Introduction
Endothelial progenitor cells (EPCs), the precursor
cells of vascular endothelial cells, migrate to the peripheral
circulation and differentiate into mature endothelial cells. These
cells are important for repair of damaged vascular endothelium,
participating in neoangiogenesis and maintaining the integrity of
the vascular endothelium (1).
Certain previous studies showed that estrogen use may reduce the
risk of heart failure in female cardiovascular disease patients,
suggesting a protective role of estrogen in the cardiovascular
system. The mechanism may involve EPCs. 17β-estradiol is an
estrogen, which promotes the homing and proliferation of vascular
endothelial cells (2).
17β-estradiol and EPCs have been found to participate in the repair
of damaged vessels and angiogenesis (3). The long-term use of high-dose
estrogen may lead to breast cancer, blood clot diseases as well as
other adverse results. Yet the effects of low-dose estrogen therapy
on circulating EPCs are unknown. The present study investigates the
effect of 17β-estradiol at various concentrations on the biological
characteristics of rat bone marrow-derived EPCs, to provide an
experimental basis and clinical reference for the application of
17β-estradiol and EPCs in vascular tissue and bone tissue
engineering.
Materials and methods
Experimental animals, reagents and
instruments
Twenty Wistar rats (4 weeks old) were provided by
the Laboratory Animal Center of China Medical University
(Shengyang, Liaoning, China). All experimental procedures were
performed in accordance with the Guidelines for the Care and Use of
Laboratory Animals, formulated by the Ministry of Science and
Technology of the People’s Republic of China. The study was
approved by the ethics committee of The First Affiliated Hospital
of China Medical University, Shenyang, China. M199 medium and fetal
bovine serum were obtained from Gibco-BRL (Carlsbad, CA, USA).
Lymphocyte separation medium was purchased from Tianjin Haoyang
Biologicals Technology Co., Ltd (Tianjin, China), 17β-estradiol was
obtained from Sigma-Aldrich (St. Louis, MO, USA), CD133, CD34 and
CD31-labeled antibodies from Wuhan Boster Biological Technology
Co., Ltd (Wuhan, Hubei, China) and Transwell chambers from Corning,
Inc. (NY, USA).
Isolation, culture and identification of
cells
The tibia and femur were separated under sterile
conditions and the marrow cavity was flushed with M199 medium. The
irrigation fluid was collected and mixed fully prior to
centrifugation with lymphocyte separation medium (density gradient,
1.077 g/cm3; volume ratio, irrigation liquid to
separated liquid was 2:1). Ficoll-Histopaque gradient
centrifugation was conducted at 20°C at 384 × g for 20 min. The
mononuclear cell layer in the middle was removed and washed in PBS
prior to inoculation into a 100 ml culture flask at a density of
1×106 cells/ml. Cells were cultured in M199 medium
containing 20% fetal bovine serum in a 5% CO2 saturated
humidity incubator at 37°C for 48 h prior to re-vaccination of
non-adherent cells. Half of the medium was changed on the third day
with whole replacement on the fifth day. Cells were digested with
0.25% trypsin (containing 1% EDTA) when cell fusion reached
>80%. On the seventh day, CD133 and CD34 phenotypes were
identified with a flow cytometer.
Proliferation assay
Adherent cells were collected and counted. EPC
suspension (200 μl) was inoculated in a 96-well plate for 48 h with
various concentrations of 17β-estradiol (0, 10, 100 nmol/l)
following 24 h culture in M199 culture medium without fetal bovine
serum. Each concentration was repeated in 5 wells. After 24 h, 20
μl MTT (5 g/l) was added and incubated for 4 h, followed by
replacement with DMSO (150 μl/well) and agitation for 10 min. The
plate was read at 560 nm on the microplate reader. The average
optical density (OD) values of five wells were used to obtain the
optimal concentration for cells. Incubation time was used as the
abscissa and the OD value as the ordinate for cell growth
curve.
