Introduction
Acute liver failure (ALF) is a lethal syndrome
characterized by the sudden cessation of hepatic function, systemic
inflammatory response syndrome and multiple organ failure (1,2). The
innate immune system is activated at an early stage and is crucial
in the pathogenesis and the outcome of ALF. Kupffer cells, the
hepatic macrophages, are key effector cells of the innate immune
system within the liver and exert a pivotal ‘scavenger’ role over
gut-derived microbial products in physiological conditions
(3). Kupffer cells may be
activated at the onset of ALF, express a range of sensing receptors
[toll-like receptor (TLR)], release various inflammatory mediators
[tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6)] and
subsequently contribute to the severe inflammatory response in
liver injury (3–5). These observations have suggested a
direct correlation between the functional status of hepatic
macrophages and the severity of ALF.
In previous years, a new hypothesis has emerged
indicating that the functional deactivation of the immune system,
termed ‘immunoparesis’, is associated with immunosuppression and
increased predisposition to infection (6). This immune paralysis is indicative of
a defect in extrahepatic and intrahepatic immune components and
reveals the existence of a counteractive response that protects
against the initial pro-inflammatory process. It has been reported
that the immune function of circulating monocytes is severely
suppressed in patients with acetaminophen-induced ALF (7,8).
However, a few studies have investigated the profiling of
functional status and fate of intrahepatic macrophages. The
mechanisms associated with the role of hepatic macrophages in
refractory immunoparesis during ALF are virtually unexplored.
In the present study, a mouse model of ALF was
established by intravenous injection of concanavalin A (con A),
which commonly serves as an animal model for studying
immune-mediated ALF (9). The
functional status and fate of hepatic macrophages was investigated
by evaluating TLR4 expression and the apoptosis of hepatic
macrophages in mice exposed to con A. In addition, the profile and
degree of inflammatory mediator production with the functional
status of hepatic macrophages in vivo and in vitro
was assessed.
Materials and methods
Animals
Adult C57BL/6 mice (6–8 weeks old, male) were
obtained from the Experimental Animal Center of Chinese Science
Academy (Shanghai, China). The mice were housed in a pathogen-free
environment. All of the procedures were approved by the Scientific
Investigation Board of Zhejiang Medical College (Hangzhou, China),
in accordance with the guidelines for the Care and Use of
Laboratory Animals (Hangzhou, China).
Reagents
Collagenase IV, con A and protease E were purchased
from Sigma-Aldrich (St. Louis, MO, USA). PE-Cy5-conjugated
anti-mouse F4/80 antibodies, Alexa Fluor 488-conjugated anti-mouse
TLR4 antibodies and the isotype control antibodies were from
eBioscience Inc. (San Diego, CA, USA). The Annexin-V Apoptosis
Detection kit was purchased from BD Biosciences (San Diego, CA,
USA). TNF-α, IL-6 and IL-12p40 enzyme-linked immunosorbent assay
(ELISA) kits were purchased from R&D Systems (Minneapolis, MN,
USA).
Preparation of an animal model
ALF was established in mice by intravenous injection
with con A. Mice were exposed to con A (20 mg/kg) for 1, 3, 6, 12
and 24 h. Mice exposed to normal saline served as the 0 h group
(control group).
Assessment of hepatotoxicity
Hepatotoxicity was assessed by histological
examination of haematoxylin and eosin (HE)-stained hepatic tissues
and by detection of serum alanine aminotransferase (ALT) and
aspartate transaminase (AST) levels using an Automatic Chemical
Analyzer 7600-100 (Hitachi, Ltd., Tokyo, Japan). Following exposure
to con A, blood was collected from the retro-orbital sinus in mice.
Hepatic sections were fixed with 10% v/v neutral-buffered formalin,
paraffin-embedded and cut into 3–5 μm slices. Following
deparaffinisation and rehydration, the slices were stained with
H&E. Histological changes in all the specimens were assessed by
two experienced pathologists.
