Introduction
Cervical cancer represents the third most common
type of cancer in females worldwide with ~500,000 new cases/year.
Cervical cancer leads to an estimated 274,000 deaths globally every
year, resulting in an increased health and economic burden,
particularly in developing countries (1,2). The
risk for the development of cervical cancer is enhanced through
infection by human papillomavirus (HPV). However, infection with
HPV alone is not sufficient for the development of this type of
cancer, since several additional host factors may affect the
persistence of HPV infection, which induces the malignant
conversion of cervical epithelial cells (3–8). DNA
hypomethylation has been shown to facilitate the integration of HPV
DNA into cells and to reduce the inhibition of HPV expression
(3). As a result, enzymes in the
one-carbon pathway have received increasing interest since
differences in metabolic properties may affect cancer risk.
Methylenetetrahydrofolate reductase (MTHFR) and
methionine synthase (MS) are two important enzymes essential for
nucleic acid synthesis, DNA repair and methylation; therefore, the
investigation of their role in folate metabolic pathways has
attracted increased interest. Two common mutations in the MTHFR
gene are C677T and A1298C, and the common variant in MS is an
A-to-G transversion at position 2756 (MS A2756G) (9,10).
Genetic variants of MTHFR and MS genes modify the activities or
other kinetic properties of the encoded enzymes (11). The functional consequences of
variant enzyme properties may include abnormalities in DNA
synthesis, repair and methylation, and, thereby, altered
susceptibility to precancerous lesions and cervical cancer
(11).
However, controversy remains concerning the role of
these polymorphisms in cervical carcinogenesis in terms of cancer
sites, racial differences and the combined influences of additional
risk factors (1,11–18).
To address such questions, we performed a meta-analysis of
published studies to determine potential associations between MTHFR
(C677T and A1298C) and MS gene polymorphisms (A2756G) with the risk
of CIN II/III and cervical cancer.
Materials and methods
Search strategy and study
identification
The present meta-analysis was conducted according to
Meta-analysis of Observational Studies in Epidemiology (MOOSE)
criteria (19). A literature
search for all studies examining the association between the
polymorphisms of MTHFR C677T, MTHFR A1298C and MS A2756G with CIN
II/III and cervical cancer was performed using electronic
databases, including PubMed, Web of Science, MEDLINE and Wanfang
Data. The following keywords and subject terms were used: ‘cervical
intraepithelial neoplasia’, ‘cervical cancer’,
‘methylenetetrahydrofolate reductase’, ‘polymorphism’, ‘variant’,
‘mutation’, ‘folate’ and ‘one-carbon metabolism’ up to November
30th, 2012. ‘Methionine synthase’ was used to replace
‘methylenetetrahydrofolate reductase’ in further searches of
related studies. References in the identified publications were
evaluated and literature retrieval was conducted in triplicate by
three independent reviewers (Jie Zhu, Wei Cai and Fangli Ye).
Selection criteria
Eligible studies were included in the present
meta-analysis when the following criteria were met: i) the study
was an unrelated case-control study examining the association
between MTHFR or MS gene polymorphisms and CIN II/III or cervical
cancer; ii) the sample size, distribution of genotype frequency or
additional information was available; iii) the genotype
distribution of the population met all the expectation of the
Hardy-Weinberg equilibrium (HWE) theory; iv) for studies where the
same or overlapping data were used, the most recent study was
included in the present meta-analysis; and v) only studies
published as full length articles or letters with adequate study
details were used.
Data extraction
Data were collected for meta-analysis according to
the selection criteria. The collected information included the year
of publication, country, ethnicity, mean age of study population,
study design, study method, sample size, source of controls, as
well as allele and genotype frequencies in case and control
groups.
Statistical analysis
Deviation from HWE was determined by Fisher’s exact
test in the control group of each study. Crude odds ratios (ORs)
with their 95% confidence intervals (CIs) were applied to evaluate
the strength of association of MTHFR C677T, MTHFR A1298C and MS
A2756G polymorphisms with CIN II/III or cervical cancer,
respectively. The pooled ORs and their 95% CIs were calculated and
compared for different genetic models for MTHFR C677T allele T
[allele model (T vs. C), homozygote model (TT vs. CC), dominant
model (TT+CT vs. CC) and recessive model (TT vs. CT+CC)]. The same
comparisons were performed for allele C of the MTHFR A1298C and
allele G of the MS A2756G polymorphism, respectively.