Migration assay
In vitro migration assay was performed in a
24-well Transwell chamber (pore size, 8 μm). EPC suspension (200
μl; 1×104 cells/ml) was added into the upper chamber
with M199 medium containing various concentrations of 17β-estradiol
(0, 10 and 100 nmol/l) in the lower chamber. Cells were cultured
for 24 h and cells attached to the upper chamber were removed with
a wet cotton swab. The cells were fixed and stained using the
Giemsa method. The experiment was repeated three times.
In vitro angiogenesis assay
Fibrin gel was used for in vitro angiogenesis
experiments. Artificial fibrinogen (30 μl) and 20 μl thrombin were
added in sequence to 96-well plates and agitated prior to
incubation at 37°C for gel formation. Cells (5×103) were
added and cultured overnight. Next, the culture medium was removed
and 30 μl artificial fibrinogen and 20 μl thrombin was added in
sequence. Culture medium containing various concentrations of
17β-estradiol (0, 10 and 100 nmol/l) was added and incubated for an
additional 24 h. Angiogenesis was observed and three visual fields
(magnification, ×200) were selected for blood vessel counting.
Effect of 17β-estradiol on EPC-induced
differentiation
Differentiation was assessed on day 4 of primary
cell culture. The culture medium was changed to M199 medium
containing various concentrations of 17β-estradiol (10 and 100
nmol/l) for 24 h and flow cytometry was performed. Adherent cells
were digested with trypsin for single cell suspension solution and
centrifuged at 384 × g for 5 min. Cells were resuspended in 50 μl
PBS, a CD31-labeling antibody was added and incubated at 4°C for 60
