Introduction
Tuberculosis (TB) is a chronic infectious disease
caused by the bacterium Mycobacterium tuberculosis, and it
remains a significant public health risk worldwide. The pestilence
of TB in the Cosmopolitan population has been eased with the
introduction of anti-TB and -HIV drugs during the 1980s (1,2).
However, the management of TB incidence has become more difficult
with the emergence of multiple drug-resistant (MDR) and
mycobacterial cell wall-deficient strains. Therefore, there is an
urgent need to develop new anti-TB agents that are effective in the
treatment of active and latent TB, and MDR-TB. Thus, the
identification of potential therapeutic agents to prevent and treat
MDR-TB infection by natural drug screening is important. Radix
Ranunculi Ternati, Radix Sophorae Flavescentis, Prunella
Vulgaris L. and Stellera Chamaejasme L. are common
traditional Chinese herbal medicines with heat-clearing and
detoxifying effects (3–5). In recent years, several studies have
demonstrated that these extracts possess anti-Mycobacterium
tuberculosis activity in vitro(6–8). In
the present study, we examined the therapeutic effects of
traditional Chinese herbal medicines for the treatment of MDR-TB.
The effect of these extracts on cell-mediated immunity in rats was
also examined, to provide preliminary data for the clinical
application of Chinese herbal medicines in the treatment of
MDR-TB.
Materials and methods
Animals
A total of 60 adult, male Kunming rats weighing
18–22 g were purchased from the Center of Experimental Animals
(Nanjing University, Nanjing, China) and bred in the university
facilities. All animal experiments were performed in accordance
with the Chinese laws for animal protection and in adherence with
the experimental guidelines and procedures approved by the
Institutional Animal Care and Use Committee (IACUC) of Nanjing
Medical University for the use of laboratory animals (permit
number: NJMU 09-1107) and The Ethical Review Committee of Nanjing
Medical University for the use of laboratory animals.
MDR-TB models
The rats were randomly divided into six groups, each
containing ten rats: The normal group (fed daily on a standard
diet); the model group (drenched with 5 ml sodium chloride and then
fed on a standard diet); and four groups that were each treated
with one of the extracts of Chinese herbal medicines (drenched with
5 ml of extract daily at a concentration of 200 mg/kg, and fed on a
standard diet for 4 weeks). The rats were then sacrificed and serum
was collected following sterilization (9). The purified MDR-TB colony was
prepared in a 1 mg/ml bacterial suspension with sterile saline.
Each rat was injected with 2 ml of suspension via the tail vein
(10).
Preparation of the Chinese herbal
medicine extracts
The extracts of Radix Ranunculi Ternati, Radix
Sophorae Flavescentis, Prunella Vulgaris L. and Stellera
Chamaejasme L. were prepared with distilled water. The solvent
was reflowed, extracted and filtrated with 20% ethanol to remove
any impurities. The extracts were then concentrated and
freeze-dried to obtain the ethanol extract (11).
ELISA analysis
The serum levels of IFN-γ, IL-4, IL-10 and IL-12
were quantified using the double antibody sandwich ELISA kit
(Jingmei Biotech, Co., Ltd., Shenzhen, Guangdong, China). The assay
was performed according to the manufacturer’s instructions.
Briefly, 150 μl sample was added to microtiter plates, which were
coated with the respective monoclonal antibody. The reaction plate
was then mixed at 37°C for 120 min to fully integrate the antigen
and antibody. Following washing, streptavidin antibody fluid
labeled with 100 μl horseradish peroxidase was kept at 37°C for 60
min and added to 100 μl o-phenylenediamine dihydrochloride at 37°C
for 5–10 min in the dark. The optical density was measured at a
wavelength of 492 nm using an ELISA microplate reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA). The serum concentrations
were calculated and expressed as pg/ml.
Isolation and culture of PBMCs
The animals were sacrificed following the removal of
blood from the femoral artery. PBMCs were isolated by density
gradient centrifugation. The cells were routinely grown in
RPMI-1640 medium supplemented with 10% FBS, 50 U/ml penicillin and
50 mg/ml streptomycin. Following resuspension, 0.5 ml cell
suspension was plated out in 24-well culture plates at a density of
1×106 cells/ml and cells were cultured in an incubator
at 37°C and 5% CO2. The medium was cultivated after 24
h.