Isolation of liver mononuclear cells and
purification of primary hepatic macrophages
Liver mononuclear cells (MNCs) were isolated by a
sequential pronase/collagenase technique as described by Liu et
al(10). Livers were perfused
with D-Hank's solution (Invitrogen Gibco, Carlsbad, CA, USA) via
the portal vein and then with HBSS (Invitrogen Gibco) containing
0.01% w/v protease E (Sigma-Aldrich) and 0.05% w/v collagenase IV
(Sigma-Aldrich). The homogenate of liver tissue was filtered
through a 200-gauge mesh. Hepatocytes were removed by
centrifugation at 50 × g for 5 min. Supernatant containing MNCs was
isolated by density gradient centrifugation with Percoll (30% v/v
over 60% v/v; Pharmacia, Uppsala, Sweden) at 800 × g for 20 min. In
the in vitro experiments, purification of hepatic
macrophages was performed by the selective adherence method
(11). The non-adherent cells were
removed 4 h later by extensive washing with medium. The cells were
incubated in 24-well plates at a density of 1.0×105
cells/well in DMEM supplemented with 10% v/v FBS, 100 U/ml
penicillin and 100 μg streptomycin at 37ºC in 5% CO2.
The purity of hepatic macrophages bound by F4/80+ antibodies was
~88%. Cell viability measured by the trypan blue exclusion assay
was ≥95%.
Flow cytometry to determine the
expression of TLR4 on F4/80+ hepatic macrophages
Following incubation with Fc-blocker, hepatic MNCs
were incubated with PE-Cy5-conjugated F4/80 antibodies and Alexa
Fluor 488-conjugated TLR4 antibodies at 4ºC in the dark for 15 min
and washed once with PBS. Isotype-matched antibodies were used as