min. Flow cytometry was performed in the dark immediately following
PBS washing and re-suspension. PBS was used as the isotype control.
WinMDI2.9 software was used to calculate the percentage of
CD31+ cells.
Statistical analysis
Data are presented as the mean ± SD and SPSS 10.0
software was used (SPSS, Inc., Chicago, IL, USA) for statistical
analysis. Univariate, multifactorial analysis of variance was
performed and t-tests were performed to compare between groups. The
LSD method was used for the multiple group comparison. P<0.05
was considered to indicate a statistically significant
difference.
Results
Identification of cell morphology and
phenotype
Freshly isolated mononuclear cells were small, round
and evenly distributed in the suspension. Cells adhered to the
wells 24 h post-inoculation and gradually grow into a slender
spindle or irregular polygon morphology. One week following the
removal of unattached cells from the suspension, a small colony was
observed and at two weeks, a large colony had formed. At three
weeks, colonies had connected and the shape of adherent cells had
changed from slender to short, representative of a typical paving
stone-like appearance. Vacuoles were observed in the cytoplasm,
indicative of aging.
Cells that adhered to the wells after being replated
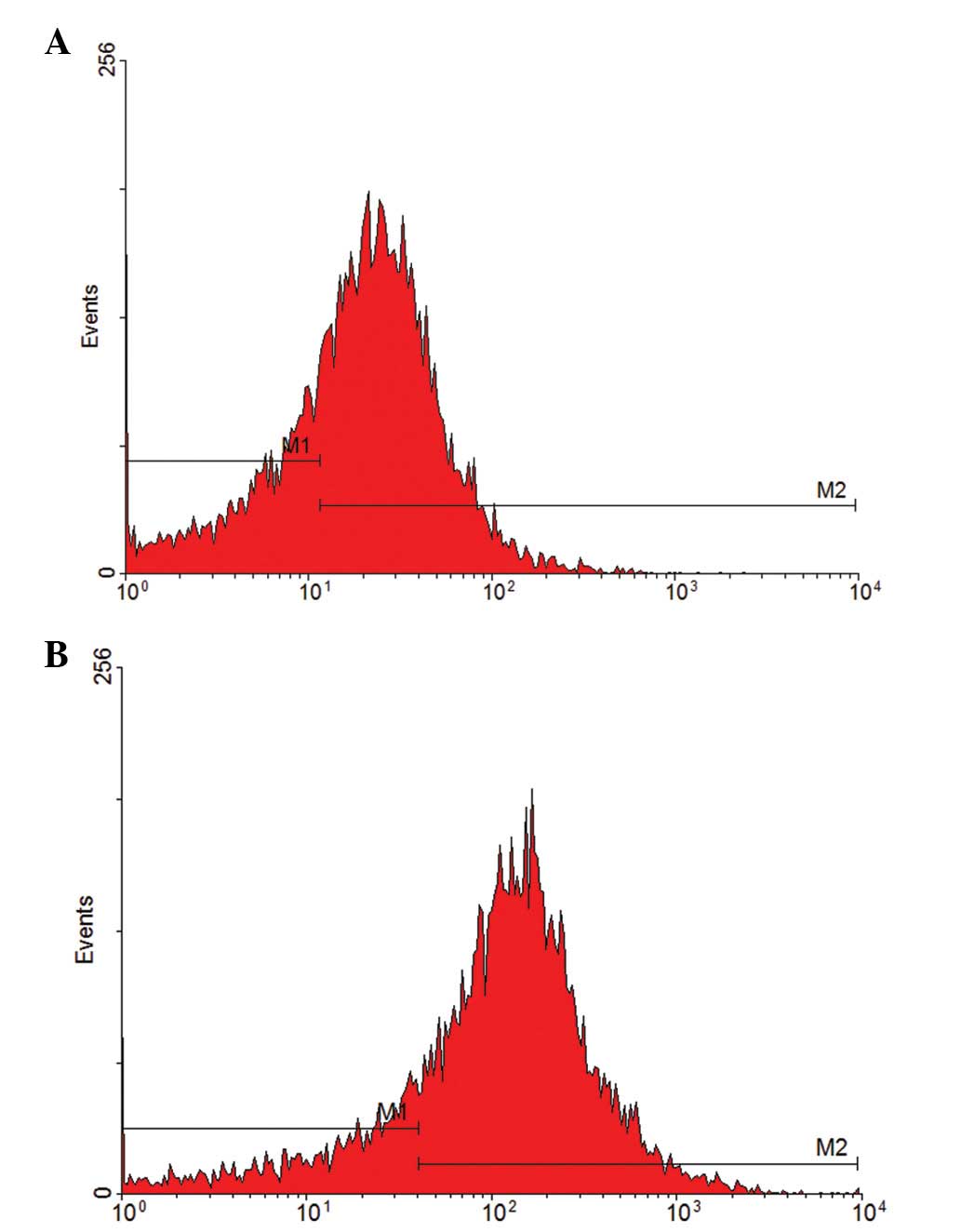
were purified on day 7 of culture. The CD133-positive rate of
primary cells was 69.44% and the CD34-positive rate was 81.05%
(Fig. 1). These results indicate
that the extracted, separated and purified cells are endothelial
progenitor cells.