Primer construction
The primer sequences used in the study were as
follows: IFN-γ forward, 5′-ACAATGAACGCT ACACACTGC-3′ and reverse,
5′-CGAATCAGCAGCGA CTCCTT-3′, product size 456 bp; IL-12 forward,
5′-GCTAACCATCTCCTGGTTTGC-3′ and reverse,
5′-CTTTCCAGAGCCTATGACTCC-3′, product size 390 bp; IL-4 forward,
5′-CTCACAGCAACGAAGAACAC-3′ and reverse,
5′-GGCTCAGTACTACGAGTAATCC-3′, product size 267 bp; IL-10 forward,
5′-CTTTCAAATGAA GGATCAGC-3′ and reverse, 5′-ATGTCAAAC
TCACTCATGGC-3′, product size 327 bp; and GLS forward,
5′-GGGAAGCTCCATAAATGTCACCT-3′ and reverse, 5′-GGTTTAGATCGGCACAT-3′,
product size 405 bp. For the housekeeping gene GAPDH, the primers
used were forward, 5′-GAAGGTGAAGGTCGGAGT-3′ and reverse,
5′-GAAGATGGTGATGGGATTTA-3′, product size 320 bp. The above primers
were synthesized by Sangon Biotech, Co., Ltd. (Shanghai,
China).
RT-PCR analysis
Total RNA was isolated from PBMCs using TRIzol
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions, and collected using a one-step method
at a concentration of 200 μg/ml. The total volume used for reverse
transcription was 50 μl and the procedure was performed using the
SYBR-Green PCR kit (Applied Biosystems, Foster City, CA, USA)
according to the manufacturer’s instructions. The RT-PCR reaction
conditions were as follows: RT reaction at 50°C for 30 min; RTase
inactivation at 94°C for 5 min, 9°C for 30 sec, 55°C for 90 sec and
72°C for 30 sec for 35 cycles in total, followed by maintenance at
72°C for 10 min. The amplified products (5 μl) were separated by
electrophoresis on 1.5% agarose gel, and were then imaged and
analyzed using ImageMaster TotalLab software (GE Healthcare
Biosciences, Pittsburgh, PA, USA). The ratios of the target genes
and the internal reference gene (GAPDH) were used to determine the
relative expression levels of the target genes.
Statistical analysis
Data are expressed as the mean ± SD, and were
analyzed using SPSS 12.0 software (SPSS, Inc., Chicago, IL, USA).
One-way ANOVA was used for statistical analysis to determine
differences between groups. P<0.05 was considered to indicate a
statistically significant result.
Results
ELISA analysis
The serum levels of IFN-γ, IL-4, IL-10 and IL-12
were examined using ELISA (Table
I). The levels of IFN-γ and IL-12 were significantly decreased
in the model group compared with the normal group, and the levels
of IL-4 and IL-10 were significantly increased (P<0.01). The
levels of IFN-γ, IL-12, IL-4 and IL-10 in the Radix Ranunculi
Ternati group demonstrated a significant increase compared with the
model group (P<0.05). In the Radix Sophorae Flavescentis group,
the levels of IFN-γ and IL-12 were significantly increased
(P<0.05), and the levels of IL-4 and IL-10 were significantly
decreased compared with the model group (P<0.05). In the
Prunella Vulgaris L. group, the levels of IFN-γ, IL-10 and
IL-12 were significantly increased compared with the model group
(P<0.05); however, no significant changes in IL-4 levels were
detected. In the Stellera Chamaejasme L. group, the levels
of IL-4, IL-10 and IL-12 were significantly increased (P<0.05)
and the levels of IFN-γ were significantly decreased (P<0.05)
compared with the model group.
 | Table ISerum levels of IFN-γ, IL-4, IL-10 and
IL-12 in different groups of rats, as determined by ELISA. |
Table I
Serum levels of IFN-γ, IL-4, IL-10 and
IL-12 in different groups of rats, as determined by ELISA.