A Chi-square-based Q-test was conducted to assess
heterogeneity between studies, which was considered significant
when P<0.05. The percentage variability of the overall OR
attributable to heterogeneity between studies was assessed by
I2 test. A fixed effects model was used to calculate the
summary OR value when heterogeneity did not exist (20). Otherwise, a random effects model
(Mantel-Haenszel method) was adopted (20). A Z-test was implemented to
determine the significance of the pooled OR and P<0.05 was
considered to indicate a statistically significant difference. In
order to estimate ethnic-specific OR, subgroup analyses were also
performed for Asian and Caucasian populations, respectively. The
MTHFR A1298C and MS A2756G comparisons for the association with CIN
II/III were not stratified for subgroup analysis due to the
limitations of the available data. Sensitivity analyses were
conducted by reassessing the significance of ORs after each study
was omitted in turn. Publication bias was examined with Egger’s
linear regression test and Begg’s funnel plot test.
All statistical analyses were performed using the
program Review Manager 5 and STATA software package (version 11.0;
StataCorp, College Station, TX, USA). All the P-values were
two-sided and P<0.05 for any test or model was considered to
indicate a statistically significant difference.
Results
Selection of eligible studies
Concerning cervical cancer, 22 studies were
retrieved for cervical cancer and 13 met our inclusion criteria; 8
studies were excluded since detailed genotyping information was not
available. Moreover, 1 study (8)
was replaced with its updated version since the subjects in these 2
studies were from the same population. The final pool of eligible
studies consisted of 13 studies with 1,936 cases and 2,858 controls
(3,6,12,13,15,17,21–27)
for MTHFR C677T polymorphism (Table
I), 5 studies with 585 cases and 1,000 controls for MTHFR
A1298C polymorphism (Table I), and
3 studies with 389 cases and 440 controls for MS A2756G
polymorphism (Table I). The
genotype distribution in the controls of all these studies was
consistent with HWE. However, not all the studies provided enough
data for the ethnicity subgroup analysis of the association of
MTHFR A1298C or MS A2756G polymorphism with the risk to cervical
cancer.
 | Table ICharacteristics of eligible studies
included in this meta-analysis. |
Table I
Characteristics of eligible studies
included in this meta-analysis.
| Study | Year of
publication | Ethnicity | Mean age
(Case/control) | Genotyping
method | Source of
controls | Ref. |
|---|
| Goodman et
al | 2001 | Mixed | NS/NS | PCR-RFLP | Hospital-based,
case-control study | (13) |
| Lambropoulos et
al | 2003 | Caucasian | 33.2/33.2 | PCR-RFLP | Hospital-based,
case-control study | (24) |
| Sull et
al | 2004 | Asian | 50.3/46.2 | SNaPshot | Population-based,
case-control study | (21) |
| Kang et
al | 2005 | Asian | NS/NS | PCR-RFLP | Hospital-based,
case-control study | (15) |
| Zoodsma et
al | 2005 | Caucasian | NS/NS | TaqMan SNP | Hospital-based,
case-control study | (17) |
| Delgado-Enciso
et al | 2006 | Mixed | 46/44 | PCR-RFLP | Hospital-based,
case-control study | (25) |
| Wang et
al | 2006 | Asian | 52.53/50.56 | PCR-RFLP | Hospital-based,
case-control study | (27) |
| Piyathilake et
al | 2007 | Mixed | 21.5/23.0 | PCR-RFLP | Hospital-based,
case-control study | (26) |
| Shekari et
al | 2008 | Asian | 48.55/48.81 | PCR-RFLP | Hospital-based,
case-control study | (22) |
| Kohaar et
al | 2010 | Asian | 49.4/48.2 | SNaPshot | Hospital-based,
case-control study | (3) |
| Tong et
al | 2011 | Asian | 50.8/45.7 | TaqMan | SNP Hospital-based,
case-control study | (6) |
| Prasad and
Wilkhoo | 2011 | Asian | NS/NS | PCR-RFLP | Population-based,
case-control study | (12) |
| Mostowska et
al | 2011 | Caucasian | 54.6/53.3 | PCR-RFLP | Hospital-based,
case-control study | (23) |
With regard to CIN II/III, 8 studies were retrieved
and 8 were included in the present meta-analysis according to the
selection criteria. One study (8)
was replaced with its updated version due to overlapping inclusion
of study subjects in the most recent study. The eligible studies
comprised 7 studies (3,6,17,21,24,26,27)
with 1,936 cases and 2,858 controls for MTHFR C677T polymorphism
(Table I) and the genotype
distribution in the controls of all these studies was consistent
with HWE. There were not enough studies available for the
meta-analysis of the contribution of MTHFR A1298C or MS A2756G
polymorphisms to susceptibility to CIN II/III.