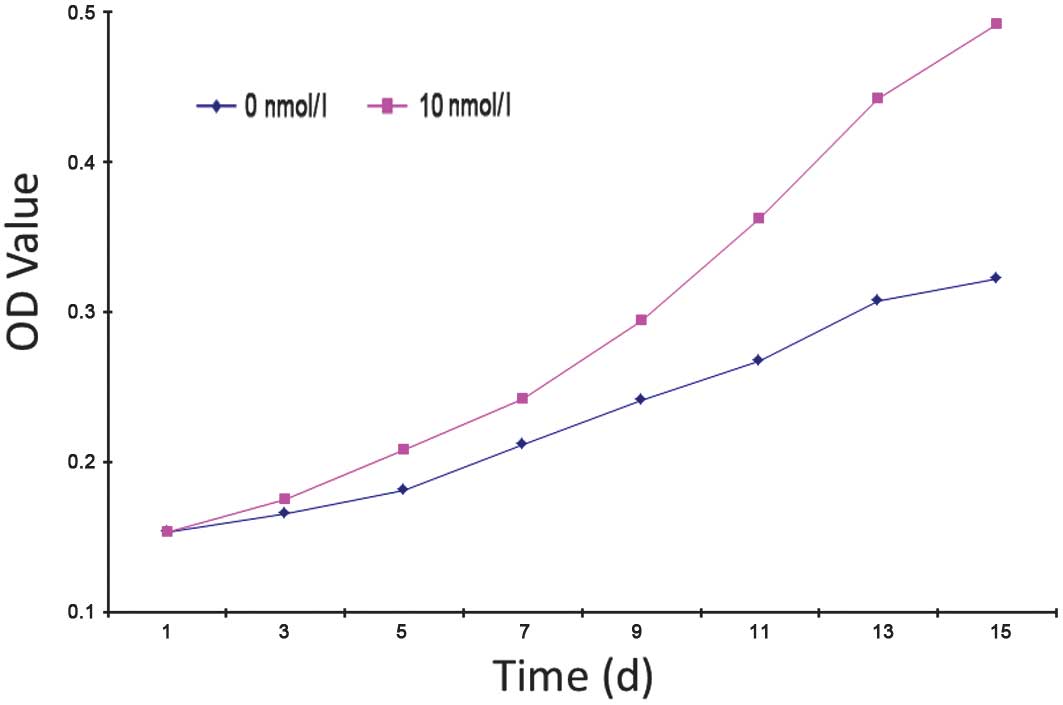
Effect of VEGF on EPC proliferation
Average OD values of the 0, 10 and 100 nmol/l groups
were 0.3490±0.0332, 0.6278±0.0796 and 0.2758±0.0125, respectively
and OD was found to be significantly different in the 10 and 100
nmol/l groups compared with the 0 nmol/l group (P<0.05).
17β-estradiol was found to have a significant effect on EPC
proliferation, improving the proliferation of EPCs; however, as the
concentration of 17β-estradiol increased, EPC proliferation
weakened. The 0 and 10 nmol/l groups were selected to generate a
cell growth curve (Fig. 2)
revealing the following observations: the first three days
post-inoculation is the incubation period, in which cells begin to
grow adherent to the well with low levels of cell proliferation;
following three days, cell proliferation accelerates and
proliferation in the 10 nmol/l group was markedly higher than that
of 0 nmol/l. This trend continued to the end of primary culture.
The rate of proliferation in the 0 nmol/l group began to reduce at
day 13 where it entered the platform stage; however, marked levels
of proliferation were maintained in the 10 nmol/l group.
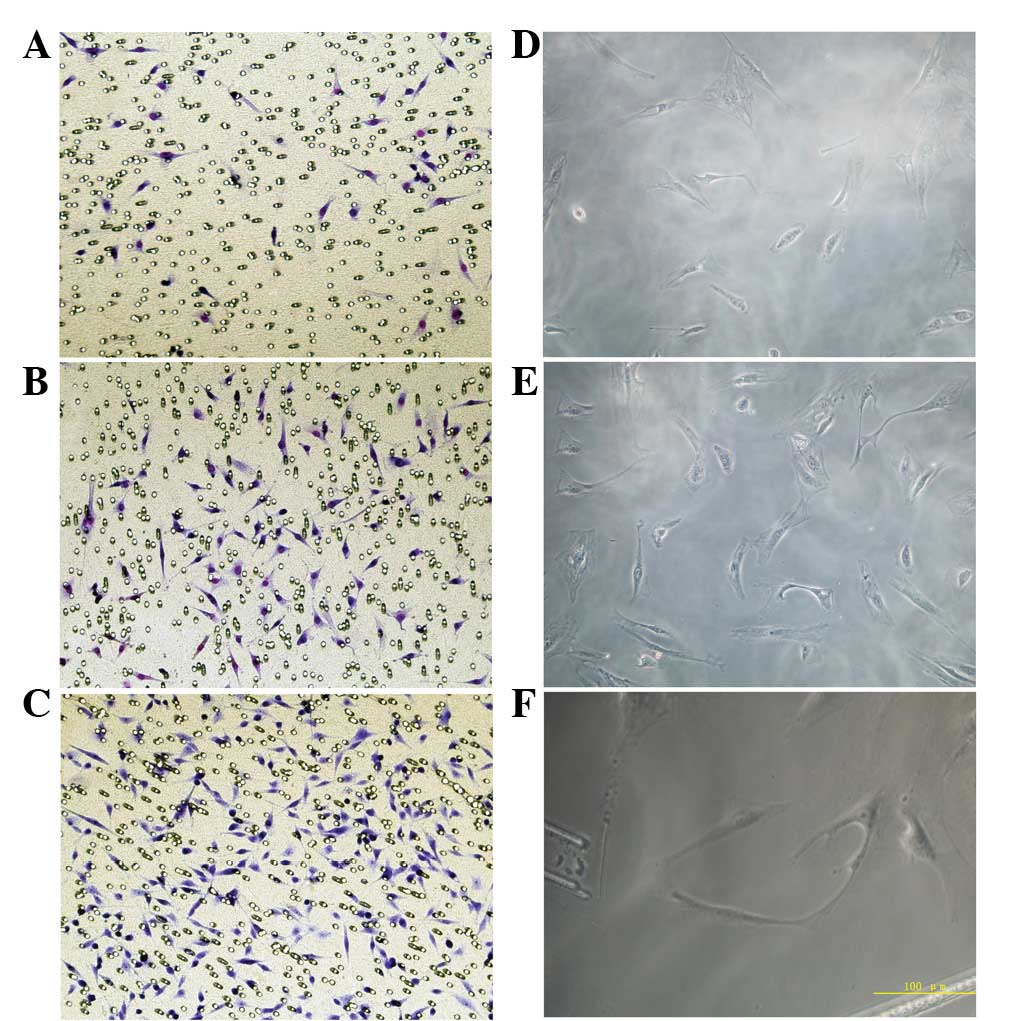
Effect of VEGF on EPC migration
Migration of EPCs was analyzed under various
concentrations of 17β-estradiol. Migration was enhanced with