| Group | Number | IFN-γ (pg/ml) | IL-12 (pg/ml) | IL-4 (pg/ml) | IL-10 (pg/ml) |
|---|
| Normal | 10 | 2.24±0.62 | 3.79±0.92 | 5.58±1.43 | 11.23±2.08 |
| Model | 10 | 1.18±0.38a | 2.19±0.57a | 8.15±2.24a | 16.10±2.21a |
| Radix Ranunculi
Ternati | 10 | 2.01±0.73 | 2.99±0.89b | 6.01±1.46 | 12.09±3.07b |
| Radix Sophorae
Flavescentis | 10 | 1.92±0.56b | 2.75±0.84b | 6.12±1.35b | 12.45±4.01b |
| Prunella
Vulgaris L. | 10 | 1.98±0.67b | 3.02±0.86b | 6.47±1.46b | 12.13±3.43b |
| Stellera
Chamaejasme L. | 10 | 1.94±0.59b | 2.89±0.75b | 6.15±1.44b | 12.54±3.78b |
RT-PCR analysis
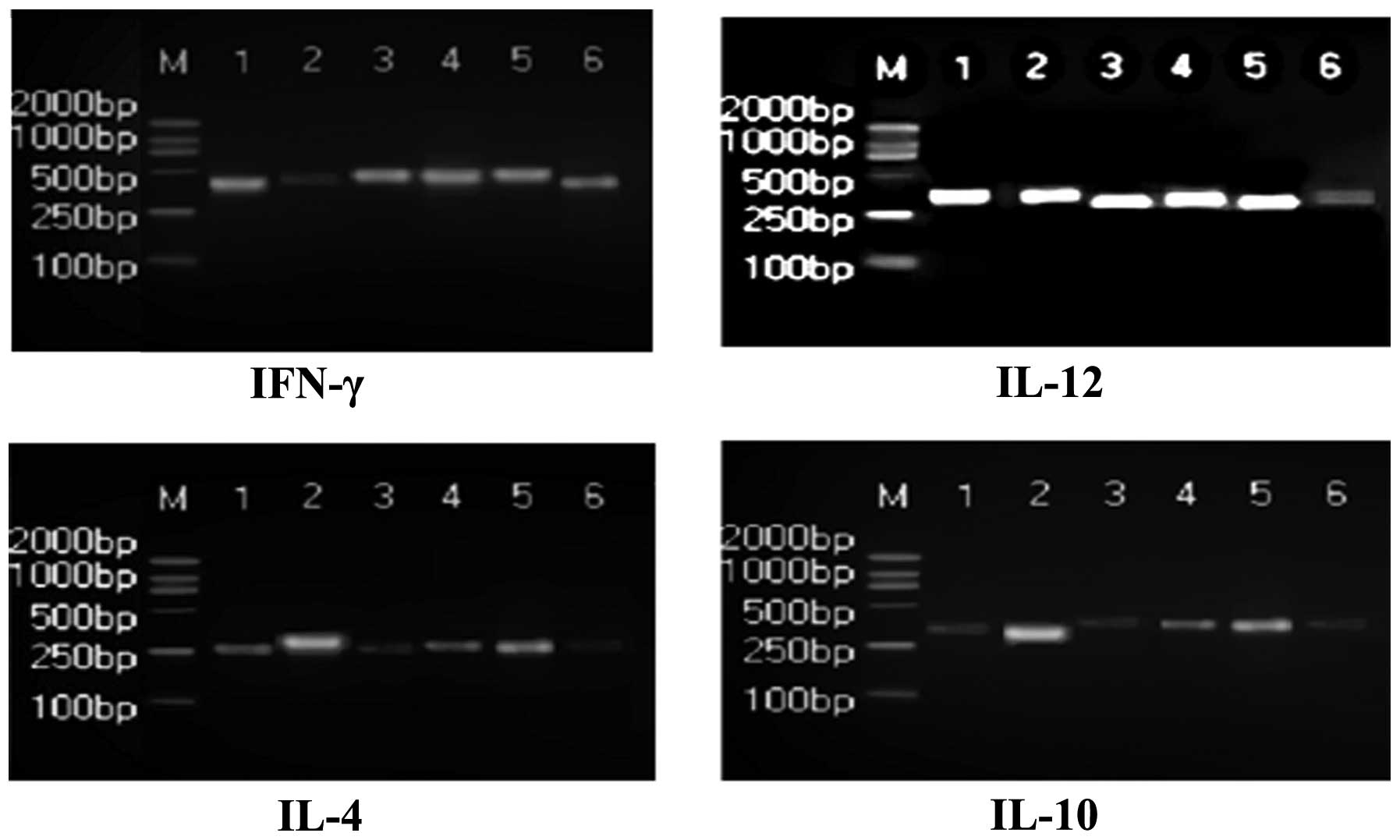
RT-PCR was used to examine the mRNA expression
