Introduction
The vertebrate multichambered heart is the first
organ to form during development (1). The zebrafish offers several distinct
advantages as a genetic and embryonic model system to study
cardiovascular disease. Zebrafish mutations have revealed specific
genetic requirements for cardiac fate determination, migration,
fusion, tube assembly, looping and remodeling. These processes
maintain functional integrity and cardiac function may affect
aspects of cardiac morphogenesis (2). In particular, the spatial and
temporal coordination is essential for the heart to ensure proper
cardiac function throughout development. Therefore, the
investigation of the genetic regulation of cardiogenesis is popular
among developmental biologists using a variety of model organisms
from flies to zebrafish.
Fatty acid binding protein 3 (FABP3), also termed
heart-type fatty acid binding protein, may be essential in fatty
acid transport, cell growth, cellular signaling and gene
transcription (3,4). FABP3 is upregulated during the
terminal differentiation of mouse cardiomyocytes (5). In our previous studies, FABP3 was
differentially expressed between ventricular septum defect
myocardium and normal ventricular septum myocardium (6). In addition, the inhibition of FABP3
expression promoted cell apoptosis and led to mitochondrial
dysfunction in P19 cells (embryonic murine carcinoma cells)
(7). However, the precise role of
FABP3 in cardiac development remains to be elucidated.
Cardiac development is controlled by evolutionarily
conserved transcription factors which connect signaling pathways
with genes for muscle growth, patterning and contractility, which
involves a series of molecular and morphogenetic events. Cardiac
lineage commitment occurs between the first and second stages of
development depending on the expression of cardiac transcription
factors, such as Nkx2.5, GATA4, MEF2C and dHAND, in which Nkx2.5 is
involved in embryo heart development and stem cell cardiomyogenesis
(8). The Wnt receptor signaling
pathway is also known to be involved in every aspect of embryonic
development, including cell-fate specification, proliferation,
survival, migration and adhesion, particularly in cardiac
development and differentiation (9,10).
Furthermore, apoptosis is an essential event in
vertebrate organ formation and the development process. Apoptosis
participates in the fundamental aspects of morphology development,
such as endocardia, epicardia and neural crest formation, and is
also essential in embryonic development and adult tissue
homeostasis (11,12). In addition, gain and loss of
function studies demonstrated that apoptosis may result in
congenital heart defects (12,13).
It has been determined that mitochondria are involved in apoptosis
by inducing a variety of pro-apoptotic conditions, including the
release of caspase activators, altered cellular oxidation-reduction
and participation of pro- and anti-apoptotic Bcl-2 family proteins
(14).
In the present study, zebrafish were used as a model
to investigate the involvement of FABP3 in cardiac development and
to determine the effects of FABP3 knockdown on cell apoptosis and
mitochondrion function in vivo. In addition, the gene
expression profile of the Wnt signaling pathway and Nkx2.5 were
analyzed to determine whether FABP3 knockdown exhibits an effect on
this pathway in zebrafish cardiac development.
Materials and methods
Embryo maintenance and
transplantation
Wild type (WT) zebrafish stocks were obtained from
the Model Animal Research Center of Nanjing University (Nanjing,
Jiangsu, China). Embryos were obtained from natural spawning of
wild type adults and were grown at 28.5°C in embryo medium which
was changed twice per day, as previously described (15). Morphological features were used to
determine the embryonic developmental stage, as described by Kimmel
et al(16). Embryos older
than 24 h post-fertilization (pf) were incubated in 0.003%
phenylthiourea to inhibit pigment formation.
Morpholinos (MOs) and microinjection
MOs were purchased from Gene Tools (Philomath, OR,
USA). The FABP3-MO was designed against the ATG start site of FABP3
to block its translation (FABP3-MO; 5′-ACG TGC CGA TAA AAG CGT CTG
CCA T-3′). A standard control MO (Wt-MO; 5′-CCT CTT ACC TCA GTT ACA
ATT TAT A-3′) served as a negative control (17). MO oligomers were dissolved in 0.3X
2 mmol/l Danieaus’s solution [58 mmol/l NaCl, 0.7 mmol/l KCl, 0.4
mmol/l MgSO4, 0.6 mmol/l Ca(NO3)2,
5.0 mmol/l N-(2-hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid)
(HEPES), pH 7.6] (18). The
oligomers were diluted to their final concentration in 0.2 mol/l
KCl and 0.5% (w/v) phenol red (Sigma-Aldrich, St. Louis, MO, USA).
Injection of 5.0 ng MO into single to four-cell stage zebrafish
embryos using back-filled fine borosilicate glass capillary needles
(Corning Inc., Corning, NY, USA) was performed as previously
described (18). Following MO
injection, embryos were incubated at 28.5°C for up to 3 days
pf.
Measurements of heart development
Following microinjection of MOs into zebrafish
embryos, the embryos were incubated at 28.5°C for up to 4 days pf.
