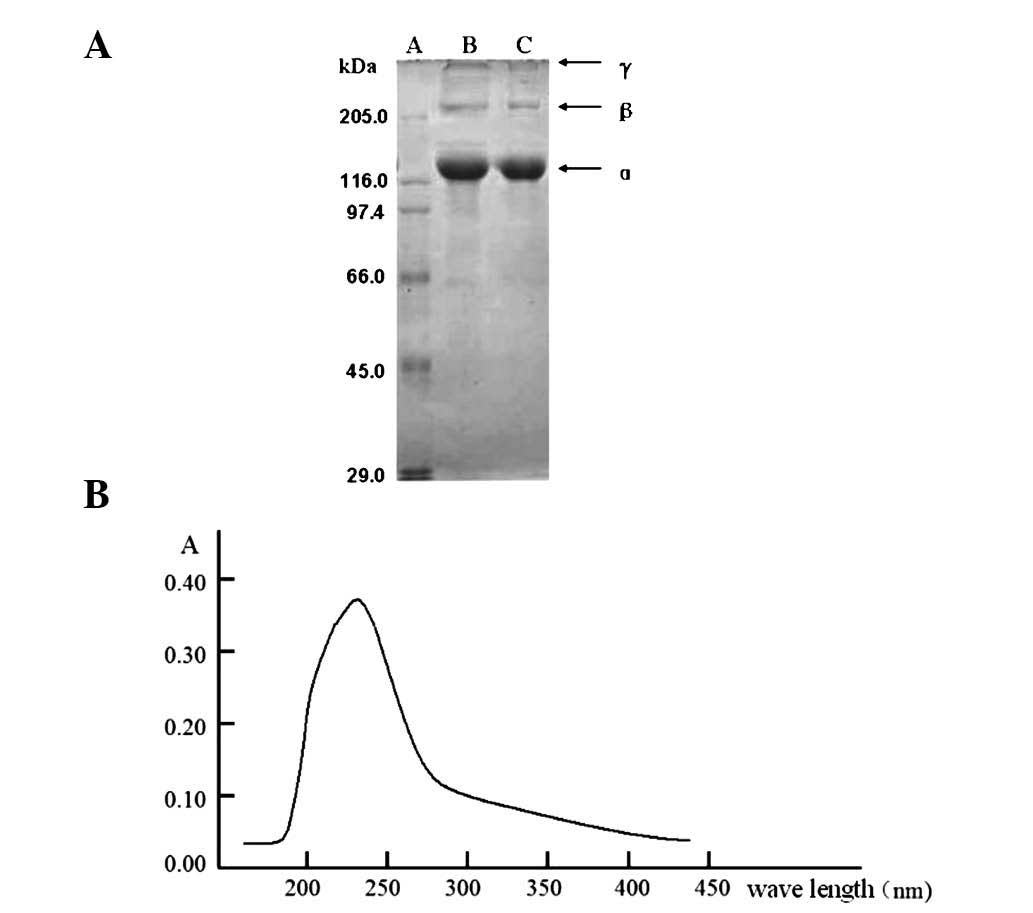
CII was successfully isolated and purified. SDS-PAGE
analysis demonstrated that CII was constituted of α, β and γ
isoforms. β forms a dimer with α and γ forms a trimer with α. The
relative molecular weight of the α monomer was 12 kDa (Fig. 1A). High performance liquid
chromatography (HPLC) was performed to analyze the amino acid
constitution of the α monomer and identified that the α monomer is
rich in glycine, proline and alanine, particularly glycine (1/3 of
the structure). Aromatic amino acid content was low, phenylalanine
was 1.53% and tyrosine was not detected. Analysis of absorption
spectrometry was in accordance with HPLC results (Fig. 1B).