controls to eliminate non-specific binding. The frequency of TLR4+
hepatic macrophages was characterized by gating on F4/80+ cells
using flow cytometric analysis. A minimum of 1×105 cells
was measured by Beckman Coulter flow cytometry (FC500 MPL; Beckman
Coulter, Inc. Brea, CA, USA) and data were analyzed by CXP
software.
Determination of apoptosis of hepatic
macrophages
Hepatic macrophages were identified using the
macrophage marker, F4/80. Hepatic MNCs were stained with
PE-Cy5-conjugated F4/80 antibodies. The apoptotic rates of hepatic
macrophages exposed to con A in vivo and in vitro
were measured using a commercial Annexin-V/PI assay according to
the manufacturer's instructions by flow cytometry.
Evaluation of the cytokine levels in
mouse serum and culture supernatant
Serum and culture supernatant levels of TNF-α, IL-6
and IL-12p40 were quantified by ELISA kits from R&D Systems.
Absorbance was measured at 450 nm using a microtiter plate reader
(Bio-Rad).
Statistical analysis
Data were expressed as mean ± SD. In vitro
experiments were repeated a minimum of three times. SPSS
statistical software 13.0 for Windows was used for data analysis
(SPSS Inc., Chicago, IL, USA). Data were compared using t-tests or
one-way ANOVA, followed by the LSD post-hoc test (equal variances)
or Dunnett's T3 post-hoc test (unequal variances). P<0.05 was
considered to indicate a statistically significant difference.
Results
Con A induces acute liver failure
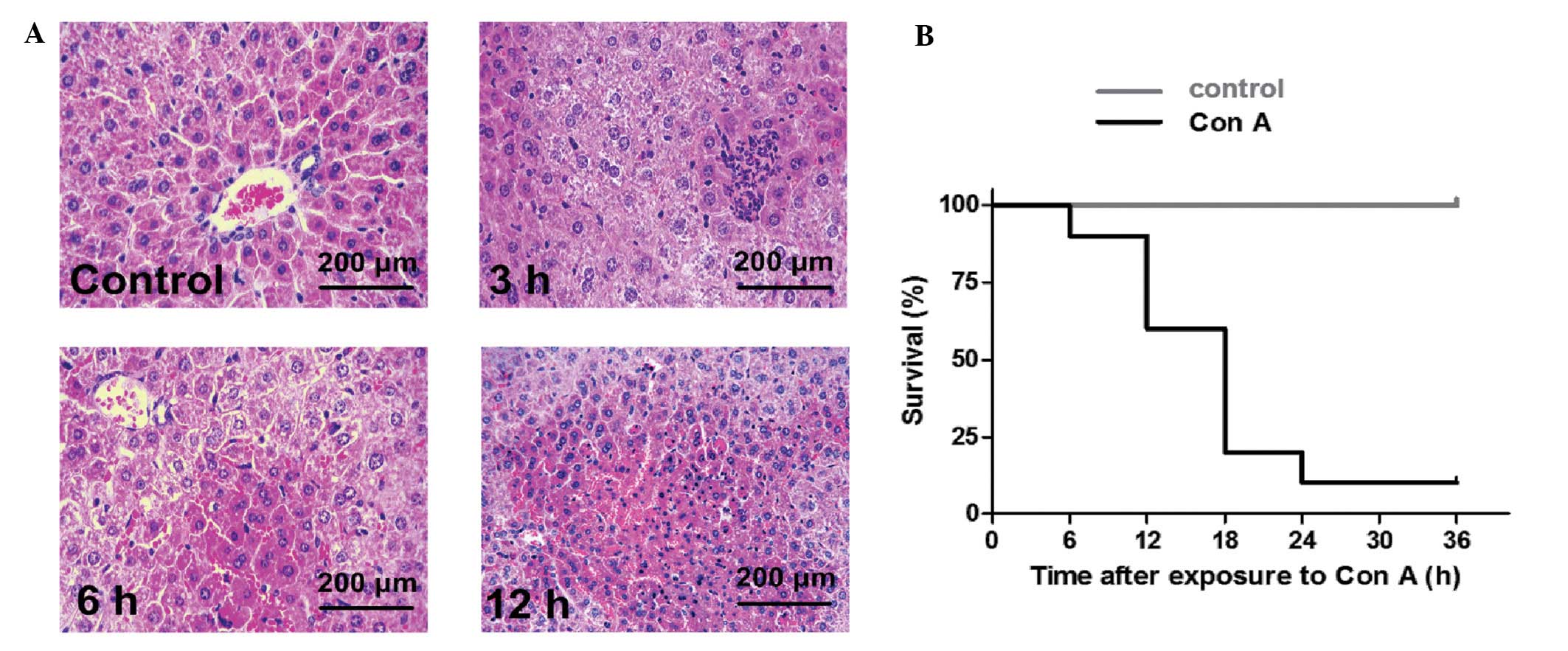
Con A induced ALF in mice with massive
necroinflammatory foci within 12 h and a high mortality within 24 h