In order to determine the effect of time on FABP3-MO-related
morphological abnormalities of the heart, histological sections
were used to identify heart-specific phenotypes. Larvae were
anesthetized with 0.05% tricaine (3-aminobenzoic acid ethylester)
and fixed in 4% paraformaldehyde in 0.1 M Sorenson’s buffer (pH
7.4). Larvae were washed in 0.01 M phosphate-buffered saline (PBS;
pH 7.2), dehydrated in a graded series of ethanol, infiltrated and
embedded in blocks. Each block was serially transverse-sectioned at
5 μm. Sections were hydrated, stained with Harris’ hematoxylin and
eosin Y, dried and mounted in neutral balsam. For larvae within the
block, measurements were made for the heart when the central
portion of the heart was visible. In each group, six larvae were
assessed for heart layer thickness and cell density at 96 h pf (4
days pf). All other slides of the selected heart were imaged at a
final magnification of ×1000 with a Pixera Pro 600ES digital camera
(Los Gatos, CA, USA) attached to a JD-801 microscope (Olympus,
Tokyo, Japan).
Apoptosis assay
Following injection of 5.0 ng MO into single to
four-cell stage zebrafish embryos, zebrafish were harvested using
collagenase I (Sigma-Aldrich) at 24 and 48 h pf, and washed with
PBS. The single-cell suspensions of zebrafish were centrifuged,
resuspended in 1 ml binding buffer and stained with 10 μl Annexin
V-fluorescein isothiocyanate (FITC; BioVision, Mountain View, CA,
USA) and 10 μl propidium iodide at room temperature for 15 min. The
stained cells were analyzed immediately using flow cytometry as
described previously (19).
ATP production assay
Zebrafish larvae were digested with collagenase I
(Sigma-Aldrich) to obtain single-cell suspensions. The ATP content
of the zebrafish was measured using a luciferase-based luminescence
assay kit (Biyuntian, Nantong, Jiangsu, China). Briefly, the
single-cell suspensions of zebrafish were homogenized in ice-cold
ATP-releasing buffer and light emission was recorded for 30 sec
using a photon-counting luminometer (Turner Biosystems, Sunnyvale,
CA, USA) pf at 24 and 48 h pf. The relative ATP level was
normalized to the protein concentration of each sample, as
determined by a bicinchoninic acid assay.
Reactive oxygen species assay
Intracellular reactive oxygen species (ROS)
generation was determined using
6-carboxy-2,7-dichlorodihydrofluorescein diacetate
(H2-DCFDA; Sigma-Aldrich), as previously described
(20). Briefly, following
injection with 5.0 ng MO into single to four-cell stage zebrafish
embryos, the embryos were collected pf at 24 and 48 h pf. The
digested single-cell suspensions of zebrafish were washed and
incubated with H2-DCFDA for 20 min. Subsequent to this,
the suspensions were washed several times and harvested in PBS. The
fluorescence of H2-DCFDA was detected using a
fluorescence-activated cell sorter (FACS; excitation, 488 nm;
emission, 530 nm).
qPCR for the assessment of mitochondrial
DNA (mtDNA) concentration
The relative quantities of mtDNA were determined by
qPCR, which was performed using an Applied Biosystems 7500 Sequence
Detection system (ABI 7500 SDS; Applied Biosystems, Foster City,
CA, USA) according to the manufacturer’s instructions (21). In brief, DNA was isolated from the
zebrafish following microinjection 24, 48, 72 h pf using the
Wizard® Genomic DNA purification kit DNA extraction kit
(Promega, Madison, WI, USA) and quantified using a NanoDrop 2.0
spectrophotometer (Thermo Scientific, Foster City, CA, USA). Two
primer sets (listed in Table I)
were used for PCR analysis. A 125 bp mtDNA fragment within the
cytochrome b (CYTB) gene was used for the quantification of
mtDNA. We previously cloned the PCR product into the plasmid
pMD18-T and verified it by DNA sequencing. Plasmid standards of
known copy number were used to generate a log-linear standard
curve, from which the CYTB copy number was determined in the
samples by qPCR. The results were normalized to a 168 bp region of
the nuclear gene for 28S rRNA using a standard curve generated from
a plasmid containing the 28S fragment. The ratio of mtDNA to
nuclear DNA was determined to reflect the concentration of
mitochondrial DNA per cell.
 | Table ISequences for primer sets used in
qPCR gene expression analysis. |
Table I
Sequences for primer sets used in
qPCR gene expression analysis.