levels of IFN-γ, IL-4, IL-10 and IL-12 in the PBMCs of rats
(Table II). Compared with the
normal group, the mRNA levels of IFN-γ and IL-12 were significantly
decreased in the model group, and IL-4 and IL-10 levels were
significantly increased (P<0.01). The mRNA expression levels of
IFN-γ, IL-12, IL-4 and IL-10 in the PBMCs of the Radix Ranunculi
Ternati group were significantly increased compared with the model
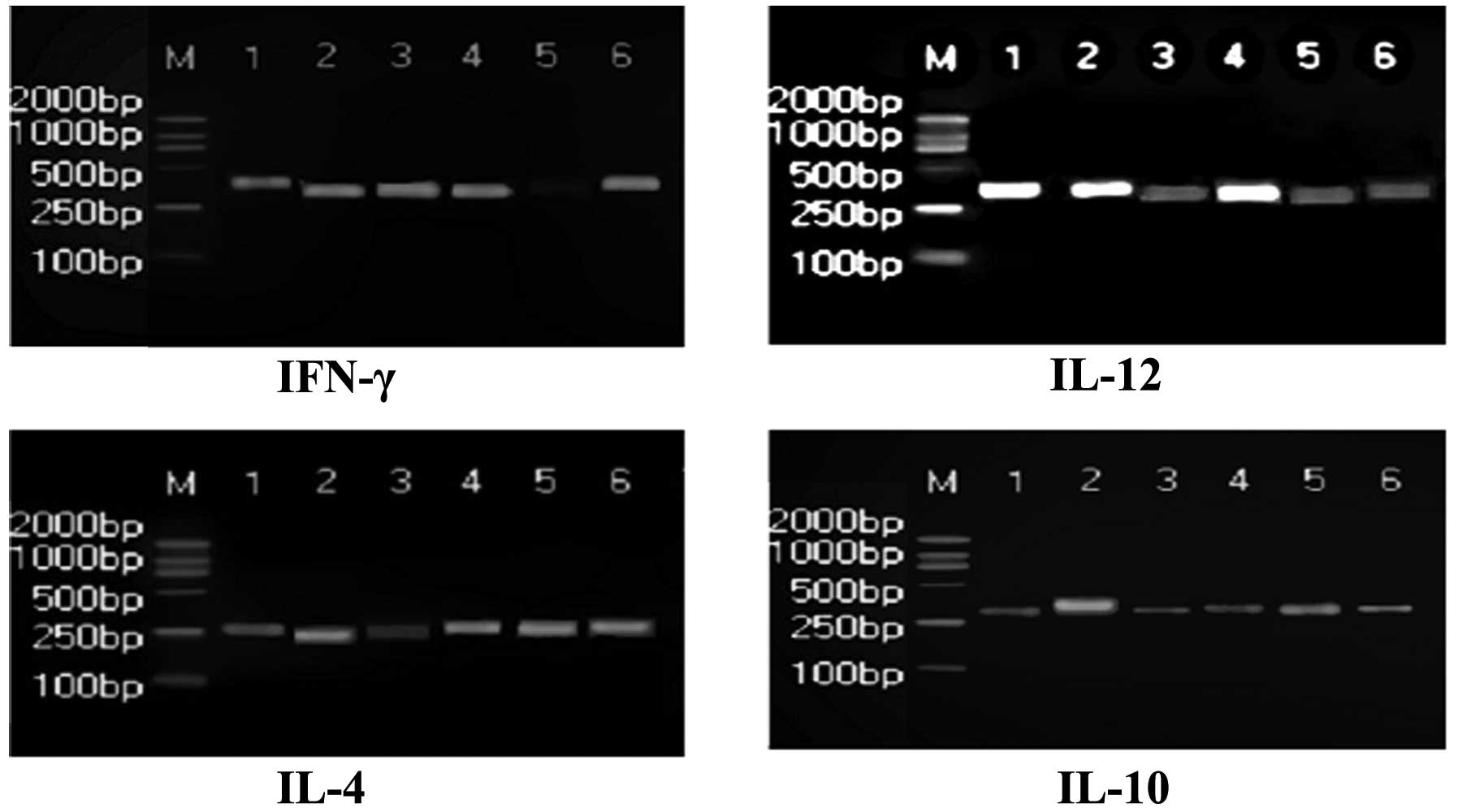
group (P<0.05; Fig. 1). The
levels of IFN-γ and IL-4 in PBMCs of the Radix Sophorae
Flavescentis group were significantly increased (P<0.05), while
IL-10 and IL-12 levels were significantly decreased compared with
the model group (P<0.05; Fig.