(Fig. 1). Table I shows that ALT levels in 3–24 h
groups increased significantly compared with 0 or 1 h groups
(P<0.05) and that there was a time-effect correlation. The AST
levels in the 6–24 h groups were significantly higher than those in
the 0–3 h groups (P<0.05) and there was a significant difference
between the 12 and 24 h groups (P<0.05).
 | Table ILevels of serum ALT and AST
(U/l) in mice
exposed to con A in vivo (mean ± SD). |
Table I
Levels of serum ALT and AST
(U/l) in mice
exposed to con A in vivo (mean ± SD).
| Groups | 0 h | 1 h | 3 h | 6 h | 12 h | 24 h |
|---|
| ALT | 108.0±35.6 | 176.0±57.3 | 751.0±82.2a,b |
4,924.0±1089.8a–c | 11,884±3234.9a–c | 21,786±3840.6a–e |
| AST | 168.0±24.9 | 232.0±68.7 | 528.8±222.4 |
3,946.0±1634.9a,b | 10,086±1622.9a–d | 16,638±2675.0a–e |
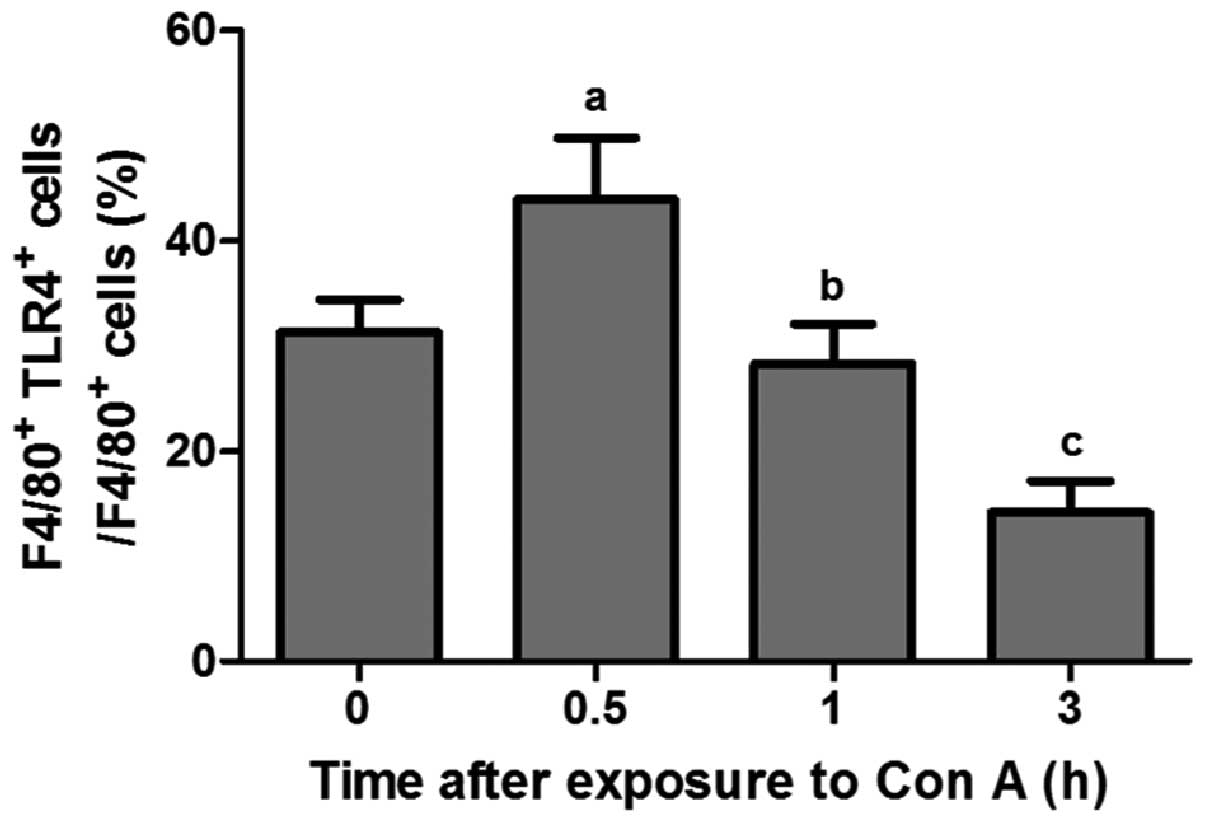
Activated status of hepatic macrophages
in the development of ALF
The activated status of hepatic macrophages was
verified with TLR4 expression which was previously used to assess
macrophage activation (2,4). Fig.
2 shows that the peak of TLR4 levels occurred ~0.5 h following
exposure to Con A, which rapidly decreased at 1 h (P<0.05) and
there was a significant difference between the 1 and 3 h groups
(P<0.05).
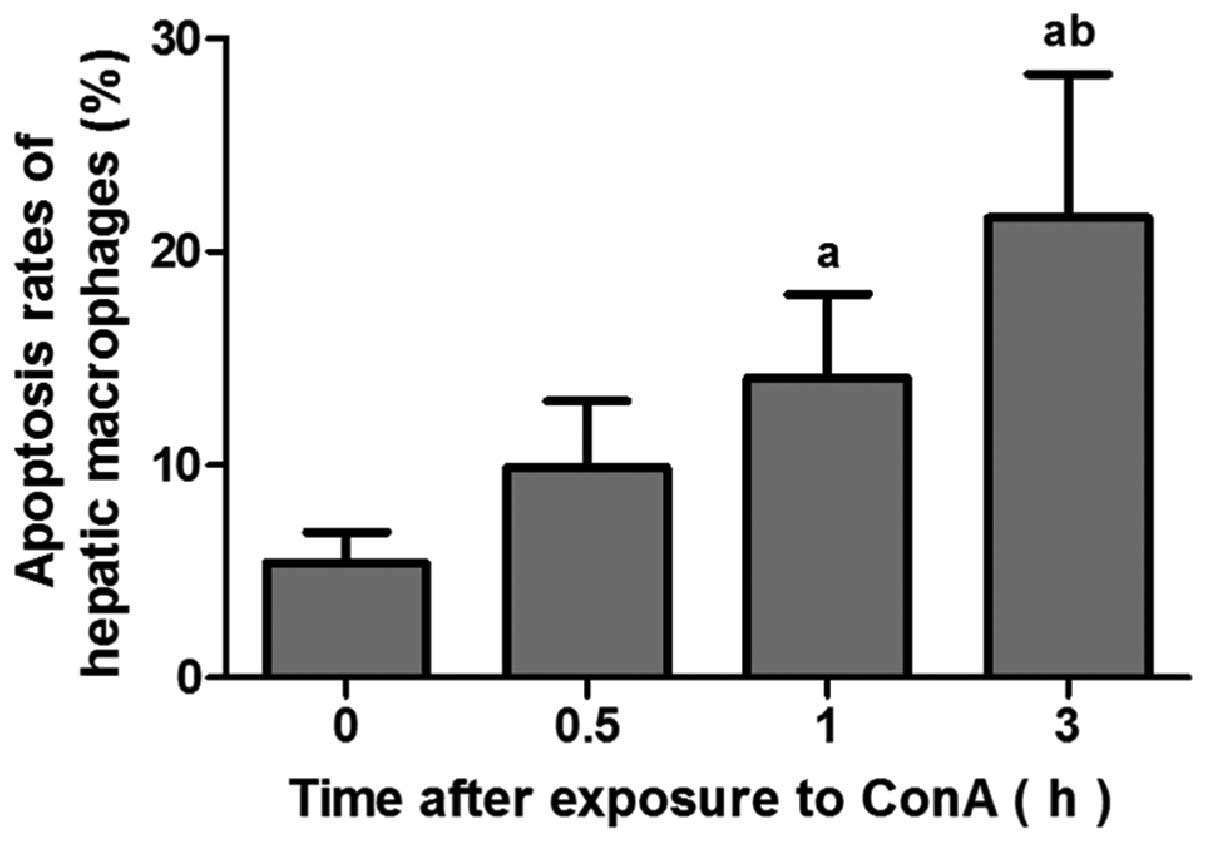
Hepatic macrophages in livers of mice
exposed to con A undergo apoptosis
Fig. 3 shows that
the apoptotic rates of F4/80+ hepatic macrophages in the 1 and 3 h
groups were significantly higher than that in the 0 h group
(P<0.05) and there was a significant difference between the 0.5
and 3 h groups (P<0.05).