| Gene name | Sense primer
(5′-3′) | Antisense primer
(5′-3′) |
|---|
| CYTB |
TATTCGCATACGCCATTC |
TGCTATTCCTCGCTGTTT |
| 28s |
ATATGCGTCCGTCCCACTT |
ATCGCCACCTTGACTTCG |
| Wnt1 |
GCTGAGAGGGAGGTGACAA |
TACAAAGGCAGGAGGGAATG |
| Wnt5a |
TACGCCTTCTTCAAGCATCC |
CTCTTTGCGCTTTTCTGTCC |
| Wnt11 |
GTAAGTGCCATGGGGTGTCT |
GCTTCCAAGTGAAGGCAAAG |
| Nkx2.5 |
GCTTTTACGCGAAGAACTTCC |
GATCTTCACCTGTGTGGAGG |
| β-actin |
CAACAGAGAGAAGATGACACAGATCA |
GTCACACCATCACCAGAGTCCATCAC |
qPCR for the assessment of Nkx2.5 and
molecules of the Wnt signalling pathway
Total RNA was extracted from zebrafish using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), and the
extracted RNA was quantified using a NanoDrop 2.0 spectrophotometer
(Thermo Scientific). Complimentary DNA was synthesized from 2 μg
total RNA by using an AMV Reverse Transcriptase kit (Promega A3500;
Promega, Madison, WI, USA) according to the manufacturer’s
instructions. qPCR was performed using SYBR-Green PCR Master mix
(Applied Biosystems) on an Applied Biosystems 7500 Sequence
Detection System (ABI 7500 SDS). qPCR reaction conditions were 95°C
for 10 min followed by 40 cycles at 94°C for 20 sec, 60°C for 20
sec and 72°C for 30 sec with a final extension of 7 min at 72°C.
NK2 homeobox 5 (Nkx2.5), Wnt1, Wnt5a and Wnt11 expression was
normalized against β-actin using the comparative CT method to
determine the relative changes in mRNA expression in the target
sample. The sequences of the primers used for each gene are shown
in Table I.
Statistical analysis
All data are presented as the mean ± standard
deviation of three independent experiments. Statistical
significance was assessed using the independent-samples t-test
using the statistical software package SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
FABP3-MO impairs heart development
To investigate the function of FABP3 in the
establishment of cardiac development, a loss-of-function study was
performed using a previously characterized anti-FABP3 (ATG) MO (in
our previous original study: knockdown of FABP3 impaired cardiac
development in zebrafish through the retinoic acid signaling
pathway). The MO oligonucleotides were injected into single to
four-cell stage zebrafish embryos directly and is effective up to
3–4 days pf (18). Thus, in the
present study, embryos were injected with 0.5 ng MO targeting FABP3
translation. Cardiac development is complete at ~96 h pf (4 days
pf) (22), and the thickness of