DNA was prepared from blood samples or tissue for
genotyping in all the studies. SNaPshot genotyping assay was
adopted in 2 studies (3,21) and a TaqMan single nucleotide
polymorphism (SNP) genotyping assay was used in 2 studies (6,17)
with polymerase chain reaction-restriction fragment length
polymorphism (PCR-RFLP) employed in all of the remaining studies
(13,15,22–27)
to validate genotype distribution (Table I).
Results of the meta-analysis
Genotype distributions, allele frequencies, summary
ORs and 95% CI for various genetic contrasts investigating the
association of MTHFR C677T and A1298C or MS A2756G polymorphisms
with cervical cancer and CIN II/III are listed in Tables II–V.
 | Table IIDistribution of MTHFR C677T genotypes
and their allelic frequency associated with the risk of cervical
cancer or CIN II/III. |
Table II
Distribution of MTHFR C677T genotypes
and their allelic frequency associated with the risk of cervical
cancer or CIN II/III.
| Study | Genotype
distribution, n | Chi-square | HWE P-value
(control) | Ref. |
|---|
|
|---|
| Total | CC | CT | TT |
|---|
| Cervical
cancer |
| Lambropoulos et
al | Case | 21 | 11 | 8 | 2 | 0.699 | 0.403 | (24) |
| Control | 91 | 42 | 37 | 12 | | | |
| Sull et
al | Case | 246 | 73 | 115 | 58 | 0 | 0.990 | (21) |
| Control | 454 | 153 | 221 | 80 | | | |
| Kang et
al | Case | 79 | 27 | 32 | 20 | 0.482 | 0.487 | (15) |
| Control | 74 | 30 | 32 | 12 | | | |
| Zoodsma et
al | Case | 636 | 357 | 230 | 49 | 0.263 | 0.608 | (17) |
| Control | 592 | 273 | 262 | 57 | | | |
| Wang et
al | Case | 111 | 20 | 53 | 38 | 1.137 | 0.286 | (27) |
| Control | 111 | 33 | 60 | 18 | | | |
| Delgado-Enciso
et al | Case | 70 | 18 | 34 | 18 | 0.910 | 0.340 | (25) |
| Control | 89 | 20 | 49 | 20 | | | |
| Shekari et
al | Case | 200 | 170 | 28 | 2 | 0.372 | 0.542 | (22) |
| Control | 200 | 125 | 68 | 7 | | | |
| Kohaar et
al | Case | 164 | 113 | 47 | 4 | 0.277 | 0.599 | (3) |
| Control | 231 | 161 | 65 | 5 | | | |
| Tong et
al | Case | 146 | 53 | 65 | 28 | 0.792 | 0.373 | (6) |
| Control | 427 | 152 | 198 | 77 | | | |
| Prasad and
Wilkhoo | Case | 62 | 0 | 5 | 57 | 3.472 | 0.062 | (12) |
| Control | 125 | 1 | 8 | 116 | | | |
| Mostowska et
al | Case | 124 | 56 | 59 | 9 | 0.649 | 0.420 | (23) |
| Control | 168 | 69 | 81 | 18 | | | |
| CIN II/III |
| Goodman et
al | Case | 150 | 73 | 67 | 10 | 0.656 | 0.418 | (13) |
| Control | 179 | 93 | 75 | 11 | | | |
| Lambropoulos et
al | Case | 64 | 27 | 29 | 8 | 0.622 | 0.430 | (24) |
| Control | 91 | 42 | 37 | 12 | | | |
| Sull et
al | Case | 176 | 50 | 90 | 36 | 0 | 0.990 | (21) |
| Control | 454 | 153 | 221 | 80 | | | |
| Zoodsma et
al | Case | 264 | 121 | 120 | 23 | 0.340 | 0.556 | (17) |
| Control | 592 | 273 | 262 | 57 | | | |
| Piyathilake et
al | Case | 80 | 59 | 16 | 5 | 0.034 | 0.853 | (26) |
| Control | 355 | 223 | 116 | 16 | | | |
| Kohaar et
al | Case | 39 | 28 | 11 | 0 | 0.277 | 0.599 | (3) |
| Control | 231 | 161 | 65 | 5 | | | |
| Tong et
al | Case | 160 | 54 | 74 | 32 | 0.792 | 0.373 | (6) |
| Control | 427 | 152 | 198 | 77 | | | |
 | Table VMeta-analysis of various genetic
comparisons investigating the association of MTHFR C677T, MTHFR
A1298C and MS A2756G polymorphisms with cervical cancer or CIN
II/III susceptibility. |
Table V
Meta-analysis of various genetic
comparisons investigating the association of MTHFR C677T, MTHFR
A1298C and MS A2756G polymorphisms with cervical cancer or CIN
II/III susceptibility.