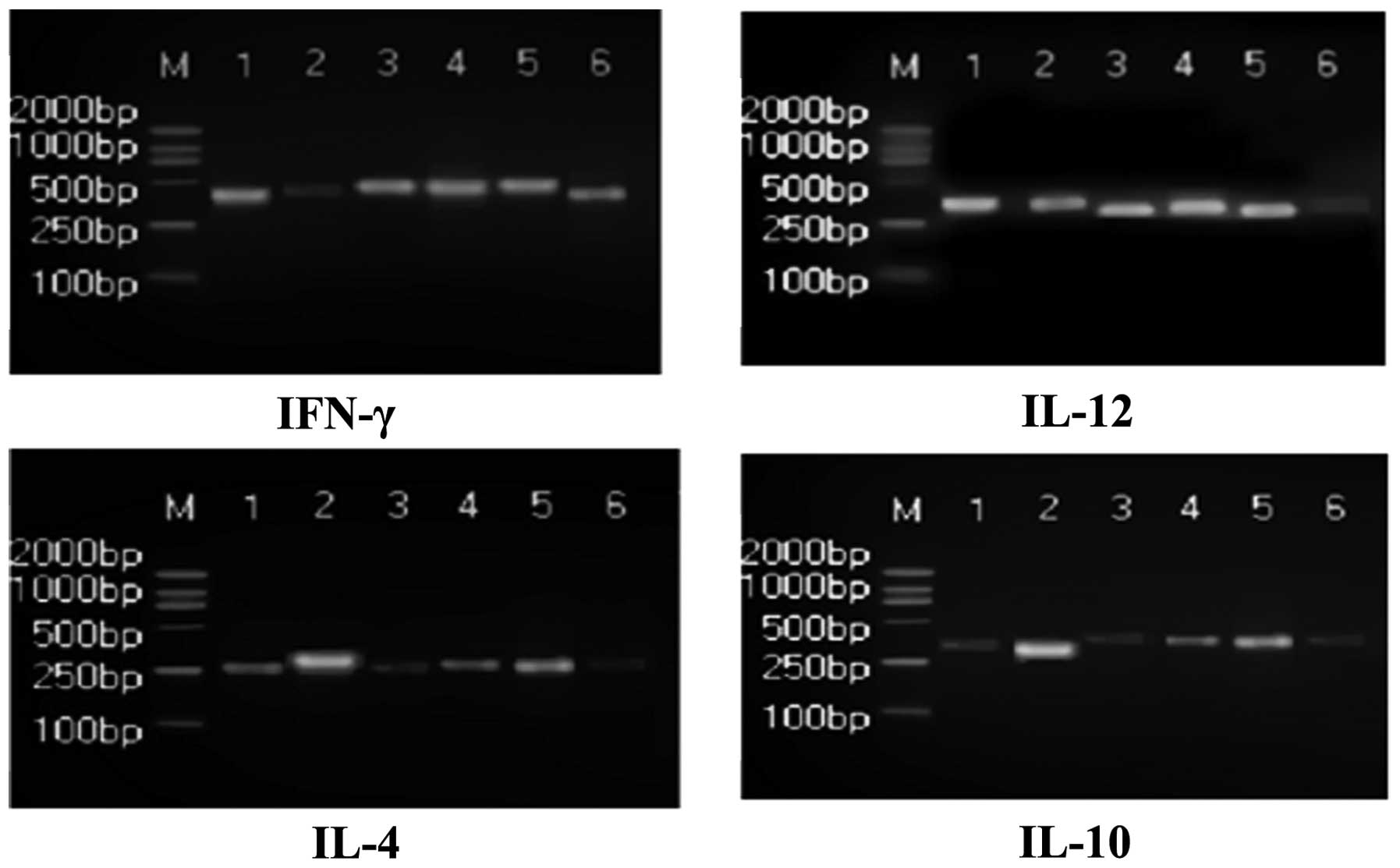
2). The mRNA expression levels of IFN-γ and IL-12 in the
Prunella Vulgaris L. group increased markedly, and the
levels of IL-4 and IL-10 decreased significantly compared with the
model group (P<0.05); however, no evident change in IL-4 levels
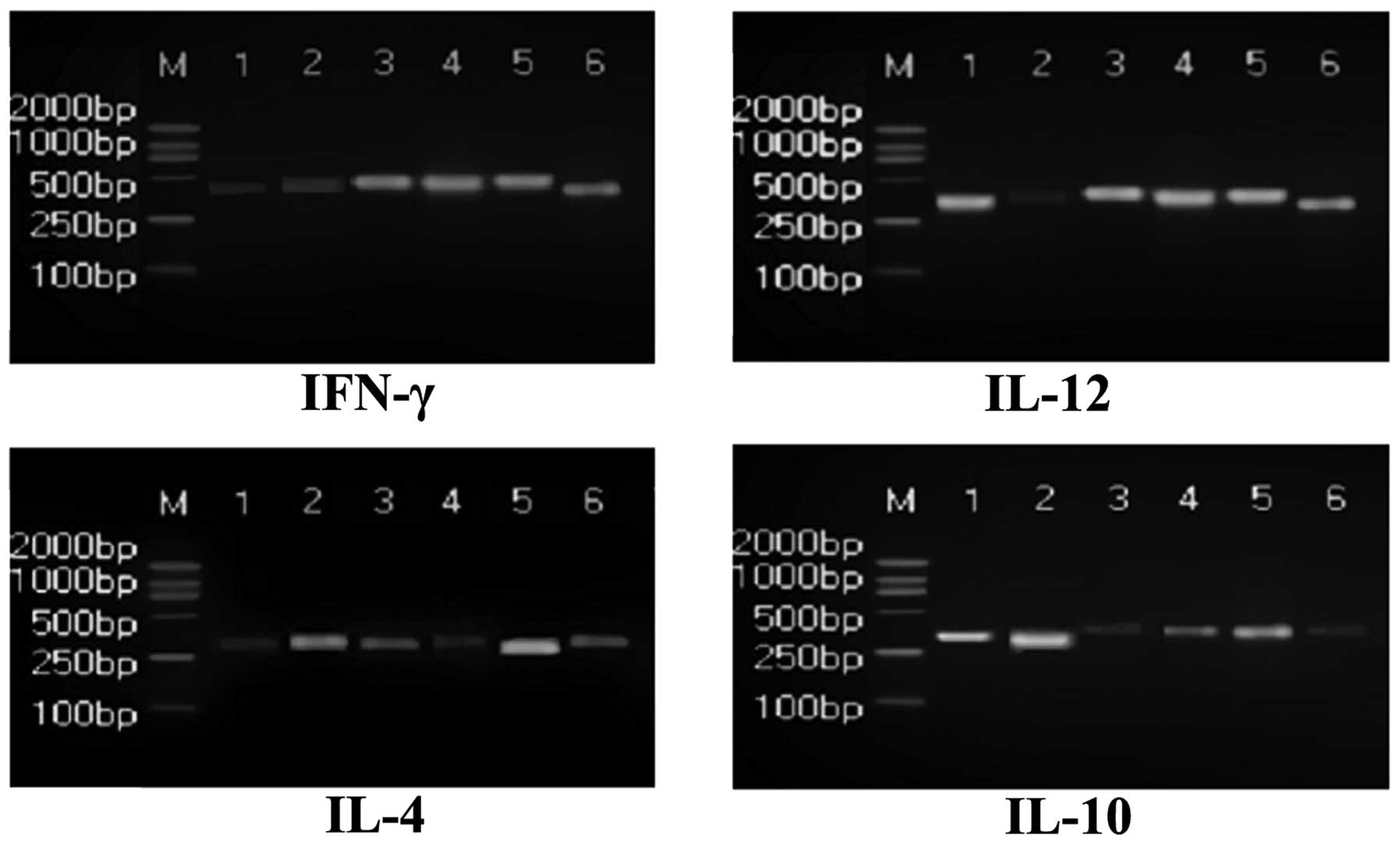
were observed (P<0.05; Fig. 3).
IL-4, IL-10 and IL-12 levels in PBMCs of the Stellera
Chamaejasme L. group increased significantly (P<0.05) and
IFN-γ levels decreased markedly compared with the model group
(P<0.05; Fig. 4). Granulysin
(GLS), a type of polypeptide whose expression is induced by
cytotoxic T lymphocytes (CTLs) and natural killer cells during late
activation, is capable of killing Mycobacterium
tuberculosis. The mRNA expression level of GLS is one of the
markers that demonstrates the molecular effectiveness of cells that
kill intracellular bacteria. Compared with the model group, the
mRNA levels of GLS increased significantly (P<0.05 or
P<0.01).
 | Table IImRNA levels of IFN-γ, IL-4, IL-10,
IL-12 and GLS in rat PBMCs, as determined by RT-PCR. |
Table II
mRNA levels of IFN-γ, IL-4, IL-10,
IL-12 and GLS in rat PBMCs, as determined by RT-PCR.
| Group | IFN-γ/GAPDH (gray
ratio) | IL-12/GAPDH (gray
ratio) | IL-4/GAPDH (gray
ratio) | IL-10/GAPDH (gray
ratio) | GLS/GAPDH (gray
ratio) |
|---|