the heart is normally ~30–40 μm at 4 days pf. By 4 days pf, we
observed defective phenotypes of embryos resulting from FABP3-MO by
using a series of continuous section slides by tissue cutting
(thickness, ~5 μm). Compared with the Wt-MO group, transverse
sections of the heart of zebrafish larvae at 4 days pf displayed
defective phenotypes, including a thin atrial-ventricular wall, a
change in the relative position of the atrium and ventricle, an
expansion of the diameter of the atrioventricular cavity and an
alteration in the morphology of the bulbus arteriosus (Fig. 1). In addition to these defects,
pericardial edema, developmental delay and incomplete looping of
the developing heart were also observed in FABP-MO zebrafish as we
demonstrated previously (23).
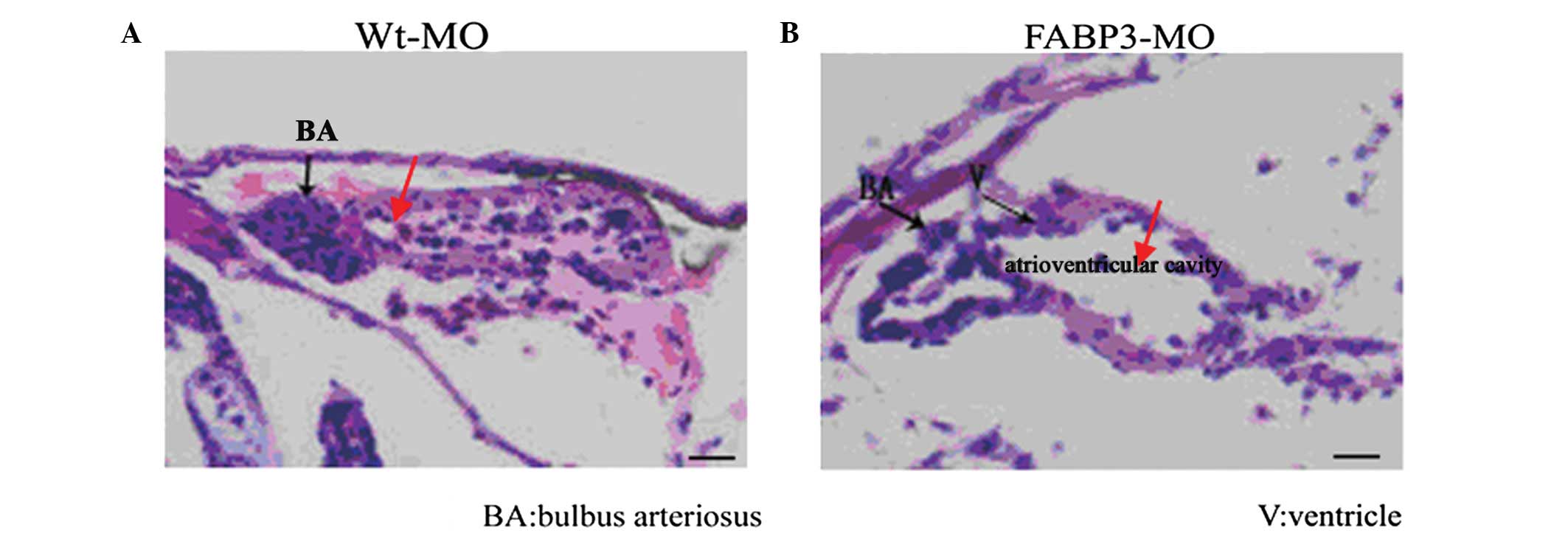 | Figure 1Histological sections of developed
heart 4 days pf. (A and B) Wt-MO and FABP3-MO are represented
respectively. Compared with the Wt-MO group, embryos injected with
0.5 ng FABP3-MO following 4 days pf displayed defective phenotypes,
including a thin atrial-ventricular wall, a change in the relative
position of the atrium and V, an expansion of the diameter of the
atrioventricular cavity and an alteration in the morphology of the
BA. Scale bar, 20 μm. BA, bulbus arteriosus; V, ventricle; pf,
post-fertilization; Wt-MO, wild type-morpholinos; FABP3-MO, fatty
acid binding protein 3. |
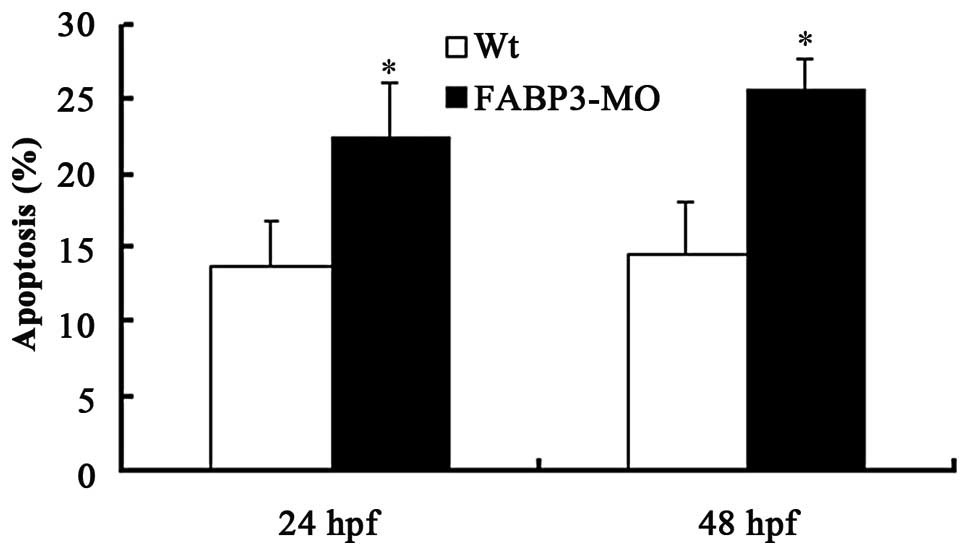
FABP3-MO promotes zebrafish cell
apoptosis
To study the effect of FABP3-MO on zebrafish cell
apoptosis, the zebrafish larvae were harvested at 24 and 48 h pf
using collagenase I. The single-cell suspensions of zebrafish
larvae were quantified by flow cytometric analysis subsequent to
staining with Annexin V-FITC. The results demonstrated that
FABP3-MO promoted zebrafish cell apoptosis compared with Wt-MO
(Fig. 2;
*P<0.05).
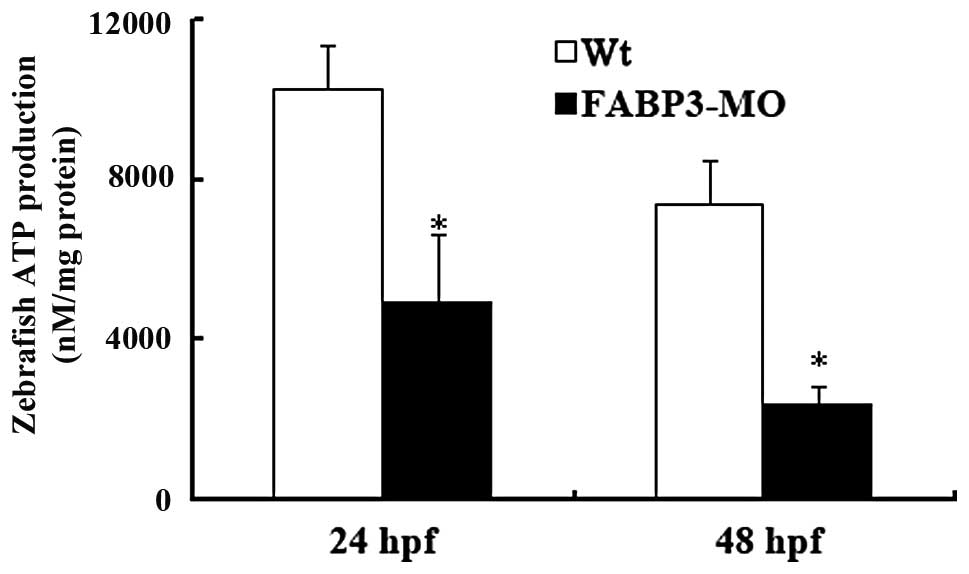
Effects of FABP-MO on cellular ATP
production in zebrafish
To further investigate whether FABP3 knockdown in
zebrafish leads to impaired mitochondrion function, the ATP content
was assayed in the FABP3-MO and Wt-MO groups in zebrafish at 24 and
48 h pf. As shown in Fig. 3, the
zebrafish ATP content in the FABP3-MO group was significantly
decreased compared with the Wt-MO group at 24 and 48 h pf
(*P<0.05). The results showed that FABP3 knockdown in
zebrafish decreased the total cellular ATP production.