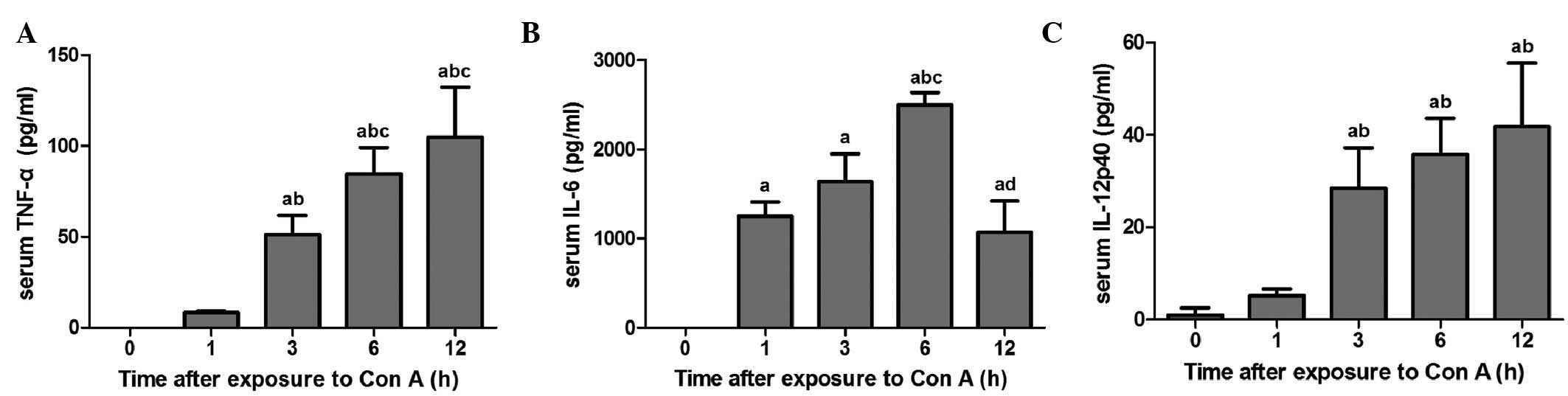
Levels of serum cytokine in mice exposed
to con A
Fig. 4 demonstrates
that TNF-α levels in the 3–12 h groups increased significantly when
compared with the 0 and 1 h groups (P<0.05). In addition, TNF-α
levels in 6–12 h groups were significantly higher than those in the
3 h group (P<0.05). IL-6 levels in all the exposure groups
increased significantly compared with the 0 h group (P<0.05).
IL-6 serum levels peaked at 6 h following con A exposure and
significant differences were identified between the 6 and 12 h
groups (P<0.05). The IL-12p40 levels in the 3–12 h groups also
increased significantly, when compared with the 0–1 h groups
(P<0.05).