| Normal | 0.31±0.06 | 0.78±0.10 | 0.45±0.05 | 0.63±0.08 | 0.22±0.04 |
| Model | 0.18±0.05a | 0.59±0.08a | 0.61±0.06a | 0.99±0.12a | 0.14±0.04a |
| Radix Ranunculi
Ternati | 0.27±0.06b | 0.75±0.09b | 0.50±0.05b | 0.69±0.08b | 0.21±0.05c |
| Radix Sophorae
Flavescentis | 0.25±0.07b | 0.64±0.09 | 0.52±0.06b | 0.83±0.11 | 0.17±0.05 |
| Prunella
Vulgaris L. | 0.26±0.06b | 0.71±0.11b | 0.58±0.05 | 0.70±0.10b | 0.18±0.04 |
| Stellera
Chamaejasme L. | 0.22±0.07 | 0.68±0.10b | 0.55±0.06b | 0.72±0.12 | 0.19±0.04b |
Discussion
Radix Ranunculi Ternati is a prescription medicine
that has been reported in the Chinese pharmacopoeia to facilitate
detumescence. Radix Ranunculi Ternati may also possess anticancer
properties; however, the mechanism by which these effects are
exerted has yet to be fully elucidated (12). Radix Sophorae Flavescentis has
diuretic, anti-inflammatory, antiviral, anticancer and antifibrotic
properties, and may also be used to treat a number of immunological
diseases (13,14). Prunella Vulgaris L. has been
shown to improve eyesight and eliminate stagnation by partially
inhibiting tumor cell proliferation in vivo, and it induces
its antitumor effects by regulating several cell signal
transduction pathways, which stimulates the activation of
macrophages (15–17). Stellera Chamaejasme L.
possesses antitumor, antiviral and immunological properties
(18).