| Analysis model | Ethnicity | Random effects
model OR (95% CI) | Fixed effects model
OR (95% CI) | P-value for
heterogeneity | P-value for fixed
effects model |
|---|
| MTHFR C677T in
cervical cancer | | | | | |
| T vs. C | Asian | 0.98
(0.73–1.32) | 1.01
(0.87–1.17) | 0.002 | 0.93 |
| Caucasian | 0.82
(0.69–0.97) | 0.82
(0.69–0.97) | 0.87 | 0.02 |
| Total | 0.94
(0.78–1.14) | 0.93
(0.83–1.103) | 0.007 | 0.17 |
| TT vs. CC | Asian | 1.4
(0.87–2.26) | 1.41
(1.07–1.86) | 0.05 | 0.01 |
| Caucasian | 0.65
(0.45–0.93) | 0.65
(0.45–0.93) | 0.99 | 0.02 |
| Total | 1.07
(0.73–1.58) | 1.06
(0.86–1.31) | 0.008 | 0.6 |
| TT+CT vs. CC | Asian | 0.98
(0.62–1.54) | 0.94
(0.79–1.13) | <0.0001 | 0.52 |
| Caucasian | 0.7
(0.58–0.86) | 0.7
(0.58–0.86) | 0.66 | 0.0005 |
| Total | 0.89
(0.66–1.18) | 0.83
(0.73–0.94) | <0.0001 | 0.004 |
| TT vs. CC+CT | Asian | 1.36
(0.95–1.95) | 1.38
(1.08–1.75) | 0.12 | 0.008 |
| Caucasian | 0.75
(0.53–1.07) | 0.75
(0.53–1.07) | 0.92 | 0.11 |
| Total | 1.13
(0.84–1.52) | 1.14
(0.94–1.38) | 0.05 | 0.18 |
| MTHFR C677T in CIN
II/III | | | | | |
| T vs. C | Total | 1.01
(0.88–1.17) | 1.01
(0.88–1.17) | 0.8 | 0.86 |
| Asian | 1.00
(0.67–1.48) | 1.07
(0.88–1.31) | 0.86 | 0.49 |
| TT vs. CC | Total | 1.13
(0.86–1.49) | 1.12
(0.86–1.48) | 0.91 | 0.4 |
| Asian | 1.25
(0.88–1.80) | 1.25
(0.87–1.78) | 0.76 | 0.22 |
| TT+CT vs. CC | Total | 1.02
(0.85–1.23) | 1.02