Isolated CII induced CIA, a Th17
cell-related disease
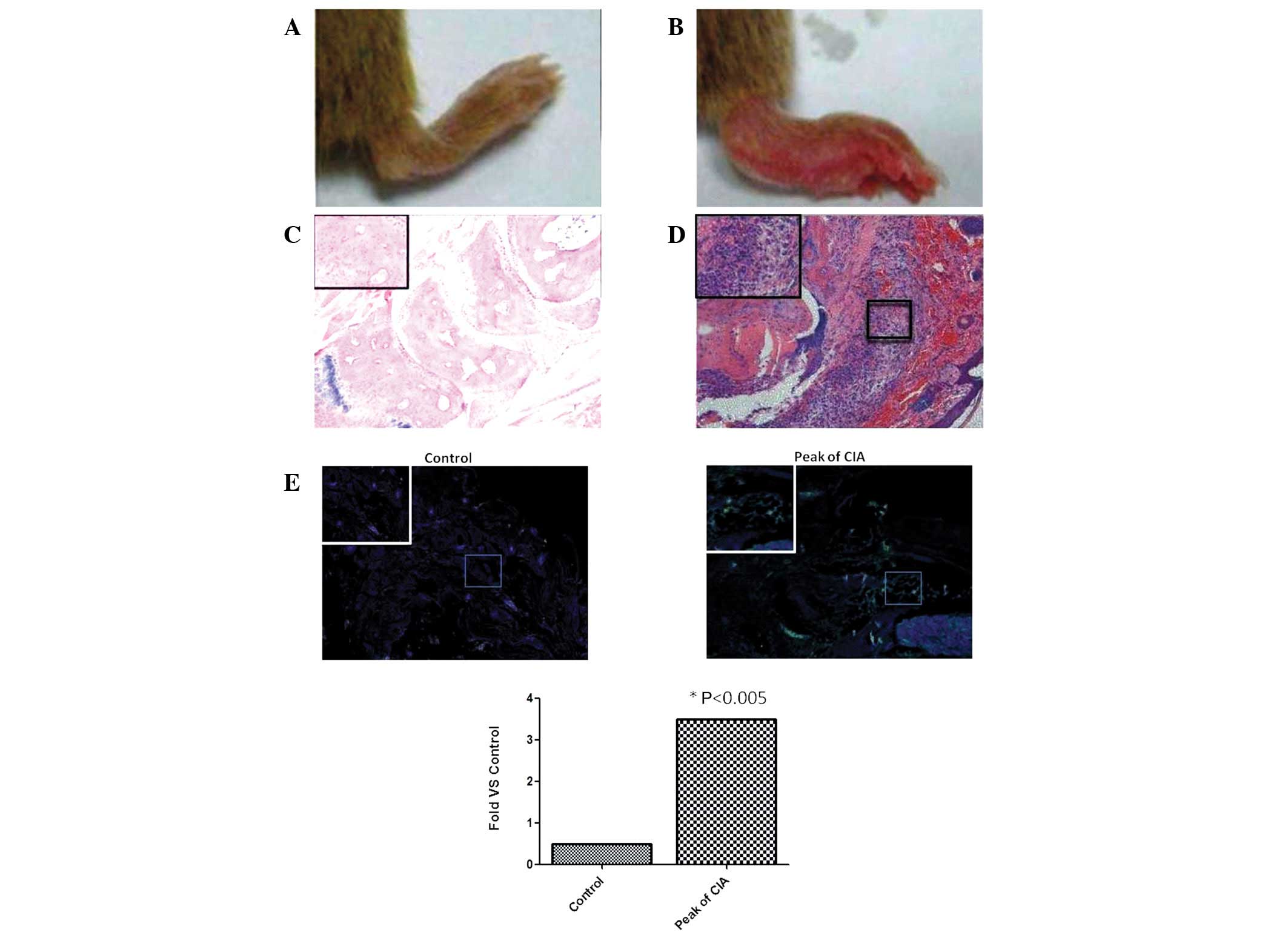
To determine whether isolated CII induced CIA in
mice, models were induced as described previously (15). On day 34, the joints of
experimental mice were swollen. Control mouse joints were unchanged
(Fig. 2A and B). On day 45,
histological examination revealed marked infiltration of
inflammatory cells in the experimental group and characteristics
typical of RA. All model mice severity scores were >1 (Fig. 2C and D).