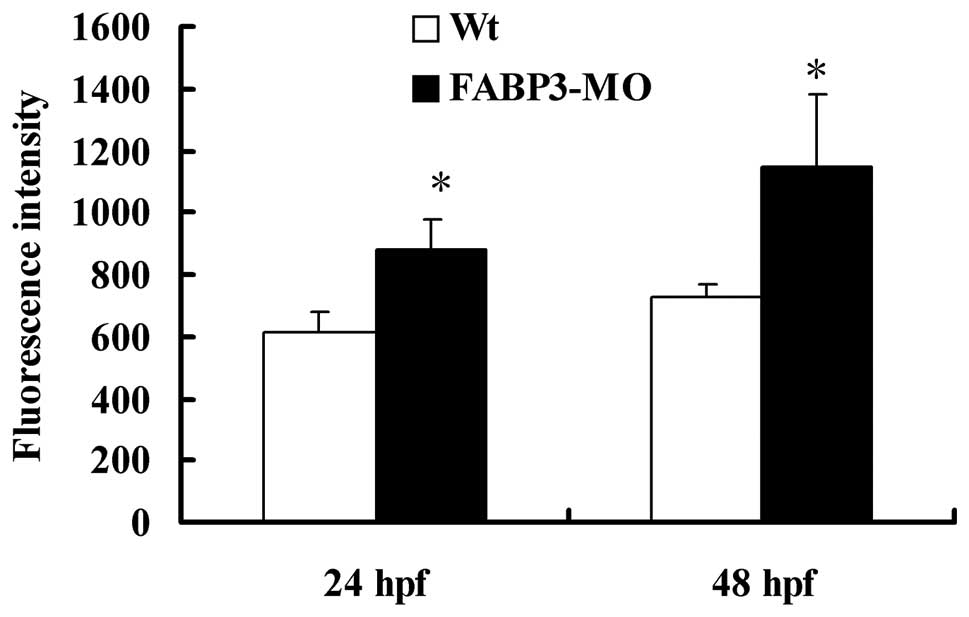
FABP3-MO affects zebrafish intracellular
ROS levels
ROS, a by-product of the electron transport chain,
is predominantly produced by mitochondria (24). As shown in Fig. 4, in contrast with Wt-MO injected
zebrafish, embryos injected with FABP3-MO at 24 and 48 h pf induced
a significantly increased concentration of intracellular ROS
levels, as indicated by increased fluorescence in the presence of
the compound DCFDA (2′,7′-dichlorofluorescein diacetate;
*P<0.05). This result further demonstrated that
FABP3-MO impaired mitochondrion function in zebrafish.
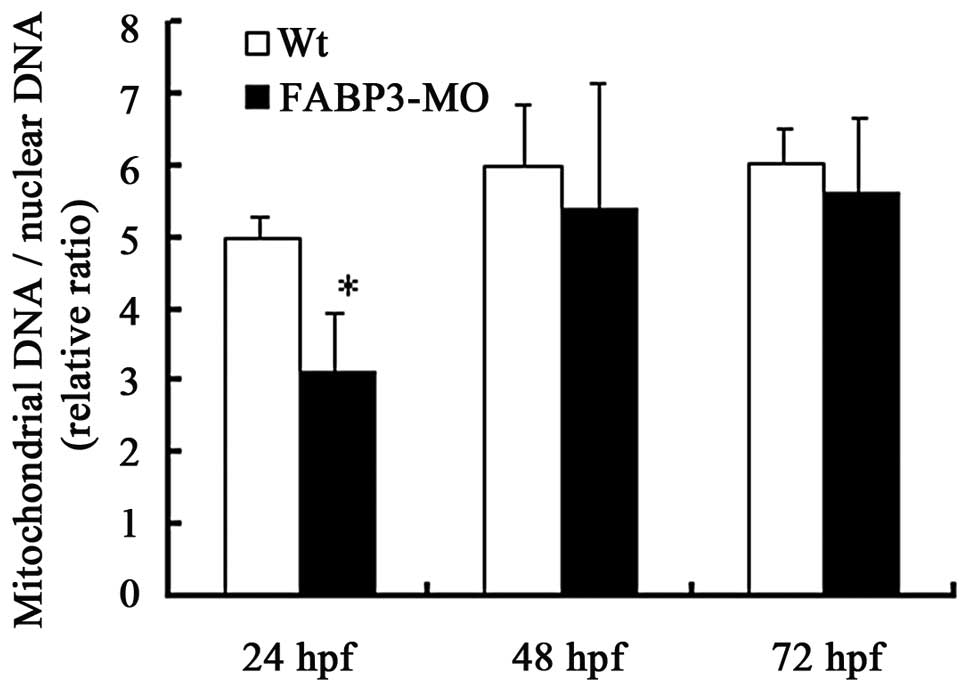
Effects of FABP3-MO on mtDNA copy number
in zebrafish
In addition to the ATP content and ROS production
detection of FABP3-MO injected zebrafish, the mitochondrial DNA
copy number is regarded to indicate the cellular mitochondrion
number and may be a possible marker of mitochondrion function. We
measured mitochondrial and genomic DNA in FABP3-MO and Wt-MO
injected embryos using qPCR. The results demonstrated that the
mtDNA copy number was decreased in the FABP3-MO group compared with
the Wt-MO group at three time points, in particular, the mtDNA copy
number was significantly decreased at 24 h pf in the FABP-3MO group
(Fig. 5; *P<0.05).