(0.86–1.22) | 0.37 | 0.79 |
| Asian | 1.14
(0.89–1.48) | 1.14
(0.89–1.47) | 0.67 | 0.3 |
| TT vs. CC+CT | Total | 1.09
(0.85–1.39) | 1.08
(0.84–1.39) | 0.93 | 0.54 |
| Asian | 1.16
(0.85–1.59) | 1.16
(0.84–1.58) | 0.85 | 0.37 |
| MTHFR A1298C in
cervical cancer | | | | | |
| C vs. A | Total | 0.99
(0.82–1.20) | 0.99
(0.82–1.20) | 0.83 | 0.94 |
| Asian | 1.06
(0.85–1.33) | 1.06
(0.85–1.33) | 0.96 | 0.59 |
| CC vs. AA | Total | 0.89
(0.55–1.41) | 0.85
(0.54–1.34) | 0.43 | 0.48 |
| Asian | 0.96
(0.40–2.27) | 0.99
(0.57–1.71) | 0.25 | 0.96 |
| CC+AC vs. AA | Total | 1.05
(0.83–1.31) | 1.04
(0.83–1.31) | 0.81 | 0.7 |
| Asian | 1.12
(0.86–1.45) | 1.12
(0.86–1.45) | 0.8 | 0.39 |
| CC vs. AA+AC | Total | 0.81
(0.52–1.24) | 0.80
(0.56–1.16) | 0.31 | 0.24 |
| Asian | 0.91
(0.35–2.38) | 0.98
(0.58–1.63) | 0.19 | 0.93 |
| MS A2756G in
cervical cancer | | | | | |
| G vs. A | Total | 0.65
(0.21–1.98) | 0.62
(0.46–0.84) | 0.0001 | 0.002 |
| GG vs. AA | Total | 0.79
(0.17–3.62) | 0.64
(0.33–1.21) | 0.03 | 0.17 |
| GG+AG vs. AA | Total | 0.63
(0.26–1.50) | 0.61
(0.47–0.79) | 0.0001 | 0.002 |
| GG vs. AA+AG | Total | 0.81
(0.25–2.62) | 0.72
(0.38–1.38) | 0.11 | 0.32 |
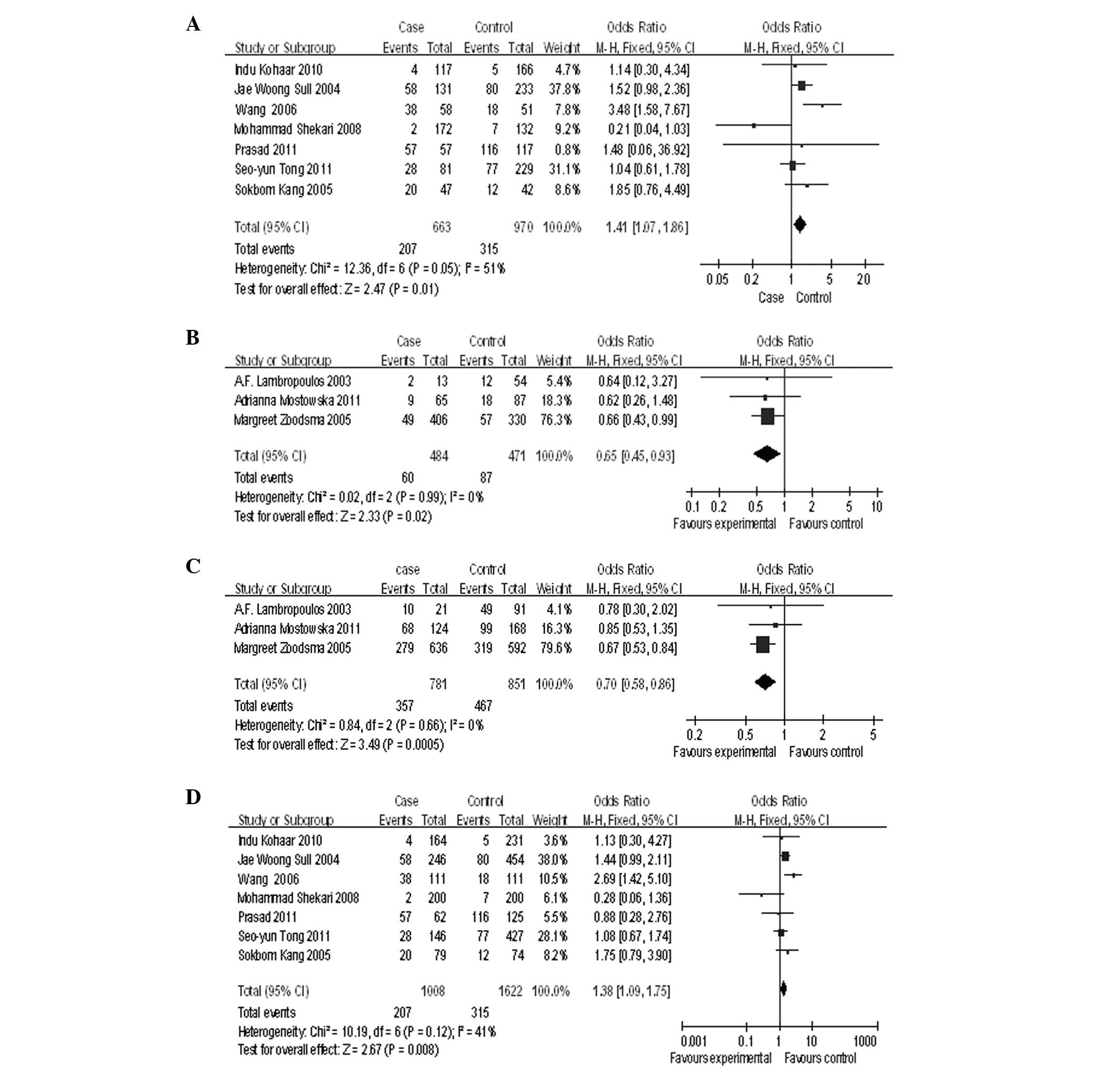
With respect to the MTHFR C677T polymorphism, no
association was found with cervical cancer when a random effects
model was adopted to conduct a worldwide allele comparison (T vs.