increases in 17β-estradiol concentration. The number of cells
migrating through the pores in the 10 (18.7±4.5) and 100 nmol/l
(37.4±9.4) groups was significantly higher than that of the 0
nmol/l group (5.8±1.2) and migration in the 100 nmol/l group was
found to be significantly higher than that of the 10 nmol/l group
(Fig. 3; P<0.05).
Effect of VEGF on in vitro EPC
angiogenesis
EPCs revealed various angiogenic abilities in the
fibrin gel following treatment with different concentrations of
17β-estradiol. In the 0 nmol/l group, a large number of isolated
cells were observed in a scattered distribution and few connections
between cells were noted. In the 10 nmol/l group, vascular lumen
formation was not found; however, the number of cells was higher
than that in the 0 nmol/l group and connections between cells were
observed. In the 100 nmol/l group, several cells are arranged in a
circle and cells exhibited ring connections, constituting a blood
vessel lumen. In addition, the formed lumen was rounder than that
of the 10 nmol/l group (Fig.
3D–F). The total number of blood vessels in three randomly
selected fields was 4.7±1.1, 16.6±2.3 and 27.8±4.2 in the 0, 10 and
100 nmol/l groups, respectively. The number of blood vessels in the
100 nmol/l group was significantly higher than that of the other
groups (P<0.05), indicating that the 100 nmol/l group markedly
promoted EPC vessel formation.