However, there was no significant difference in mtDNA copy number
at 48 and 72 h pf between the two groups (Fig. 5; P>0.05).
Alteration of expression patterns of
Nkx2.5 and Wnt signaling molecules in FABP-MO zebrafish
To further investigate the effect of FABP3-MO on
cardiac development, the expression level of the cardiac
development-specific gene, Nkx2.5 (the earliest known marker of
vertebrate heart development), was determined (25). Notably, as shown in Fig. 6A, qPCR results demonstrated that
Nkx2.5 was upregulated in zebrafish embryos injected with FABP3-MO
compared with those injected with Wt-MO.
In addition, the mechanism through which FABP3
knockdown may affect cardiac development was investigated by
focusing on the Wnt signaling pathway. Wnt signals exhibit
developmental stage-specific and biphasic involvement in
cardiomyogenesis and are also involved in heart disease (26–28).
Wnt signaling consists of the canonical (Wnt1) and non-canonical
(Wnt5 and Wnt11) Wnt signaling pathways. Thus, Wnt1, Wnt5a and
Wnt11 were chosen to investigate the involvement of Wnt signaling
in zebrafish cardiac development in injected FABP3-MO zebrafish
embryos using qPCR at 24, 48 and 72 h pf, respectively. As shown in
Fig. 6B–D, the expression pattern
of the three Wnt signaling molecules was enhanced in FABP3-MO
injected zebrafish at 24, 48 and 72 h pf. These results indicated
that FABP3-MO affected the Wnt signaling pathway which may also
have contributed to abnormal cardiac development.
Discussion
The heart is the first organ to form and function in
vertebrate embryo development. The transparency of the zebrafish
embryo and the fact that zebrafish survive without circulating
blood until the larval stage, are advantages that allow the
high-resolution visualization of the heart during its rapid
development and facilitates the extended study of heart dysfunction
(29–31). Therefore, zebrafish are commonly
selected as model organisms in the study of cardiac
development.
It has previously been demonstrated that FABP3 was
highly expressed in patients with ventricular-septal defects, when
compared with normal controls (6).
It has also been demonstrated that inhibition or overexpression of
FABP3 promoted cell apoptosis and resulted in mitochondrial
dysfunction in P19 cells, which indicated that FABP3 promoted
apoptosis through the induction of mitochondrial impairment in P19
cells (7). The function of FABP3
in cardiac development in vivo remains to be elucidated
despite observations in vitro. In the present study, cardiac
development was investigated in FABP3-MO injected zebrafish embryos
and the cause of heart defects was studied at the molecular
level.
Apoptosis is hypothesized to be important during the
critical stages of heart development and excess apoptosis directly
or indirectly results in congenital heart disease (CHD) (32). In the present study, results
demonstrated that FABP3-MO promoted zebrafish cell apoptosis which
was concordant with our previous study in vitro(23). Furthermore, over the past few
decades, studies have focused on the correlation between
mitochondrial function and apoptosis, including the release of
caspase activators (such as cytochrome c), the alteration of
cellular oxidation-reduction and the involvement of pro- and
antiapoptotic Bcl-2 family proteins (14). Thus, in the present study, it was
determined whether mitochondrial function was predominant in
triggering or inhibiting apoptosis in zebrafish. In addition, FABP3
is predominantly involved in the mitochondrial energy metabolism
and in transferring long-chain fatty acids to the mitochondria in
the process of ATP production in myocardial cells (33). Therefore, ATP content, ROS
production and mtDNA copy number was investigated in FABP3-MO
zebrafish to determine the effect of FABP3-MO on mitochondrial
function during the process of cardiac development. The results
indicated that significant dysfunction in mitochondria was observed
in FABP3-MO injected zebrafish, including a marked decrease in ATP
content, enhanced ROS production and reduced mtDNA, which was
concordant with the results of our in vitro experiments
(23). In addition, the inhibition
efficiency of the FABP3-MO was greatest at 48 h pf. Thus,
mitochondrion dysfunction may be responsible for the development of
FABP3-induced apoptosis in zebrafish.