C: P=0.53, OR=0.94, 95% CI=0.78–1.14, P=0.007 for heterogeneity;
Table V). In the ethnicity
subgroup analysis, an enhanced risk was demonstrated in Asian women
(TT vs. CC: P=0.01, OR=1.41, 95% CI=1.07–1.86, P=0.05 for
heterogeneity; Table V and
Fig. 1A). By contrast, an inverse
association was observed in Caucasian women (TT vs. CC: P=0.02,
OR=0.65, 95% CI=0.45–0.93, P=0.99 for heterogeneity; Table V and Fig. 1B). Furthermore, meta-analyses of
the contrasts in a recessive genetic model revealed that the 677T
allele is more likely to reduce the risk of Caucasian women (TT+CT
vs. CC: P=0.0005, OR=0.7, 95% CI=0.58–0.86, P=0.66 for
heterogeneity; Table V and
Fig. 1C), while the 677T allele is
more likely to increase the risk of Asian women (TT vs. CC+CT:
P=0.008, OR=1.38, 95% CI=1.08–1.75, P=0.12 for heterogeneity;
Table V and Fig. 1D). No significant effect of MTHFR
A1298C polymorphism on the susceptibility was found in worldwide
populations (C vs. A: P=0.94, OR=0.99, 95% CI=0.82–1.20, P=0.83 for
heterogeneity) and in Asian females (C vs. A: P=0.59, OR=1.06, 95%
CI=0.85–1.33, P=0.96 for heterogeneity; Table V). Similarly, no association
between MS A2756G polymorphism and cervical cancer was detected in
the worldwide population (G vs. A: P=0.002, OR=0.65, 95%
CI=0.21–1.98, P=0.0001 for heterogeneity; Table V).
With respect to association with CIN II/III,
meta-analyses did not provide evidence to support an association
between C677T polymorphism and susceptibility to CIN II/III in the
worldwide population (T vs. C: P=0.86, OR=1.01, 95% CI=0.88–1.17,
P=0.8 for heterogeneity; Table V)
and in Asian women (T vs. C: P=0.49, OR=1.07, 95% CI=0.88–1.31,
P=0.86 for heterogeneity; Table
V).
Sensitivity analysis
Sensitivity analysis was conducted by sequentially
omitting each study in turn under homozygote and recessive
contrasts performed on a worldwide population and on ethnically
defined subgroups to evaluate the robustness and plausibility of
the meta-analysis. The pooled ORs (including 95% CI) from various
contrasts were not significantly altered (data not shown),
indicating that the summary estimate of the effect of MTHFR C677T,
MTHFR A1298C and MS A2756G polymorphisms on the risk of cervical
cancer and CIN II/III was not altered during the sensitivity
analysis.
Publication bias
The Begg’s funnel plot and Egger’s test were
conducted to estimate the publication bias of the included studies.
The funnel plot for the comparison of the 677C allele with the 677T
allele provided limited evidence on obvious asymmetry. No
publication bias by Egger’s test was detected for the comparison in
homozygote (TT vs. CC), dominant (TT+CT vs. CC) and recessive
models (TT vs. CT+CC) for both cervical cancer and CIN II/III
(Table VI). Similarly, there was
no statistical evidence suggesting publication bias for the
comparison of the three models of MTHFR A1298C polymorphism for
cervical cancer (Table VI).
Furthermore, no publication bias was observed for the G vs. A
allele contrast of MS A2756G polymorphism for cervical cancer
(t=0.61, P=0.654).
 | Table VIPublication bias detection for MTHFR
C677T and MTHFR A1298C polymorphisms. |
Table VI
Publication bias detection for MTHFR
C677T and MTHFR A1298C polymorphisms.