Mycobacterium tuberculosis undergoes an
initial replicative phase inside alveolar macrophages. Following
this stage, it then enters a non-replicative, drug-resistant state
of dormancy. It is able to survive in this dormant state for
decades until the immune system of the host is weakened, at which
point the bacterium reactivates and causes the infectious disease.
The immunological response to Mycobacterium tuberculosis
infection in humans is predominantly mediated by Th1 cells. Th1
cells predominantly secrete IFN-γ and INF-α, which stimulate
macrophages to kill intracellular micro-organisms. Th2 cells mainly
participate in humoral immunity, and secrete IL-4, IL-10 and
several other cytokines that stimulate the phagocytosis of
extracellular bacteria and parasites. Mycobacterium
tuberculosis is a facultative intracellular bacterium that
parasitizes macrophages. The immune response to Mycobacterium
tuberculosis infection is mainly exerted by T cells and antigen
presentation by MHC class II molecules. It is well known that IFN-γ
produced by TB-specific CD4+ T cells (Th1) is able to
activate macrophages, secreting IL-12 to promote the
differentiation of Th0 cells to Th1 cells, further expanding the
Th1 cell immune response. Thus, Th1 cells possess an important
anti-TB protective role; however, the cytokines secreted by Th2
cells, including IL-4 and IL-10, are also secreted by
Mycobacterium tuberculosis(19–21).
Therefore, the regulation of immune homeostasis by Th1/Th2 cells
may affect the type of immunological response initiated by the
body’s immune system.
In the present study, we demonstrated that infection
with Mycobacterium tuberculosis significantly affects the
serum levels of cytokines in rats. The levels of IFN-γ and IL-12 in
the model group were markedly decreased and the levels of IL-4 and
IL-10 were significantly increased compared woth the normal group
(Table I). Experimental results
demonstrated that the occurrence of TB is correlated with the
balance of Th1/Th2 cell responses. The four extracts of Chinese
herbal medicines used in this study, particularly Radix Ranunculi
Ternati, significantly enhanced the cell-mediated immunological
response of rats infected by MDR-TB through the adjustment the
Th1/Th2 balance. Therefore, these extracts may be important in the
treatment of TB.
Furthermore, results of the RT-PCR analysis
demonstrated that the mRNA expression levels of IFN-γ, IL-12 and
GLS in the PBMCs of MDR-TB-infected rats were significantly
increased, and the levels of IL-4 and IL-10 were significantly
decreased compared with the normal group (Table II); these differences were
significant (P<0.05 or P<0.01). The four extracts of Chinese
herbal medicines were found to be capable of stimulating the
expression of IFN-γ, IL-12 and GLS mRNA and downregulating the
expression of IL-4 and IL-10. The results demonstrated the
regulation of cellular immunity of the extracts is accomplished at
the level of gene transcription. It was also demonstrated that
Radix Ranunculi Ternati is able to stimulate the expression of GLS
mRNA, thus enhancing the sterilization ability of CTLs, so as to
kill Mycobacterium tuberculosis(22).
In conclusion, appropriate concentrations of the
Radix Ranunculi Ternati, Radix Sophorae Flavescentis, Prunella
Vulgaris L. and Stellera Chamaejasme L. extracts are
capable of enhancing cell-mediated immunity in rats infected by
MDR-TB, providing an experimental basis for the clinical treatment
of TB using these extracts. However, further research is required
with regard to the role of other cytokines.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (NSFC 81172778) and the Natural
Science Foundation of Anhui Province (KJ2010A087).
References
|
1
|
Telenti A and Iseman M: Drug-resistant
tuberculosis: what do we do now? Drugs. 59:171–179. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Du Toit LC, Pillay V and Danckwerts MP:
Tuberculosis chemotherapy: current drug delivery approaches. Respir
Res. 7:1182006.PubMed/NCBI
|
|
3
|
Zhang ZL, Wu XJ, Wang L and Zhang HX:
Study on immunocompetence of active constituent of Radix Ranunculi
Ternati. Zhong Hua Zhong Yi Yao Xue Hui She. 22:120–122. 2007.(In
Chinese).
|
|
4
|
Fu XR, Li JC and Zhang MZ: Advances in
modern research on prunella spike. Zhong Yi Yan Jiu Bian Ji Bu.