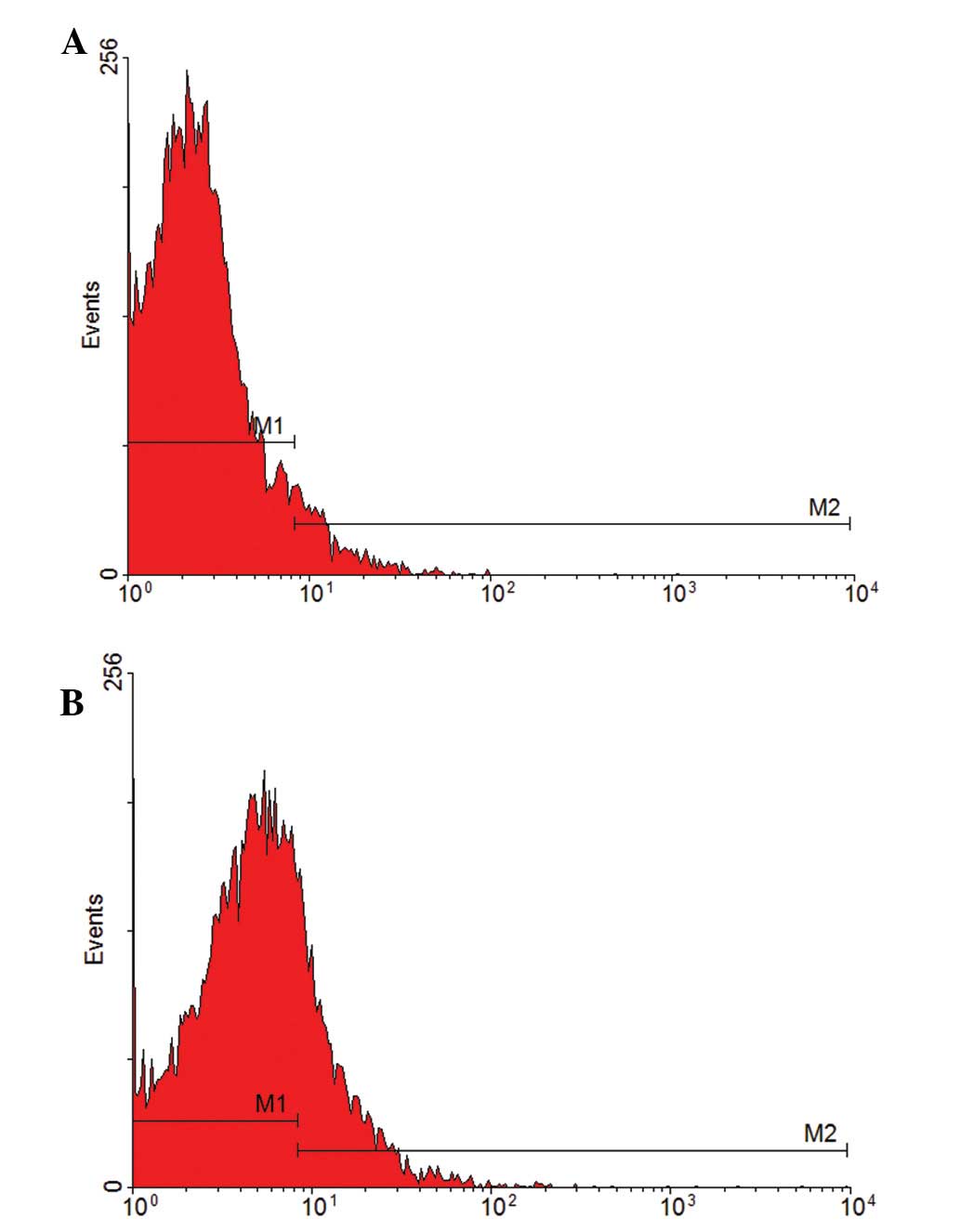
Effect of VEGF on EPC-induced
differentiation
The specific surface marker of mature vascular
endothelial cells, CD31, was analyzed to determine EPC
differentiation. EPCs were cultured with various concentrations of
17β-estradiol (10 and 100 nmol/l) and 0 nmol/l was used as a blank
control to test the CD31-positive rate by flow cytometry. The
CD31-positive rate was 6.65 and 23.30% in the 10 and 100 nmol/l
groups, respectively (Fig. 4),
indicating that 17β-estradiol induces EPC differentiation into
mature endothelial cells. Compared with the 10 nmol/l group, the
CD31-positive rate in 100 nmol/l was higher, indicating that EPC
differentiation is accelerated by increasing 17β-estradiol
concentrations.