| Genetic type | Coefficient | SE | t-value | P-value | 95% CI of
intercept |
|---|
| C677T for cervical
cancer |
| TT vs. CC | 5.480 | 4.639 | 1.18 | 0.268 | (−5.015 to
15.976) |
| TT+CT vs. CC | −1.352 | 2.836 | −0.48 | 0.645 | (−7.766 to
5.063) |
| TT vs. CT+CC | 0.948 | 1.621 | 0.58 | 0.573 | (−2.720 to
4.616) |
| C677T for CIN
II/III |
| TT vs. CC | −1.978 | 2.113 | −0.94 | 0.418 | (−8.701 to
4.746) |
| TT+CT vs. CC | 13.775 | 8.494 | 1.62 | 0.180 | (−9.807 to
37.358) |
| TT vs. CT+CC | 0.427 | 1.846 | 0.23 | 0.832 | (−5.448 to
6.302) |
| A1298C for cervical
cancer |
| CC vs. AA | −0.867 | 1.051 | −0.92 | 0.385 | (−3.393 to
1.459) |
| CC+CA vs. AA | 6.484 | 4.034 | 1.61 | 0.206 | (−6.355 to
19.323) |
| CC vs. CA+AA | −2.077 | 1.541 | −1.35 | 0.271 | (−6.980 to
2.828) |
Discussion
Cervical cancer is one of the three major
malignancies found in female cancer patients worldwide, accounting
for 250,000 deaths/year, with higher incidences being observed in
developing countries compared with developed countries (17,26,28).
Therefore, it would be useful to have precise susceptibility
information on cervical carcinogenesis to develop effective,
specific and individualized disease prevention programs for
different populations (37,38).
Infection with oncogenic subtypes of HPV has been
confirmed to play a crucial etiological role in the development of
cervical cancer (32,38,39).
However, infection with high-risk HPV alone is not sufficient to
cause cervical neoplasia. An increasing number of studies suggest
that oral contraceptives, smoking, host genetic factors and
epigenetic changes enhance susceptibility to the development of
cervical intraepithelial neoplasia and invasive cancer (23,30).
Recently, epidemiological studies have reported that heritable
factors, including genetic polymorphisms, contributed to ~64% of
the familial risks for cervical cancer (23,30).
Variation of several candidate genes involved in the
one-carbon metabolism pathway may explain some of the individual
differences in cervical tumorigenesis, including MTR, BHMT, MTHFR,
MTHFD1 and MS, among which MTHFR and MS are two of the most
commonly investigated candidate genes.
MTHFR catalyzes the conversion of
5,10-methylenetetrahydrofolate (5,10-methylene-THF) to
5-methyltetrahydrofolate (5-methyl-THF). 5,10-methylene-THF is a
substrate necessary for thymidine synthesis, while 5-methyl-THF
acts as a substrate for the remethylation of homocysteine under the
catalysis of MS, resulting in methionine synthesis, which plays a
role as a substrate for S-adenosyl methionine (SAM) synthesis. SAM
is a universal methyl donor necessary for DNA and protein
methylation. Increased MTHFR function results in low levels of
5,10-methylene-THF, thereby leading to the misincorporation of dUTP
into DNA, which in turn causes double strand breaks (9,31).
Conversely, decreased MTHFR activity may result in low levels of
5-methyl-THF and, thus, be responsible for DNA hypomethylation.
These are common features in cancer development (9,21,23,24).
The gene for MTHFR is located on chromosome 1p36
with 11 exons (44) and SNPs within the coding region are associated
with DNA hypomethylation, which constitutes a hallmark of human
cancer cells (1,24,25).
Two common mutations in the MTHFR gene are C677T and A1298C. MTHFR
C677T polymorphism leads to substitution of alanine by valine at
the amino acid position 222, which affects the catalytic domain of
the enzyme and decreases its affinity for its cofactor (9). This altered form of enzyme results in
a thermolabile protein and is associated with reduced enzymatic
activity (9,10). Thus, elevated homocysteine levels
may be attributed to a correspondingly decreased activity of this
enzyme in individuals homozygous and heterozygous for the variation
(9), which results in
abnormalities in DNA methylation (23). MTHFR A1298C polymorphism converts a
glutamine to alanine at the amino acid position 429 (9), which is located within the regulatory
domain of this protein (9).
However, this alteration does not appear to affect the function of
MTHFR by itself, but may reduce MTHFR activity when it is
heterozygous with the 1556 G→A, 1743 G→A and 1958 C→T polymorphisms
(9,34,35).
Due to both MS and MTHFR functioning in the same
metabolic pathway sequentially, the affect of MS gene polymorphism
constitutes an additional interesting research issue. MS catalyzes
the methyl transfer from homocysteine to methionine with cobalamine
as a co-factor to maintain adequate intracellular SAM levels for
DNA methylation, which is believed to suppress cancer development.