In order to determine the mitochondrial function and
apoptosis in FABP3-MO injected zebrafish, the effect of FABP3-MO on
Nkx2.5 and Wnt gene expression was also determined. Nkx2.5 is a
transcription factor which is essential for cardiac precursor
formation and differentiation (34), regulates multiple aspects of
cardiac cell structure, function, and development (8,35).
Deletion of this gene results in the failure of normal heart
development and the disorder of cardiomyocyte maintenance (25). As the Nkx2.5 transcription factor
is one of the earliest genes expressed in the heart cells (36), the expression level of Nkx2.5 was
determined in FABP3-MO zebrafish. The results demonstrated that the
expression level of Nkx2.5 was upregulated in FABP3-MO compared
with Wt-MO injected zebrafish.
Furthermore, a large number of studies have
demonstrated that blocking Wnt signaling (canonical and
non-canonical) in the anterior mesoderm of Xenopus embryos
influenced the expression of early cardiac genes, including Nkx2.5,
Gata4 and Tbx5 (37,38). Thus, to determine why the
expression level of Nkx2.5 was enhanced, the expression profile of
Wnt signaling molecule (Wnt1, Wnt5a and Wnt11) was analyzed.
Concordant with the hypothesis, the expression pattern of the three
Wnt signaling molecules were enhanced in FABP3-MO injected
zebrafish. Moreover, Wnt signals exhibited developmental
stage-specific and biphasic effects (both positive and negative) on
cardiomyogenesis (26–28). At least ten types of FABPs have
been identified in vertebrates (39), each FABPs gene showed a specific
pattern of expression during development and in adulthood in
mammals (40). Thus, FABP3-MO led
to an abnormal expression profile in zebrafish. Additionally,
Wnt/β-catenin is pro-cardiogenic in the early precardiac mesoderm
and inhibitory later in cardiac differentiation. The non-canonical
Wnts are essential in cardiac morphogenesis. Recent studies from
the Morrisey lab demonstrated that the non-canonical Wnts inhibit
canonical Wnt signaling in heart development (41). Therefore, the balance of canonical
and non-canonical Wnt was key in heart development. Dohn and Waxman
(42) indicated that canonical
Wnts promoted apoptosis through p53 and caspase-3 independent
mechanisms in zebrafish, which suggested that FABP3-MO induced
apoptosis in zebrafish, which may occur through the promotion of
the Wnt signaling pathway.
In the present study, FABP3-MO led to defects in
cardiac development of zebrafish embryos. In addition, the
molecular mechanism underlying such defects involved in
mitochondrial dysfunction induced-apoptosis. Moreover, these
studies have demonstrated that Wnt activity is a key regulator of
the morphogenesis of the heart.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81070138), the
Natural Science Foundation of Jiangsu Province, China (grant no.
BK2010582) and the Talent Foundation of Jiangsu Province, China
(grant no. WSN-020).
References
|
1
|
Srivastava D: Making or breaking the
heart: from lineage determination to morphogenesis. Cell.
126:1037–1048. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glickman NS and Yelon D: Cardiac
development in zebrafish: coordination of form and function. Semin
Cell Dev Biol. 13:507–513. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qian Q, Kuo L, Yu YT and Rottman JN: A
concise promoter region of the heart fatty acid-binding protein
gene dictates tissue-appropriate expression. Circ Res. 84:276–289.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Besnard P, Niot I, Poirier H, Clément L
and Bernard A: New insights into the fatty acid-binding protein
(FABP) family in the small intestine. Mol Cell Biochem.
239:139–147. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang MK, Kindler PM, Cai DQ, Chow PH, Li M
and Lee KK: Heart-type fatty acid binding proteins are upregulated
during terminal differentiation of mouse cardiomyocytes, as
revealed by proteomic analysis. Cell Tissue Res. 316:339–347. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Zhou L, Yang R, et al:
Identification of differentially expressed genes in human heart
with ventricular septal defect using suppression subtractive
hybridization. Biochem Biophys Res Commun. 342:135–144. 2006.
View Article : Google Scholar
|
|
7
|
Shen YH, Song GX, Liu YQ, et al: Silencing
of FABP3 promotes apoptosis and induces mitochondrion impairment in
embryonic carcinoma cells. J Bioenerg Biomembr. 44:317–323. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bruneau BG: Transcriptional regulation of
vertebrate cardiac morphogenesis. Circ Res. 90:509–519. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gessert S and Kühl M: The multiple phases
and faces of wnt signaling during cardiac differentiation and
development. Circ Res. 107:186–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nusse R: Wnt signaling in disease and in
development. Cell Res. 15:28–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poelmann RE and Gittenberger-de Groot AC:
Apoptosis as an instrument in cardiovascular development. Birth
Defects Res C Embryo Today. 75:305–313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fiorina P, Corradi D, Pinelli S, et al:
Apoptotic/mytogenic pathways during human heart development. Int J
Cardiol. 96:409–417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Westerfield M: The Zebrafish Book: A guide
for the laboratory use of zebrafish
Danio(Brachydanio) rerio. 4th edition.