Hepatic marophages undergo apoptosis
following exposure to con A in vitro
When the exposure doses were higher than 20 μg/ml
for 3 h, 5 μg/ml for 6 h and 10 μg/ml for 12 or 24 h, the early
apoptotic rates of exposure groups were significantly higher than
those of the control groups (P<0.05) and increased in a
dose-dependent manner. When the exposure doses were higher than 20
μg/ml for 3 or 6 h or 10 μg/ml for 12 or 24 h, the proportion of
late apoptotic and necrotic cells of exposure groups was
significantly higher than those of the control groups (P<0.05)
and increased in a dose-dependent manner (Table II). These observations indicate
that the early and late apoptotic or necrotic rates of hepatic
macrophages increased in a con A dose-dependent manner and
apoptosis of hepatic macrophages exposed to con A may be involved
in con A-induced ALF.
 | Table IIApoptosis rates (%) of hepatic
macrophages exposed to Con A at various doses and times in
vitro (mean ± SD). |
Table II
Apoptosis rates (%) of hepatic
macrophages exposed to Con A at various doses and times in
vitro (mean ± SD).
| Dose (μg/ml) | Proportion of early
apoptotic cells (%) | Proportion of late
apoptotic and necrotic cells (%) |
|---|
|
|
|---|
| 3 h | 6 h | 12 h | 24 h | 3 h | 6 h | 12 h | 24 h |
|---|
| 0 | 3.27±2.10 | 7.17±1.45 | 5.33±2.00 | 7.42±4.40 | 1.08±0.34 | 0.76±0.40 | 1.14±0.55 | 1.90±1.72 |
| 5 | 6.10±2.51 | 13.39±1.20a | 14.41±4.67 | 13.27±4.28 | 1.94±1.23 | 2.95±1.94 | 3.58±1.45 | 6.52±2.35 |
| 10 | 8.40±2.76 | 19.72±2.16a,b | 23.72±2.78a | 21.13±3.05a,b | 2.33±1.84 | 3.39±2.02 | 7.74±2.67a,b | 11.02±2.92a |
| 20 | 10.86±2.25a | 25.41±1.18a,b | 28.51±9.88a,b | 24.09±5.08a,b | 3.34±0.83a | 4.20±0.50a | 8.98±2.17a,b | 11.70±3.41a,b |
| 40 | 21.13±5.25a–d | 36.70±6.99a–d | 33.52±7.28a,b | 34.50±4.47a–d | 5.89±0.41a–d | 7.39±1.63a–d | 12.33±3 27a–c | 11.84±3.22a,b |
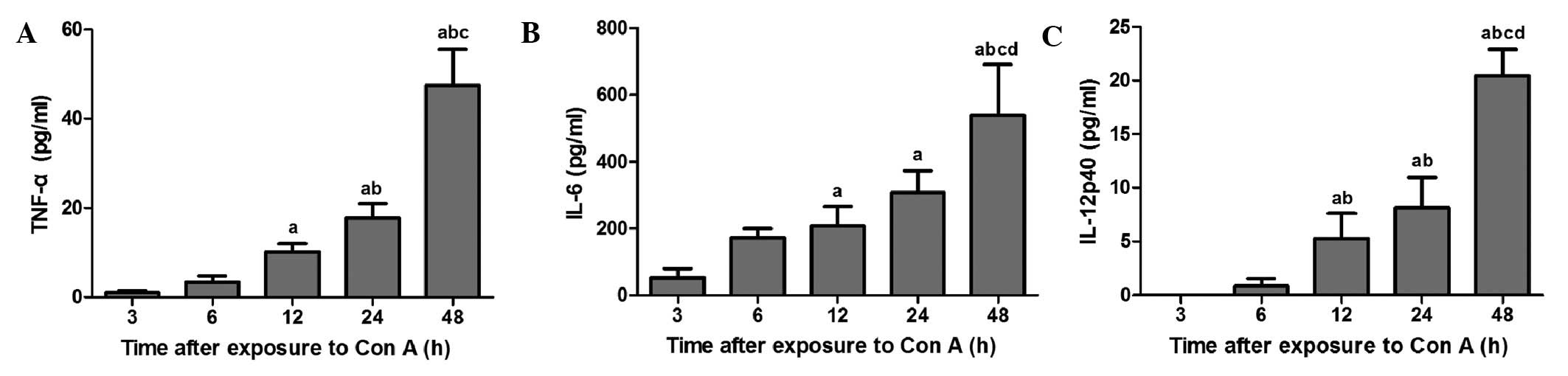
Hepatic macrophages release cytokines
following exposure to con A
Fig. 5 shows the
levels of the cytokines, TNF-α, IL-6 and IL-12p40, in hepatic
macrophage culture exposed to con A (10 μg/ml). TNF-α levels in
hepatic macrophage culture exposed to con A for 12, 24 and 48 h
were significantly higher than those in the 3 h group (P<0.05).
Maximum levels of TNF-α were observed at 48 h following exposure of
hepatic macrophages to con A. IL-6 levels in the 12–48 h groups
increased significantly compared with the 3 h group (P<0.05).