18:60–62. 2005.(In Chinese).
|
|
5
|
Ji RL, Xia SH and Li F: Oxymatrine
inhibits MMP-2 expression and reduces cell invasion in human
pancreatic carcinoma cell line SW1990. Shi Jie Hua Ren Xiao Hua Za
Zhi Bian Ji Bu. 19:19–24. 2011.(In Chinese).
|
|
6
|
Wu HD and Wang L: Research of
antituberculotic. Medical Review. 13:475–478. 2007.
|
|
7
|
Lu J, Ye S, Deng Y, et al: Effects of four
extracts of Chinese herbal medicines on cellular immunity in rats
induced by multiple drugs resistant bacillus tuberculosis
among pneumoconiosis patients complicated with tuberculosis. Zhong
Hua Wei Sheng Wu Xue He Mian Yi Xue Za Zhi Bian Ji Bu. 31:893–897.
2011.(In Chinese).
|
|
8
|
Bai J, Sun HF and Chen XF: Studies on the
anti-mycobacterium tuberculosis activity of 4 Chinese
medicinal herbs. Shi Zhen Guo Yi Guo Yao Bian Ji Bu. 18:77–78.
2007.
|
|
9
|
Liu MX, Sun FJ, Wang CX, et al: Effects of
the extract of Coptidis Decoction for detoxification in MDR model
rats on cell apoptosis and correlative study. Zhong Yao Cai Bian Ji
Bu. 32:1270–1272. 2009.(In Chinese).
|
|
10
|
Li JG, Gao AL, Liu XH and Li NN: The
effect of crewels pills on protection and pathological changes
degree of internal organs for mouse suffering MDR-TB. He Nan Zhong
Yi Xue Yuan Xue Bao Bian Ji Bu. 23:28–29. 2008.(In Chinese).
|
|
11
|
Guo XM, Hong YH and Zhou ZL: Advances in
studies on chemical constituents from plants of Ranunculus.
Zhong Cao Yao Bian Ji Bu. 32:748–750. 2001.(In Chinese).
|
|
12
|
Wang AW, Wang M, Yuan JR, et al: The study
on antitumor effects in vitro of different extracts in Radix
Ranunculi Ternati. Tian Ran Chan Wu Yan Jiu Yu Kai Fa Bian Ji Bu.
16:529–531. 2004.(In Chinese).
|
|
13
|
Liu XL, Zhang Y and Liu XL:
Anti-coxsackievirus B3 effects of Sophora flavescens
alkaloids in vitro. Shen Yang Yao Ke Da Xue Xue Bao Bian Ji Bu.
23:724–730. 2006.(In Chinese).
|
|
14
|
Liu D, Jiang YP and Cheng ZL: Sheltering
effect on acute and chronic liver injury of mice due to drug
combination of glycyrrhizin with matrine to carbon tetrachloride.
30:581–582. 2007.
|
|
15
|
Feng L, Jia XB, Cheng Y and Li X: Advances
in chemical constituents of Prunella vulgaris and antitumor
mechanisms. Zhong Hua Zhong Yi Yao Xue Hui She. 23:428–434.
2008.(In Chinese).
|
|
16
|
Zhang MZ and Wang XQ: Effects of extract
from Prunella vulgaris combined with chemotherapeutic agents
on proliferation of lymphoma cells. Zhong Liu Bian Ji Bu.
299:961–964. 2009.(In Chinese).
|
|
17
|
Fang X, Chang RC, Yuen WH, et al: Immune
modulatory effects of Prunella vulgaris L. Int J Mol Med.
15:491–495. 2005.PubMed/NCBI
|
|
18
|
Yang K, Wang YS, Wang LP, et al: Brief
review and clinical analysis of pharmacological action of
Stellera chamaejasme L. Yi Xue Xin Xi Bian Ji Bu.
23:2496–2498. 2010.(In Chinese).
|
|
19
|
Wu SZ and Song JF: Mycobacterium
tuberculosis pathogenesis and immunity. Zhong Hua Jie He He Hu
Xi Za Zhi Bian Ji Bu. 26:101–103. 2003.(In Chinese).
|
|
20
|
Cole ST, Brosch R, Parkhill J, et al:
Deciphering the biology of Mycobacterium tuberculosis from
the complete genome sequence. Nature. 393:537–544. 1998.
|
|
21
|
Cooper AM, Dalton DK, Stewart TA, et al:
Disseminated tuberculosis in interferon-γ gene-disrupted mice. J
Exp Med. 178:2243–2247. 1993.
|
|
22
|
Zhan L, Dai HC, Yang ZP, et al: The study
of expression of SHSP and GLS in peripheral blood lymphocytes of
drug resistant tuberculosis patients with ternatolide. Zhong Guo
Zhong Yao Za Zhi She. 27:677–679. 2002.(In Chinese).
|