Immunofluorescence analysis demonstrated that Th17
cell infiltration in the CIA group was significantly increased
compared with the control (P<0.05; Fig. 2E). Serum IL-17 levels were
321.43±19.32 and 34.76±10.23 pg/ml in the experimental and control
groups, respectively (P<0.05).
Th17 cell induction by a TLR2-dependent
pathway
Next, the mechanism by which CII induces Th17 cell
infiltration was determined. Peritoneal macrophages were isolated
and treated with 0.5 μg/ml CII in vitro. The pattern recognization
receptors, TLR2, 4, 5, 7, 8 and 9 and Dectin 1 and 2 were detected.
Results revealed that TLR2 significantly increased at 90 min and
peaked at 120 min (P<0.05), indicating that CII may be
recognized by TLR2 expressed on macrophages and associated with
induction of the Th17 cell response.
Following this, an anti-TLR2 antibody was utilized
to block TLR2 expressed on the peritoneal macrophages and the cells
were stimulated using CII. After 3 days, TGF-β and IL-6 levels were
determined in the cell culture supernatant. The results were as
follows: TGF-β, 258.12±32.13 pg/ml (stimulated group) vs.
38.32±17.34 pg/ml (stimulated + blockade group); IL-6, 435.79±13.34
pg/ml (stimulated group) vs. 123.56±21.13 pg/ml (stimulated +
blockade group), respectively. Following TLR2 blockade, TGF-β and
IL-6 levels were significantly decreased (P<0.05; Table II).
 | Table IIConcentration of IL-6 and TGF-β in
cell culture supernatant. |
Table II
Concentration of IL-6 and TGF-β in
cell culture supernatant.
| Protein (pg/ml) | Collagen II | Collagen II +
blockade TLR2 | P-value |
|---|
| TGF-β | 258.12±32.13 | 38.32±17.34 | <0.005 |
| IL-6 | 435.79±13.34 | 123.56±21.13 | <0.005 |
Discussion
Animal models of autoimmune arthritis have proven to
be valuable research tools for the analysis of the pathogenesis of
diseases as well as exploring new therapeutic targets. More
recently, transgenic and knockout mice have also enabled important
advances and led to a greater understanding of the role of a number
of cytokines, particularly IFN-γ, in disease pathogenesis. In
animal models, one mechanism by which IFN-γ functions is by
suppressing IL-17 production, which is emerging as a key pathogenic
cytokine of lymphoid tissue inducer-like Th17 cells (16–19).
Animal models have also proved useful for
understanding the complexity of the immune system, particularly
various subgroups of CD4+ T cells in disease
pathogenesis. It is well known that specific CD4+ T
cells and their associated cytokines are able to promote or negate
the development of RA in a number of models (20). For example, IFN-γ and IL-4 are able
to inhibit Th17 infiltration in the adjuvant-induced arthritis,
SKG, CIA and proteoglycan-induced arthritis models (21–23).
In addition, human and mouse CD4+ T cells are known to
possess a certain degree of plasticity (24–26),
for example Th17 cells are able to convert into Th1 cells.
Therefore, crosstalk between CD4+ T cells may be
necessary to fine-tune T cell lineage commitment to control
diseases.
In mice with CIA, CD4+ T cells are
important in disease induction and Th1 cells are considered as the
major mediator. However, the hypothesis that CIA is a Th1-mediated
disorder has been challenged (27–30).
In the present study, Th17 cells were demonstrated to represent the
dominant effector T helper cell subset in the CIA model. Results
are consistent with previous studies in IL-17-deficient mice or
mice treated with anti-IL-17 antibody demonstrating the importance
of IL-17 in the pathology of CIA (31,32).
Following this, the mechanism by which Th17 cells mediate induction
of the immune response was investigated as well as the hypothesis
that macrophages induce Th17 cells.