Discussion
EPCs are the precursor cells of vascular endothelial
cells and originate from the mesoderm, differentiating and
developing from the angioblast. EPCs migrate to the peripheral
circulation and differentiate into endothelial cells, repair
damaged vascular endothelial cells and are important for
maintaining the integrity of vascular endothelial cells (4). At present, there is no standard
method to identify EPCs. One method involves the analysis of cell
markers, including CD133, CD34 and other cell surface antigens.
CD133 is expressed in the bone marrow, peripheral blood stem cells
and early EPCs, but not in mature endothelial cells (5). Following differentiation of EPCs into
mature endothelial cells, expression levels of CD133 decline. CD34
is a well-known marker of hematopoietic stem cells and is expressed
in endothelial cells (6,7). In the present study, CD133 and CD34
were used as specific markers for the identification of EPCs.
Expression of the stem cell marker was found to gradually reduce as
EPC culture progressed. However, the expression of CD31, a specific
marker of endothelial cells was observed to increase (8), indicating that the cells had
undergone differentiation into mature endothelial cells. As a
result, CD31 was selected as a specific marker to determine the
differentiation of EPCs towards a mature endothelial cell
lineage.
Studies on the mechanisms by which estrogens
mobilize endothelial cells have largely focused on the estrogen
receptor (ER). However, to date, the number of ERs on endothelial
progenitor cells has not been determined. As estrogen levels in
normal tissues and vascular endothelia are low, the binding rate of
estrogen and ERs is extremely low (9).
In the current study, EPCs were cultured in various
concentrations of 17β-estradiol (0, 10 and 100 nmol/l) and
17β-estradiol was found to enhance EPC migration, improve in
vitro angiogenesis and promote EPC differentiation towards
mature vascular endothelial cells. With increases in the
concentration of 17β-estradiol, this effect increased, revealing a
dose-dependent correlation. When the concentration of 17β-estradiol
was low (10 nmol/l), the number of EPCs increased and proliferation
was enhanced. However, increased 17β-estradiol (100 nmol/l)
concentration did not result in further increases in EPC
proliferation. By contrast, proliferation was highest in the low
concentration group and high concentrations of 17β-estradiol were
revealed to have an inhibitory effect on EPC proliferation. It is
possible that other signaling pathways are recruited at high
estrogen concentrations (10).
In addition to regulating gene expression at the
genomic level via nuclear receptors, α and β, estrogens also
activate extracellular signals via non-genomic effects. Previous
studies have reported that the association of estrogens with ERβ
leads to the activation of the extracellular signal-regulated
kinase/mitogen activated protein kinase (ERK/MAPK) (11). As a key signaling axis in signal
transduction pathways involved in the growth, differentiation and
apoptosis of cells, the MAPK family is activated by a number of
mechanisms. Activated ERK improves the activity of the cytosolic
target protein phosphorus to acidize or regulate other protein
kinases, including activating the phospholipase A2 and regulating
kinase translation, for example (12). In addition, activated ERK enters
the cell nucleus to promote the phosphorylation of various
transcription factors. For example, ERK promotes phosphorylation of
serum response factor (SRF), enabling it to bind serum response
elements in target gene promoters, enhancing transcriptional
activity as well as accelerating cell proliferation. SRF not only
regulates the transcription of a number of cell proliferation
factors, but also exclusively controls the transcription of actin
(13). Previous studies have found
that RhoA is important for the reconstruction of actin in
fibroblasts (14). Rho GTPases are
involved in the dynamic regulation of the cytoskeleton in smooth
muscle cells by activation of SRF (15), regulating the migration and
angiogenesis of endothelial progenitor cells.
Estrogen promotes the proliferation, migration,
angiogenesis and differentiation of EPCs, and complications,
including thromboembolism, sodium and water retention and cancer,
which may appear following the systemic application of estrogen. In
the present study, in vitro administration of an appropriate
concentration of 17β-estradiol to EPCs was found to maximize
biological activity. The development of cell therapy combined with
genetic tools has made it possible to use EPCs as the carriers for
17β-estradiol based gene therapy. This will allow for improved
treatments to vascular system diseases, ischemia and would
healing.