University of Oregon Press; 1993
|
|
16
|
Kimmel CB, Ballard WW, Kimmel SR, Ullmann
B and Schilling TF: Stages of embryonic development of the
zebrafish. Dev Dyn. 203:253–310. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu F, Li K, Tian M, et al: N-CoR is
required for patterning the anterior-posterior axis of zebrafish
hindbrain by actively repressing retinoid signaling. Mech Dev.
126:771–780. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nasevicius A and Ekker SC: Effective
targeted gene ‘knockdown’ in zebrafish. Nat Genet. 26:216–220.
2000.
|
|
19
|
Vermes I, Haanen C, Steffens-Nakken H and
Reutelingsperger C: A novel assay for apoptosis. Flow cytometric
detection of phosphatidylserine expression on early apoptotic cells
using fluorescein labelled Annexin V. J Immunol Methods. 184:39–51.
1995. View Article : Google Scholar
|
|
20
|
Sundaresan M, Yu ZX, Ferrans VJ, Irani K
and Finkel T: Requirement for generation of
H2O2 for platelet-derived growth factor
signal transduction. Science. 270:296–299. 1995.
|
|
21
|
Kaaman M, Sparks LM, van Harmelen V, et
al: Strong association between mitochondrial DNA copy number and
lipogenesis in human white adipose tissue. Diabetologia.
50:2526–2533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bakkers J: Zebrafish as a model to study
cardiac development and human cardiac disease. Cardiovasc Res.
91:279–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Zhou L, Jin J, et al: Knockdown of
FABP3 Impairs Cardiac Development in Zebrafish through the Retinoic
Acid Signaling Pathway. Int J Mol Sci. 14:13826–13841. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andreyev AY, Kushnareva YE and Starkov AA:
Mitochondrial metabolism of reactive oxygen species. Biochemistry
(Mosc). 70:200–214. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akazawa H and Komuro I: Cardiac
transcription factor Csx/Nkx2-5: Its role in cardiac development
and diseases. Pharmacol Ther. 107:252–268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Naito AT, Shiojima I, Akazawa H, et al:
Developmental stage-specific biphasic roles of Wnt/beta-catenin
signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci
USA. 103:19812–19817. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tzahor E: Wnt/beta-catenin signaling and
cardiogenesis: timing does matter. Dev Cell. 13:10–13. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ueno S, Weidinger G, Osugi T, et al:
Biphasic role for Wnt/beta-catenin signaling in cardiac
specification in zebrafish and embryonic stem cells. Proc Natl Acad
Sci USA. 104:9685–9690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stainier DY and Fishman MC: The zebrafish
as a model system to study cardiovascular development. Trends
Cardiovasc Med. 4:207–212. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stainier DY: Zebrafish genetics and
vertebrate heart formation. Nat Rev Genet. 2:39–48. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pelster B and Burggren WW: Disruption of
hemoglobin oxygen transport does not impact oxygen-dependent
physiological processes in developing embryos of zebra fish
(Danio rerio). Circ Res. 79:358–362. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fisher SA, Langille BL and Srivastava D:
Apoptosis during cardiovascular development. Circ Res. 87:856–864.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McCann CJ, Glover BM, Menown IB, et al:
Novel biomarkers in early diagnosis of acute myocardial infarction
compared with cardiac troponin T. Eur Heart J. 29:2843–2850. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lyons I, Parsons LM, Hartley L, et al:
Myogenic and morphogenetic defects in the heart tubes of murine
embryos lacking the homeo box gene Nkx2-5. Genes Dev. 9:1654–1666.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harvey RP, Lai D, Elliott D, et al:
Homeodomain factor Nkx2-5 in heart development and disease. Cold
Spring Harb Symp Quant Biol. 67:107–114. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Biben C and Harvey RP: Homeodomain factor
Nkx2-5 controls left/right asymmetric expression of bHLH gene eHand
during murine heart development. Genes Dev. 11:1357–1369. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eisenberg CA and Eisenberg LM: WNT11
promotes cardiac tissue formation of early mesoderm. Dev Dyn.
216:45–58. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marvin MJ, Di Rocco G, Gardiner A, Bush SM
and Lassar AB: Inhibition of Wnt activity induces heart formation
from posterior mesoderm. Genes Dev. 15:316–327. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schaap FG, van der Vusse GJ and Glatz JF:
Evolution of the family of intracellular lipid binding proteins in
vertebrates. Mol Cell Biochem. 239:69–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Glatz JF and van der Vusse GJ: Cellular
fatty acid-binding proteins: their function and physiological
significance. Prog Lipid Res. 35:243–282. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Z, Shu W, Lu MM and Morrisey EE:
Wnt7b activates canonical signaling in epithelial and vascular
smooth muscle cells through interactions with Fzd1, Fzd10, and
LRP5. Mol Cell Biol. 25:5022–5030. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dohn TE and Waxman JS: Distinct phases of
Wnt/β-catenin signaling direct cardiomyocyte formation in
zebrafish. Dev Biol. 361:364–376. 2012.
|