The common variant in MS is an A-to-G transversion at position 2756
(MS A2756G), which leads to the replacement of aspartate by
glycine, resulting in altered enzyme activity and, thus, affecting
DNA methylation (1,35).
There is an increasing number of studies
demonstrating that MTHFR and MS polymorphisms play different roles
in influencing susceptibility to breast, colorectal, pancreatic,
hepatocellular and prostate cancers. Particularly, the effect of
MTHFR and MS polymorphisms on the risk of CIN II/III and cervical
cancer also remains inconsistent (12,15–20).
The MTHFR C677T gene variant has been associated with a risk of
cervical carcinogenesis in certain cohorts (13,17,21,23),
while this MTHFR gene variant has been associated with protection
against CIN II/III or cervical cancer (15), or has not been confirmed as a risk
factor for this type of cancer in other populations (3,9,11,23,24,35).
Similarly, the influence of MS A2756G polymorphism on the
susceptibility of cervical tumorigenesis also remains controversial
(11,15,23).
Therefore, a systematic meta-analysis with regard to the three most
investigated polymorphisms of MTHFR (C677T and A1298C) and MS
(A2756G) genes is needed in order to determine the influence of
MTHFR or MS polymorphisms on susceptibility to CIN II/III or
cervical cancer.
A total of 13 studies with 1,936 cases and 2,858
controls were identified for the investigation of MTHFR C677T and
A1298C polymorphisms, while 3 studies with 389 cases and 440
controls were eligible for the investigation of MS A2756G
polymorphism in the present study. No significant associations were
found in the worldwide population between polymorphisms of the
MTHFR gene (C677T and A1298C) or the MS gene (A2756G), and CIN
II/III or cervical cancer. However, stratified analysis by
ethnicity demonstrated that increased susceptibility was restricted
to Asian females when homozygous and recessive genetic model
contrasts were conducted. By contrast, an inverse association of
the MTHFR C677T polymorphism and cervical cancer was observed in
Caucasian females. It has been established that one-carbon
metabolism may be affected not only by genes encoding enzymes
involved in this pathway, but also by dietary factors such as
folate, vitamin B and alcohol intake (36). A previous study demonstrated that
the MTHFR TT genotype constitutes a protective factor against
susceptibility to colorectal cancer in folate-replete subjects,
while this genotype conferred an enhanced risk of colorectal cancer
in combination with a folate-deficient status (37). Therefore, the dipartite results of
the effects of MTHFR C677T polymorphism between Asian and Caucasian
females in the present study may be due to the higher folate intake
in North America and Europe in dietary supplement use and food
fortification (38). This may also
be due to ethnic differences in additional genetic factors and
different environments. No associations were found between MTHFR
A1298C or MS A2756G gene polymorphisms and cervical cancer in the
different ethnic groups partially due to the fact that each of
these single gene variations do not appear to affect the function
of MTHFR or MS by itself until it is heterozygous with other gene
polymorphisms on the same sequence.
The sensitivity analysis in the present study
indicated that the summary estimate of effect of MTHFR C677T, MTHFR
A1298C and MS A2756G polymorphisms on the risk of cervical cancer
and CIN II/III was robust, and was not significantly altered
following various comparisons. Moreover, the evaluation of
publication bias in the present study did not indicate the
existence of such bias towards the observed association between
MTHFR C677T or A1298C variant and the risk for CIN II/III or
cervical cancer in the comparison of variant alleles, suggesting
that the results were credible and stable.
However, there are several limitations that may
limit the strength of the conclusions. Firstly, the statistical
power of the meta-analysis is relatively low, due to the fact that
the number of cases of many of the included studies was relatively
small and the fact that the controls were not defined uniformly and
were not representative enough (1,13,24).
Secondly, different racial distributions and genetic heterogeneity
existed among the studied populations, which resulted in
conflicting results and led to the inability to examine the
potential susceptibility of MTHFR or MS polymorphisms (23,39).
Thirdly, the present study focused only on the three most
investigated SNPs associated with CIN II/III or cervical cancer due
to the limited number of informative studies. Fourthly, most data
were not stratified according to the investigated SNPs by
behavioral and environmental cofactors, such as dietary folate
intake, folate and other micronutrient status within the body, HPV
infection, hormone or oral contraceptives, smoking, which might
make it difficult to investigate the joint effects among pairs of
variables modifying the susceptibility of cervical cancer or
precancerous conditions. Moreover, variations in laboratory
procedures, such as methods of data collection and genotyping,
could also explain the inconsistent results. