Introduction
Curcumin, the major phytochemical in turmeric, has a
diverse pharmacological role, including anticancer,
anti-inflammatory, antioxidant and anti-proliferative activities
(1). In a previous study, curcumin
was identified to exhibit anticancer effects by interfering with
signaling pathways associated with the initiation, promotion and
progression of multistage carcinogenesis (2). In addition, curcumin has been found
to induce apoptosis in various types of cancer cells, including
colon, breast, lung and ovarian (3–6), and
suppresses tumor growth in various cancer xenograft models
(7,8). Curcumin inhibits metastasis and
angiogenesis by suppression of matrix metalloproteinase-9, vascular
endothelial growth factor and hypoxia-inducible factor 1α
expression in hepatocarcinoma (9).
The Wnt signaling pathway is important in cell
growth, proliferation, differentiation and development. Several
studies have reported that overactivation of β-catenin in the
cytosol is associated with cancer metastasis (10–13).
Under normal conditions, β-catenin is phosphorylated at the
Ser33/37 residue by GSK3β in the GSK3β/Axin/Ck1 complex, triggering
subsequent proteasomal degradation. Phosphorylated β-catenin
translocates into the nucleus, where it activates target genes
coding for proteins, including cyclin D and c-Myc in the Wnt
pathway, by binding with the transcription factor, T cell factor
(TCF) (14). The Akt/mTOR pathway,
in addition to overactivated Wnt signaling, inhibits GSK3β
activity. Phosphorylation of GSK3β by Akt has been demonstrated in
a number of cell lines to promote angiogenesis, metastasis and cell
survival by activation of the NF-κB signaling pathway (15–18).
Although several anticancer effects of curcumin in
cancer cells have been reported, studies on the molecular
mechanisms of these effects via the survival pathways in
hepatocarcinoma have not been performed. Curcumin possesses the
ability to suppress Wnt signaling and may be important for the
development of anti-proliferative and anti-metastatic drugs.
In the current study, curcumin was observed to
induce apoptosis by cell proliferation assay and cell cycle
analysis. Curcumin induced expression of apoptotic proteins and
suppressed Wnt signaling by the reduction of β-catenin and
phospho-GSK3β in vitro. In addition, in vivo
suppression of the Wnt signaling pathway by curcumin was
identified. Curcumin significantly suppressed
12-O-tetradecanoylphorbol-13-acetate (TPA)-induced cell
migration by blocking the Wnt signaling pathway in Hep3B
hepatocarcinoma cells.
Materials and methods
Cell culture and reagents
Hep3B cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). Cells were grown in DMEM
containing 10% fetal bovine serum and 1% antibiotics at 37°C in a
5% CO2 incubator. Curcumin, TPA,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
propidium iodide (PI) and BIO were obtained from Sigma-Aldrich (St.
Louis, MO, USA). LY294002 was purchased from Tocris Bioscience
(Bristol, UK).
MTT assay
Cells seeded in 12-well plates at 1×105
cells/ml were incubated with curcumin for indicated times and
concentrations. Respective medium was removed and then incubated
with 20 μl MTT solution (5 mg/ml MTT in PBS) for 1 h. Converted
purple formazan dye from MTT was solubilized in DMSO and optical
densities were measured at 595 nm.
Cell cycle analysis
Cells treated with curcumin at various
concentrations (10, 20 and 40 μg/ml for 24 h) were harvested,
washed with phosphate-buffered saline (PBS) and fixed in 70% cold
ethanol. Following washing with PBS, cells were resuspended in PBS
and incubated with RNase A and PI. Cells were analyzed with flow
cytometry.
Wound healing assay
Hep3B cells were grown to 90% confluence in a 6-well
plate at 37°C in a 5% CO2 incubator. A wound was created
by scratching cells with a sterile 200 μl pipette tip. Cells were
washed with PBS to remove floating cells and then added to a
medium. The distance between wound edges was measured at a fixed
region. Images of the wound were captured under a microscope
(magnification, ×100).