Macrophages are important antigen presenting cells
as well as an important link between the innate and adaptive immune
response. Stimulation of macrophages by antigen presentation
induces secretion of proinflammatory molecules which induce
CD4+ T cell differentiation and determine the type of
immune response. In addition, macrophages are also responsible for
numerous immunological and inflammatory processes (33). A number of previous studies have
demonstrated that macrophages perform important roles in RA
development (34–36). Therefore, in the present study,
peritoneal macrophages were isolated and treated with CII. After 2
h, TLR2 mRNA levels were identified to be significantly increased,
indicating that TLR2 is involved in recognition of CII. In mice, it
is well known that Th17 differentiation from naïve T-cells requires
TGF-β and IL-6, molecules which are critical in CIA (16,24).
To confirm the observation that CII binds TLR2 to activate
macrophages and induce Th17 cells, anti-TLR2 was used to block TLR2
on macrophages. Following blockage, levels of TGF-β and IL-6 were
identified to be significantly decreased, consistent with the
hypothesis.
In the current study, CII was isolated from chicken
sternal cartilage and successfully induced RA via a TLR2-dependent
pathway. In addition, the RA model was found to represent a Th17
cell-related disease.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81001319 and
81101677), the Post-Doctoral Foundation of China (no. 2012M511705),
the Post-Doctoral Foundation of Jiangsu Province (no. 1102129C),
Natural Science Foundation of Colleges and Universities in Jiangsu
Province (Grant NO. 10KJB310003) and High-Tech Professional of
Jiangsu University (no. 11JDG128).
References
|
1
|
Chabaud M, Durand JM, Buchs N, et al:
Human interleukin-17: A T cell-derived proinflammatory cytokine
produced by the rheumatoid synovium. Arthritis Rheum. 42:963–970.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miossec P: Interleukin-17 in fashion, at
last: ten years after its description, its cellular source has been
identified. Arthritis Rheum. 56:2111–2115. 2007.PubMed/NCBI
|
|
3
|
Van Boxel JA and Paget SA: Predominantly
T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med.
293:517–520. 1975.PubMed/NCBI
|
|
4
|
Hovdenes J, Gaudernack G, Kvien TK, et al:
A functional study of purified CD4+ and CD8+
cells isolated from synovial fluid of patients with rheumatoid
arthritis and other arthritides. Scand J Immunol. 29:641–649.
1989.PubMed/NCBI
|
|
5
|
Imboden JB: The immunopathogenesis of
rheumatoid arthritis. Annu Rev Pathol. 4:417–434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Courtenay JS, Dallman MJ, Dayan AD, et al:
Immunisation against heterologous type II collagen induces
arthritis in mice. Nature. 283:666–668. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holmdahl R, Rubin K, Klareskog L, et al:
Characterization of the antibody response in mice with type II
collagen-induced arthritis, using monoclonal anti-type II collagen
antibodies. Arthritis Rheum. 29:400–410. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keystone EC, Schorlemmer HU, Pope C and
Allison AC: Zymosan-induced arthritis: a model of chronic
proliferative arthritis following activation of the alternative
pathway of complement. Arthritis Rheum. 20:1396–1401.
1977.PubMed/NCBI
|
|
9
|
Sakaguchi N, Takahashi T, Hata H, et al:
Altered thymic T-cell selection due to a mutation of the ZAP-70
gene causes autoimmune arthritis in mice. Nature. 426:454–460.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andersson M, Cremer MA, Terato K, et al:
Analysis of type II collagen reactive T cells in the mouse. II
Different localization of immunodominant T cell epitopes on
heterologous and autologous type II collagen. Scand J Immunol.
33:505–510. 1991.PubMed/NCBI
|
|
11
|
Andersson M and Holmdahl R: Analysis of
type II collagen-reactive T cells in the mouse. I Different
regulation of autoreactive vs non-autoreactive anti-type II
collagen T cells in the DBA/1 mouse. Euro J Immunol. 20:1061–1066.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iwai H, Kozono Y, Hirose S, et al:
Amelioration of collagen-induced arthritis by blockade of inducible
costimulator-B7 homologous protein costimulation. J Immunol.
169:4332–4339. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng GM, Zheng L, Chan FK and Lenardo M:
Amelioration of inflammatory arthritis by targeting the pre-ligand
assembly domain of tumor necrosis factor receptors. Nat Med.