References
|
1
|
Callaghan MJ, Ceradini DJ and Gurtner GC:
Hyperglycemia- induced reactive oxygen species and impaired
endothelial progenitor cell function. Antioxid Redox Signal.
7:1476–1482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li HQ, Zhao Q and Sun XN: 17β-Estradiol
enhances migratory capacity of bone marrow-derived endothelial
progenitor cells by up-regulating CXCR4 expression via estrogen
receptors pathway. Zhong Hua Shi Yan Wai Ke Za Zhi She.
26:1407–1409. 2009.
|
|
3
|
Iwakura A, Shastry S, Luedemann C, et al:
Estrogen enhances recovery after myocardial infarction by
augmenting incorporation of bone marrow-derived endothelial
progenitor cells into sites of ischemic-inducible
neovascularization via endothelial nitric oxide synthase-mediated
activation of matrix metalloproteinase-9. Circulation.
113:1605–1614. 2006.
|
|
4
|
Krenning G, van Luyn MJ and Harmsen MC:
Endothelial progenitor cell-based neovascularization: implications
for therapy. Trends Mol Med. 15:180–189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friedrich EB, Walenta K, Scharlau J,
Nickenig G and Werner N:
CD34−/CD133+/VEGFR-2+ endothelial
progenitor cell subpopulation with potent vasoregenerative
capacities. Circ Res. 98:e20–e25. 2006.PubMed/NCBI
|
|
6
|
Kwon SM, Eguchi M, Wada M, et al: Specific
Jagged-1 signal from bone marrow microenvironment is required for
endothelial progenitor cell development for neovascularization.
Circulation. 118:157–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nevskaya T, Bykovskaia S, Lyssuk E, et al:
Circulating endothelial progenitor cells in systemic sclerosis:
relation to impaired angiogenesis and cardiovascular
manifestations. Clin Exp Rheumatol. 26:421–429. 2008.
|
|
8
|
Whittaker A, Moore JS, Vasa-Nicotera M,
Stevens S and Samani NJ: Evidence for genetic regulation of
endothelial progenitor cells and their role as biological markers
of atherosclerotic susceptibility. Eur Heart J. 29:332–338. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li HQ, Zhao Q, Sun X-N, et al: Effect of
physiological estrogen on the migratory capacity of bone
marrow-derived endothelial progenitor cells. Mol Cardiol Chin.
45:79–83. 2009.
|
|
10
|
Sims NA, Dupont S, Krust A, et al:
Deletion of estrogen receptors reveals a regulatory role for
estrogen receptors-beta in bone remodeling in females but not in
males. Bone. 30:18–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schwartz B, Smirnoff P, Shany S, et al:
Estrogen controls expression and bioresponse of
1,25-dihydroxyvitamin D receptors in the rat colon. J Mol Cell
Biochem. 203:87–93. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walter DH, Haendeler J, Reinhold J, et al:
Impaired CXCR4 signaling contributes to the reduced
neovascularization capacity of endothelial progenitor cells from
patients with coronary artery disease. Cirs Res. 97:1142–1151.
2005. View Article : Google Scholar
|
|
13
|
Posern G, Sotiropoulos A and Treisman R:
Mutant actins demonstrate a role for unpolymerized actin in control
of transcription by serum response factor. Mol Biol Cell.
13:4167–4178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amano M, Chihara K, Kimura K, et al:
Formation of actin stress fibers and focal adhesions enhanced by
Rho-kinase. Science. 275:1308–1311. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu HW, Halayko AJ, Fernandes DJ, et al:
The RhoA/Rho kinase pathway regulates nuclear localization of serum
response factor. Am J Respir Cell Mol Biol. 29:39–47. 2003.
View Article : Google Scholar : PubMed/NCBI
|