Western blot analysis
Cells were washed with PBS and lysed with RIPA lysis
buffer [50 mM Tris-HCl (pH 8.0), 1% NP-40, 0.5% sodium
deoxycholate, 150 mM NaCl and 1 mM PMSF]. Protein concentrations
were determined using the Bradford assay. All samples were
separated by sodium dodecyl sulfate polyacrylamide gel
electrophoresis and transferred onto nitrocellulose membrane. The
membrane was incubated overnight with primary antibodies. Secondary
antibodies, conjugated to horseradish peroxidase, against IgG were
used. Proteins were visualized by enhanced chemiluminescence
(Intron Biotechnology, Seong nam, Korea) and detected using the
LAS4000 chemiluminescence detection system (Fujifilm, Tokyo,
Japan).
Xenograft and immunohistochemistry
Hep3B cells (2×106) were inoculated
subcutaneously in 4-week nu/nu mice at the left flank. After 1
week, mice were administered curcumin or PBS (control) by
injection. Tumor size was measured in two perpendicular diameters
by using a caliper every 3 days and tumor volume was calculated
using the following formula: Volume = 1/2 × (length ×
width2).
Tumor specimens from mice were fixed in 10%
formaldehyde, embedded in paraffin and sectioned into 5-μm thick
slices. Sections were deparaffinized with xylene and dehydrated
with 98% ethanol. Serial sections were stained using standard
immunoperoxidase techniques with primary antibodies against CD31.
For epitope retrieval, specimens were microwave treated for 25 min
prior to incubation with primary antibody. Pre-immune serum was
used as a negative control for immunostaining and positive staining
was visualized with diaminobenzidine, followed by a light
counterstaining with hematoxylin. Sections were evaluated by a
pathologist, determining stain intensity and percentage of reactive
cells. Representative images were captured. All animal experiments
were approved by the Ethics Committee for Animal Experimentation of
the Hannam University (Daejeon, South Korea).
Statistical analysis
Cell viability data were statistically analyzed
using unpaired t-tests (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Effect of curcumin on cell viability and
apoptosis in Hep3B cells
To examine the effects of curcumin on Hep3B cell
proliferation, cells were seeded in 12-well plates at a density of
1×105 cells/well. The effect of curcumin on cell
viability was determined by MTT assay. Fig. 1A demonstrates significant
inhibition of cell proliferation in curcumin-treated cells in a
dose- and time-dependent manner, compared with control cells. Cell
cycle analysis revealed that curcumin induced dose-dependent
apoptosis. The percentage of apoptotic cells (sub-G1
peak) increased from 19.5% (control) to 35.53% (curcumin 20 μM) and
32.15% (curcumin 40 μM) in the Hep3B hepatocarcinoma cells
(Fig. 1B). To determine the
mechanism of curcumin-induced apoptosis, the expression levels of
apoptotic proteins, including procaspase-3, PARP, Bax and Bcl-2,
were detected by western blot analysis. Activation of caspase-3
plays a critical role in apoptosis, as it proteolytically cleaves
PARP. In Hep3B cells treated with curcumin, the levels of
procaspase-3, PARP and Bcl-2 decreased and Bax increased.
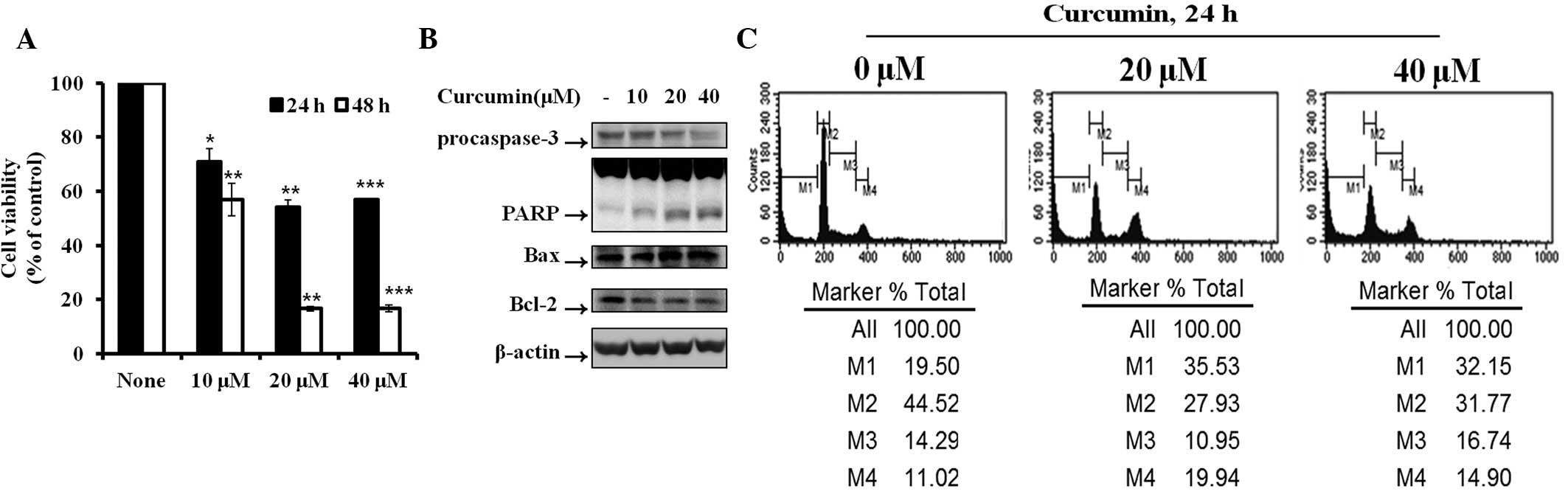 | Figure 1Cytotoxicity and cell apoptotic
effects in Hep3B cells induced by curcumin. (A) Hep3B cells were
treated with various concentrations of curcumin for various times
and cell viability was detected by MTT assay. Data are presented as
the mean ± SD. (B) Protein expression of procaspase-3, PARP, Bax
and Bcl-2 in Hep3B cells treated with various concentrations of
curcumin for 6 h was determined. (C) Hep3B cells were treated with
various concentrations of curcumin for 24 h and the cell cycle
percentage was determined by FACS. M1, sub-G1 phase; M2,
G0/G1 phase; M3, S phase; M4, G2/M
phase; FACS, fluorescence-activated cell sorting; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
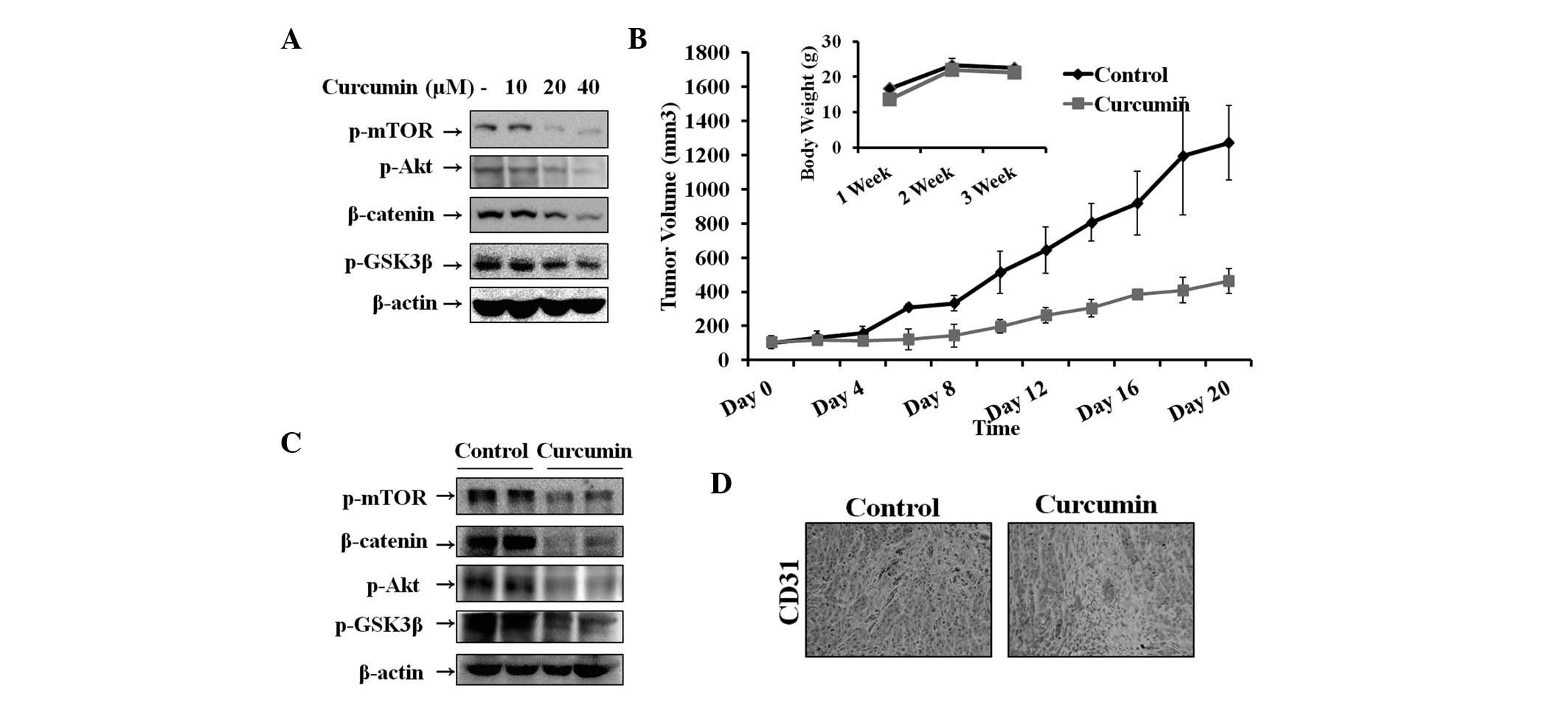
Effect of curcumin on the Wnt signaling
pathway in vitro and in vivo
The Wnt signaling pathway plays a critical role in
tumor cell growth and survival in hepatocarcinoma. Therefore, the
inhibitory effect of curcumin on Wnt signaling in Hep3B cells was
determined by analyzing the levels of β-catenin and GSK3β, which
play key roles in the pathway. Previous studies have demonstrated
that GSK3β is inactivated by Akt and regulated by mTOR (25). In addition, Akt and mTOR are
associated with cell survival and growth. Therefore, levels of
β-catenin, p-GSK3β, p-Akt and p-mTOR were assessed by western blot
analysis. As demonstrated in Fig.
2A, curcumin increased phosphorylation of GSK3β, Akt and mTOR
and thus, the level of β-catenin, in a dose-dependent manner. To
explore the therapeutic effects of curcumin, hepatocarcinoma tumors
were established in nude mice and tumors were treated by injecting
curcumin. As revealed in Fig. 2B,
tumor volume of the curcumin-treated group was significantly
reduced compared with that of the control group. No significant
differences in the body weight of mice were observed between
control and curcumin-treated groups. Furthermore, curcumin was
observed to induce antitumor activity by suppression of Wnt
signaling molecules. Fig. 2C
demonstrates that β-catenin, p-mTOR, p-Akt and p-GSK3β expression
was significantly decreased in the curcumin-treated group compared
with the control. To further correlate these observations, in
vivo tumor therapeutic effects were assessed in vitro.
Thus, the expression levels of biomarkers were assessed in tumor
tissues by immunohistochemical analysis. The results indicated that
CD31-positive microvessels were significantly suppressed following
curcumin treatment (Fig. 2D).
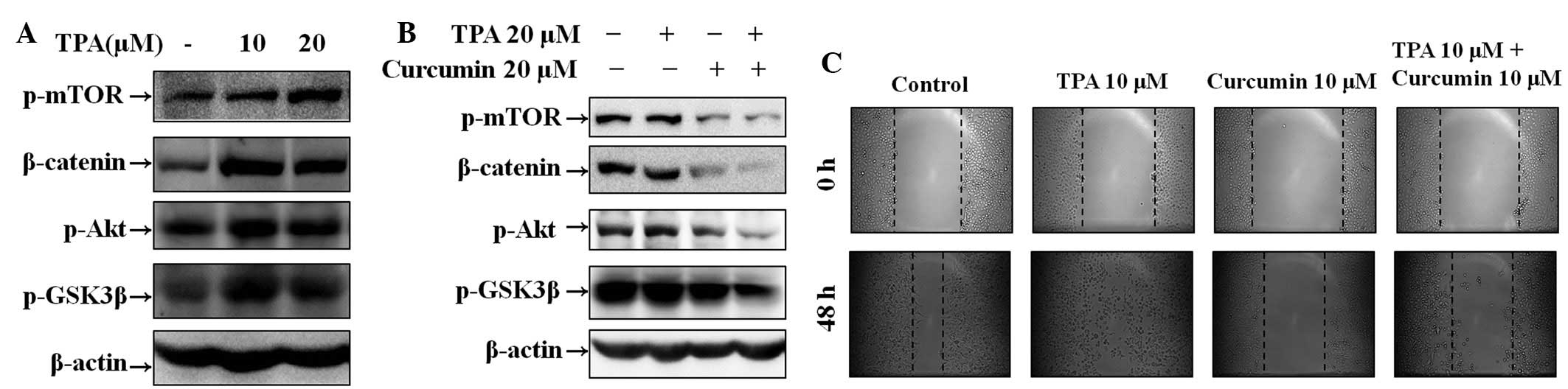
Effect of curcumin on TPA-induced Wnt
signaling pathway activation and cell migration in Hep3B cells
To investigate the effect of curcumin on TPA-induced
Wnt signaling activation and cell migration, wound healing assays
and western blot analysis were performed in Hep3B cells. Western
blot analysis demonstrated that TPA increased β-catenin and
phosphorylation of mTOR, Akt and GSK3β, and that curcumin inhibited
TPA-induced Wnt signaling pathway activation at 6 h in a
concentration-dependent manner (Fig.
3A and B). As demonstrated in Fig.
3C, cell migration was increased in cells treated with TPA.
However, the number of migrated cells was significantly decreased
in the group treated with curcumin alone and the group pre-treated
with TPA and then followed with curcumin for 48 h (for 12 and 24 h;
data not shown).
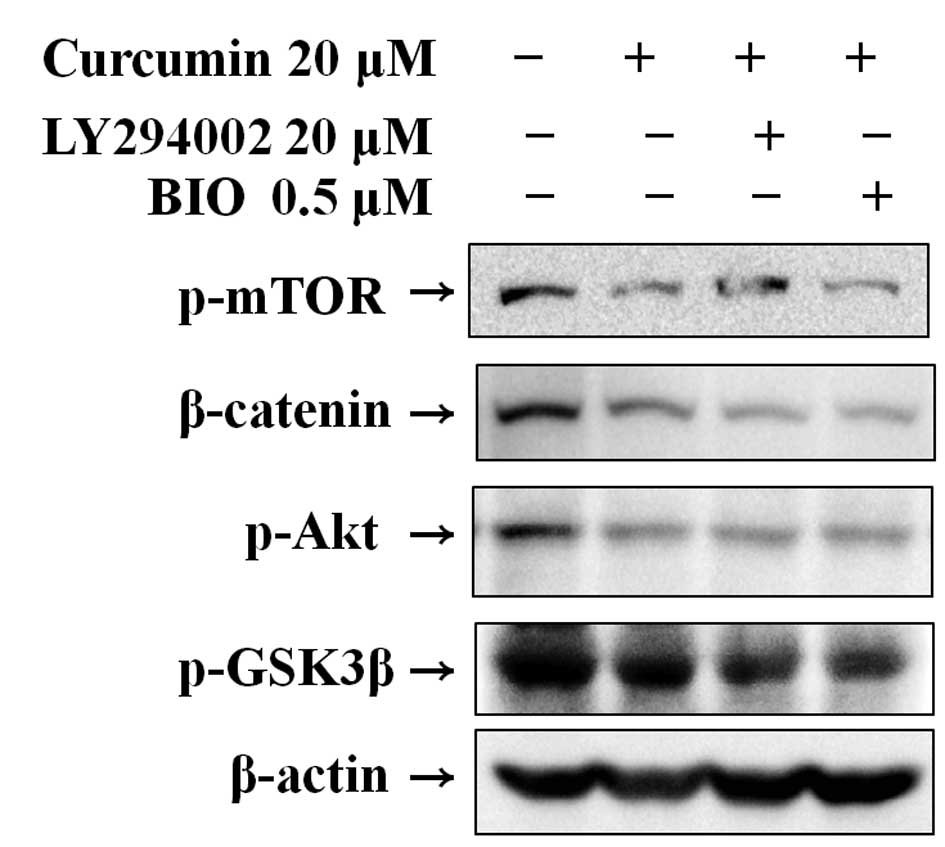
Effect of curcumin with LY294002 or BIO
on Wnt signaling
Western blot analysis was used to clarify the
mechanism of curcumin-mediated suppression of the Wnt signaling
pathway in Hep3B cells by the suppression of specific molecules.
The results indicate that curcumin significantly suppressed the
expression of p-mTOR, p-Akt, β-catenin and p-GSK3β. Pre-treatment
using LY294002 and BIO, specific PI3K and GSK3 inhibitors,
respectively, followed by curcumin treatment, effectively reduced
β-catenin and phosphorylation of mTOR, Akt and GSK3β (Fig. 4).
Discussion
Liver cancer is the leading cause of mortality from
cancer and is more prevalent in Asia and Africa. To date, a number
of signaling pathways, including Ras/Raf/MEK/ERK, PI3K/Akt/mTOR and
NF-κB have been hypothesized to represent potential targets for
hepatocarcinoma therapy. Thus, the Wnt signaling pathway is
markedly involved in the development of normal cells as well as
tumorigenesis. In addition, mutation and aberrant activation of the
Wnt signaling pathway has been identified in various types of
cancer and is frequently activated in hepatocarcinoma (19). Activation of Wnt signaling results
in phosphorylation of GSK3β (inactive form), which in turn leads to
transcription of oncogenes by β-catenin translocation and binding
with TCF in the nucleus. Following this, the complex activates the
transcription of growth-promoting genes, including cyclin D and
c-Myc. Previous studies have reported that curcumin represents a
potent anticancer agent. In addition, studies have demonstrated
that Bcl-2, Bax, procaspase-3, PARP, Noxa and PUMA may serve as
predictive markers for the evaluation of apoptosis in various
cancer cells (20,21). Thus, in the present study, curcumin
was hypothesized to suppress cell migration and proliferation by
inhibiting Wnt signaling. Curcumin was found to suppress cell
proliferation in vitro by blocking Wnt signaling and
inhibition of the pathway, which correlated with the suppression of
cell migration. In addition, analysis of the cell cycle and
expression of apoptotic proteins was performed, confirming that
curcumin induced apoptosis. Curcumin was also identified to
suppress the expression of p-mTOR, p-Akt, β-catenin and p-GSK3β
regardless of treatment with inhibitors (LY294002 and BIO).
TPA, a tumor-promoting phorbol ester, enhances
cellular signaling pathways, including PI3K/Akt, PKC and MAPK
(22). TPA also activates the PI3K
pathway and downstream Akt, leading to inhibition of GSK3β. In
addition, TPA-induced isoforms of PKC has been observed to
inactivate GSK3β. This inactivation leads to β-catenin accumulation
and Wnt target gene expression, which are involved in cell
proliferation (23,24). In the present study, migration
triggered by TPA was found to be suppressed by curcumin, confirming
that curcumin inhibits TPA-induced Wnt signaling activation. In
addition, curcumin was observed to suppress tumor growth and
microvessel density by blocking the Wnt signaling pathway in
vivo.
In conclusion, results of the present study indicate
that curcumin induces apoptosis, as confirmed by cell proliferation
assays, analysis of the cell cycle and expression of apoptotic
proteins. Curcumin was also found to inhibit TPA-induced migration
in vitro as well as tumor growth in vivo through
inhibition of the Wnt signaling pathway. Thus, curcumin treatment
may be developed as a novel strategy for the suppression of cell
proliferation and survival in hepatocarcinoma.
Acknowledgements
The present study was supported by a grant from the
National Research Foundation of Korea (KRF-2012-0021402).
References
|
1
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thangapazham RL, Sharma A and Maheshwari
RK: Multiple molecular targets in cancer chemoprevention by
curcumin. AAPS J. 8:443–449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson JJ and Mukhtar H: Curcumin for
chemoprevention of colon cancer. Cancer Lett. 255:170–181. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Radhakrishna Pillai G, Srivastava AS,
Hassanein TI, Chauhan DP and Carrier E: Induction of apoptosis in
human lung cancer cells by curcumin. Cancer Lett. 208:163–170.
2004.PubMed/NCBI
|
|
5
|
Shi M, Cai Q, Yao L, Mao Y, Ming Y and
Ouyang G: Antiproliferation and apoptosis induced by curcumin in
human ovarian cancer cells. Cell Biol Int. 30:221–226. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mehta K, Pantazis P, McQueen T and
Aggarwal BB: Antiproliferative effect of curcumin
(diferuloylmethane) against human breast tumor cell lines.
Anticancer Drugs. 8:470–481. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perry MC, Demeule M, Régina A, Moumdjian R
and Béliveau R: Curcumin inhibits tumor growth and angiogenesis in
glioblastoma xenografts. Mol Nutr Food Res. 54:1192–1201.
2010.PubMed/NCBI
|
|
8
|
Dujic J, Kippenberger S, Ramirez-Bosca A,
Diaz-Alperi J, Bereiter-Hahn J, Kaufmann R, Bernd A and Hofmann M:
Curcumin in combination with visible light inhibits tumor growth in
a xenograft tumor model. Int J Cancer. 124:1422–1428. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stagos D, Amoutzias GD, Matakos A, Spyrou
A, Tsatsakis AM and Kouretas D: Chemoprevention of liver cancer by
plant polyphenols. Food Chem Toxicol. 50:2155–2170. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.
|
|
11
|
Fatima S, Lee NP and Luk JM: Dickkopfs and
Wnt/β-catenin signalling in liver cancer. World J Clin Oncol.
2:311–325. 2011.
|
|
12
|
Esufali S and Bapat B: Cross-talk between
Rac1 GTPase and dysregulated Wnt signaling pathway leads to
cellular redistribution of beta-catenin and TCF/LEF-mediated
transcriptional activation. Oncogene. 23:8260–8271. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Whittaker S, Marais R and Zhu AX: The role
of signaling pathways in the development and treatment of
hepatocellular carcinoma. Oncogene. 29:4989–5005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cadigan KM and Nusse R: Wnt signaling: a
common theme in animal development. Genes Dev. 11:3286–3305. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim D and Chung J: Akt: versatile mediator
of cell survival and beyond. J Biochem Mol Biol. 35:106–115. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nojima H, Tokunaga C, Eguchi S, Oshiro N,
Hidayat S, Yoshino K, Hara K, Tanaka N, Avruch J and Yonezawa K:
The mammalian target of rapamycin (mTOR) partner, raptor, binds the
mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR
signaling (TOS) motif. J Biol Chem. 278:15461–15464. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung EM, Lim JH, Lee TJ, Park JW, Choi KS
and Kwon TK: Curcumin sensitizes tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)-induced apoptosis through
reactive oxygen species-mediated upregulation of death receptor 5
(DR5). Carcinogenesis. 26:1905–1913. 2005. View Article : Google Scholar
|
|
18
|
Yu S, Shen G, Khor TO, Kim JH and Kong AN:
Curcumin inhibits Akt/mammalian target of rapamycin signaling
through protein phosphatase-dependent mechanism. Mol Cancer Ther.
7:2609–2620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
20
|
Chiu TL and Su CC: Curcumin inhibits
proliferation and migration by increasing the Bax to Bcl-2 ratio
and decrasing NF-Bp65 expression in breast cancer MDA-MB-231 cells.
Int J Mol Med. 23:468–475. 2009.PubMed/NCBI
|
|
21
|
Shankar S and Srivastava RK: Involvement
of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and
mitochondrial p53 in curcumin (diferulolylmethane)-induced
apoptosis in prostate cancer. Int J Oncol. 30:905–918. 2007.
|
|
22
|
Murayama K, Kimura T, Tarutani M, Tomooka
M, Hayashi R, Okabe M, Nishida K, Itami S, Katayama I and Nakano T:
Akt activation induces epidermal hyperplasia and proliferation of
epidermal progenitors. Oncogene. 26:4882–4888. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inoki K, Ouyang H, Zhu T, Lindvall C, Wang
Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He
X, MacDougald OA, You M, Williams BO and Guan KL: TSC2 integrates
Wnt and energy signals via a coordinated phosphorylation by AMPK
and GSK3 to regulate cell growth. Cell. 126:955–968. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen RH, Ding WV and McCormick F: Wnt
signaling to beta-catenin involves two interactive components.
Glycogen synthase kinase-3beta inhibition and activation of protein
kinase C. J Biol Chem. 275:17894–17899. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dal Col J and Dolcetti R: GSK-3beta
inhibition: at the crossroad between Akt and mTOR constitutive
activation to enhance cyclin D1 protein stability in mantle cell
lymphoma. Cell Cycle. 7:2813–2816. 2008.PubMed/NCBI
|