11:1066–1072. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su Z, Sun C, Zhou C, Liu Y, et al: HMGB1
blockade attenuates experimental autoimmune myocarditis and
suppresses Th17-cell expansion. Eur J Immunol. 41:3586–3595. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brand DD, Latham KA and Rosloniec EF:
Collagen-induced arthritis. Nat Protoc. 2:1269–1275. 2007.
View Article : Google Scholar
|
|
16
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 cells. Annu Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lane P, Kim MY, Withers D, et al: Lymphoid
tissue inducer cells in adaptive CD4 T cell dependent responses.
Semin Immunol. 20:159–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lane PJ, McConnell FM, Withers D, et al:
Lymphoid tissue inducer cells: bridges between the ancient innate
and the modern adaptive immune systems. Mucosal Immunol. 2:472–477.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takatori H, Kanno Y, Watford WT, et al:
Lymphoid tissue inducer-like cells are an innate source of IL-17
and IL-22. J Exp Med. 206:35–41. 2009.PubMed/NCBI
|
|
20
|
Alzabin S and Williams RO: Effector T
cells in rheumatoid arthritis: lessons from animal models. FEBS
Lett. 585:3649–3659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kageyama Y, Ichikawa T, Nagafusa T, et al:
Etanercept reduces the serum levels of interleukin-23 and
macrophage inflammatory protein-3 alpha in patients with rheumatoid
arthritis. Rheumatol Int. 28:137–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sarkar S, Cooney LA, White P, et al:
Regulation of pathogenic IL-17 responses in collagen-induced
arthritis: roles of endogenous interferon-gamma and IL-4. Arthritis
Res Ther. 11:R1582009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Doodes PD, Cao Y, Hamel KM, et al:
IFN-gamma regulates the requirement for IL-17 in
proteoglycan-induced arthritis. J Immunol. 184:1552–1559. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu J and Paul WE: CD4 T cells: fates,
functions and faults. Blood. 112:1557–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Locksley RM: Nine lives: plasticity among
T helper cell subsets. J Exp Med. 206:1643–1646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peck A and Mellins ED: Plasticity of
T-cell phenotype and function: the T helper type 17 example.
Immunology. 129:147–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamada H, Nakashima Y, Okazaki K, et al:
Th1 but not Th17 cells predominate in the joints of patients with
rheumatoid arthritis. Ann Rheum Dis. 67:1299–1304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kato H and Fox DA: Are Th17 cells an
appropriate new target in the treatment of rheumatoid arthritis?
Clin Transl Sci. 3:319–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Selmi C: Autoimmunity in 2009. Autoimmun
Rev. 9:795–800. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scheinecker C and Smolen JS: Rheumatoid
arthritis in 2010: from the gut to the joint. Nat Rev Rheumatol.
7:73–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lubberts E, Koenders MI, Oppers-Walgreen
B, et al: Treatment with a neutralizing anti-murine interleukin-17
antibody after the onset of collagen-induced arthritis reduces
joint inflammation, cartilage destruction and bone erosion.
Arthritis Rheum. 50:650–659. 2004. View Article : Google Scholar
|
|
32
|
Nakae S, Nambu A, Sudo K and Iwakura Y:
Suppression of immune induction of collagen-induced arthritis in
IL-17-deficient mice. J Immunol. 171:6173–6177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arnett FC, Edworthy SM, Bloch DA, et al:
The American Rheumatism Association 1987 revised criteria for the
classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Corr M and Firestein GS: Innate immunity
as a hired gun: but is it rheumatoid arthritis? J Exp Med.
195:F33–F35. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Falgarone G, Jaen O and Boissier MC: Role
for innate immunity in rheumatoid arthritis. Joint Bone Spine.
72:17–25. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maciejewska Rodrigues H, Jungel A, Gay RE
and Gay S: Innate immunity, epigenetics and autoimmunity in
rheumatoid arthritis. Mol Immunol. 47:12–18. 2009.PubMed/NCBI
|