IL-6 levels in the 48 h group were significantly higher than those
in the 6–24 h groups (P<0.05). IL-12p40 levels in the 3–6 h
group were significantly lower compared with those in the 12–48 h
groups (P<0.05). Levels of IL-12p40 at 48 h following exposure
of hepatic macrophages to con A were the highest of all the time
points (P<0.05).
Discussion
The massive inflammatory infiltration of hepatic
macrophages in the initial phase of ALF emphasizes the important
role of these cells in the pathogenesis of ALF (3,4). It
has been hypothesized that macrophages are activated by various
stimuli through TLR expression to initiate the pro-inflammatory
response, promoting tissue injury. TLR4 has been used to identify
macrophage activation (2,4,12).
The present study has confirmed that the expression of TLR4 in
hepatic macrophages increased in the initial phase (0.5 h) of con
A-induced ALF. However, it was also observed that TLR4 expression
in hepatic macrophages decreased rapidly at 1 h following con A
exposure. These observations indicate that the initial activated
status of hepatic macrophages is followed by subsequent
immunosuppression during the development of ALF.
Previous studies have demonstrated that dysfunction
of monocytes has been observed in patients with acute-on-chronic
liver failure and the apoptosis of hepatic macrophages is involved
in liver inflammation and fibrosis (7,10,13).
However, the apoptosis of hepatic macrophages in ALF has been
largely ignored and is rarely reported. To determine the fate of
the deactivated hepatic macrophages, apoptosis of hepatic
macrophages was further investigated. In vivo experiments
revealed that the apoptotic rates of hepatic macrophages and the
levels of the macrophage-related pro-inflammatory cytokines, TNF-α,
IL-6 and IL-12p40, in serum significantly increased with con A
exposure time. Apoptosis associated with TLR4 expression profiling
in hepatic macrophages indicates the complexity between cell
activation and apoptosis. Fig. 1
and Table I reveal the marked
necrotic foci and enhanced serum levels of ALT and AST in mice
exposed to con A at ~6–24 h, whereas the functional switch and
apoptosis of hepatic macrophages was observed at 0.5–1 h following
con A exposure. Functional macrophage deactivation and apoptosis of
hepatic macrophages occurred prior to biochemical and pathological
changes, which further indicated that apoptosis of hepatic
macrophages is important in the subsequent development of
hepatitis.
ALF is characterized by sudden and persistent
cellular apoptosis. When a large amount of the early apoptotic
cells may not be scavenged and eliminated immediately, late
apoptotic and necrotic cells inevitably occur, which may passively
release inflammatory mediators further and lead to the exacerbation
of liver damage (14). Thus, it is
necessary to observe whether apoptotic hepatic macrophages may also
release pro-inflammatory cytokines. Table II shows that con A induces early
cell apoptosis, late apoptosis or necrosis. In addition, in
vitro experiments in the present study revealed that apoptotic
or necrotic hepatic macrophages also release a range of
pro-inflammatory cytokines, TNF-α, IL-6 and IL-12p40, which are
associated with hepatocyte injury, respiratory burst and adaptive
immune response (15–19), suggesting a link between
inflammation, cell apoptosis and immunoparesis in the development
of ALF. However, the kinetic changes of the three cytokine levels
in supernatants were slightly different from those in the serum of
mice exposed to con A. It has been hypothesized that the serum
cytokines were not only released from the apoptotic hepatic
macrophages but also from other cells (9,20–22).
These observations indicate that pro-inflammatory cytokines, TNF-α,
IL-6 and IL-12p40, released by apoptotic or necrotic hepatic
macrophages may contribute to a further pathophysiological response
in con A-induced ALF.
In conclusion, the present in vivo studies
measuring the expression of TLR4 on hepatic macrophages and the
apoptosis of hepatic macrophages, in combination with the ex
vivo experiments, demonstrate that deactivation and apoptosis
of hepatic macrophages is involved in the pathogenesis of con
A-induced ALF. These observations are likely to improve our
understanding of the essential roles of hepatic macrophages in the
progression of ALF, as well as explain the potential leakage among
inflammation, apoptosis and immunoparalysis in ALF and emphasize
the requirement for the development of therapeutic manipulations to
inhibit overactivated hepatic macrophages and apoptosis for the
treatment of ALF.
Acknowledgements
The present study was supported by the 12-5 State S
and T Projects of China (no. 2012ZX10002007) and the National
Natural Science Foundation of China (no. 81000730).
References
|
1
|
Rolando N, Wade J, Davalos M, Wendon J,
Philpott-Howard J and Williams R: The systemic inflammatory
response syndrome in acute liver failure. Hepatology. 32:734–739.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verbeke L, Nevens F and Laleman W:
Bench-to-beside review: acute-on-chronic liver failure - linking
the gut, liver and systemic circulation. Crit Care. 15:2332011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Q, Shi Y, He J and Chen Z: The
evolving story of macrophages in acute liver failure. Immunol Lett.
147:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laskin DL: Macrophages and inflammatory
mediators in chemical toxicity: a battle of forces. Chem Res
Toxicol. 22:1376–1385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zimmermann HW, Trautwein C and Tacke F:
Functional role of monocytes and macrophages for the inflammatory
response in acute liver injury. Front Physiol. 3:562012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wasmuth HE, Kunz D, Yagmur E, et al:
Patients with acute on chronic liver failure display ‘sepsis-like’
immune paralysis. J Hepatol. 42:195–201. 2005.
|
|
7
|
Antoniades CG, Berry PA, Davies ET,
Hussain M, Bernal W, Vergani D and Wendon J: Reduced monocyte
HLA-DR expression: a novel biomarker of disease severity and
outcome in acetaminophen-induced acute liver failure. Hepatology.
44:34–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Antoniades CG, Berry PA, Wendon JA and
Vergani D: The importance of immune dysfunction in determining
outcome in acute liver failure. J Hepatol. 49:845–861. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tiegs G, Hentschel J and Wendel A: A T
cell-dependent experimental liver injury in mice inducible by
concanavalin A. J Clin Invest. 90:196–203. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu C, Tao Q, Sun M, et al: Kupffer cells
are associated with apoptosis, inflammation and fibrotic effects in
hepatic fibrosis in rats. Lab Invest. 90:1805–1816. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Canbay A, Feldstein AE, Higuchi H,
Werneburg N, Grambihler A, Bronk SF and Gores GJ: Kupffer cell
engulfment of apoptotic bodies stimulates death ligand and cytokine
expression. Hepatology. 38:1188–1198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaisho T and Akira S: Toll-like receptors
and their signaling mechanism in innate immunity. Acta Odontol
Scand. 59:124–130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xing T, Li L, Cao H and Huang J: Altered
immune function of monocytes in different stages of patients with
acute on chronic liver failure. Clin Exp Immunol. 147:184–188.
2007.PubMed/NCBI
|
|
14
|
Savill J, Dransfield I, Gregory C and
Haslett C: A blast from the past: clearance of apoptotic cells
regulates immune responses. Nat Rev Immunol. 2:965–975. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Streetz K, Leifeld L, Grundmann D, et al:
Tumor necrosis factor alpha in the pathogenesis of human and murine
fulminant hepatic failure. Gastroenterology. 119:446–460. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chastre A, Bélanger M, Beauchesne E,
Nguyen BN, Desjardins P and Butterworth RF: Inflammatory cascades
driven by tumor necrosis factor-alpha play a major role in the
progression of acute liver failure and its neurological
complications. PLoS One. 7:e496702012. View Article : Google Scholar
|
|
17
|
Bettelli E, Carrier Y, Gao W, et al:
Reciprocal developmental pathways for the generation of pathogenic
effector TH17 and regulatory T cells. Nature. 441:235–238. 2006.
View Article : Google Scholar
|
|
18
|
Sekiyama KD, Yoshiba M and Thomson AW:
Circulating proinflammatory cytokines (IL-1 beta, TNF-alpha and
IL-6) and IL-1 receptor antagonist (IL-1Ra) in fulminant hepatic
failure and acute hepatitis. Clin Exp Immunol. 98:71–77. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cooper AM and Khader SA: IL-12p40: an
inherently agonistic cytokine. Trends Immunol. 28:33–38. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knolle PA, Gerken G, Loser E, et al: Role
of sinusoidal endothelial cells of the liver in concanavalin
A-induced hepatic injury in mice. Hepatology. 24:824–829. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gantner F, Leist M, Küsters S, Vogt K,
Volk HD and Tiegs G: T cell stimulus-induced crosstalk between
lymphocytes and liver macrophages results in augmented cytokine
release. Exp Cell Res. 229:137–146. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hatada S, Ohta T, Shiratsuchi Y, Hatano M
and Kobayashi Y: A novel accessory role of neutrophils in
concanavalin A-induced hepatitis. Cell Immunol. 233:23–29. 2005.
View Article : Google Scholar : PubMed/NCBI
|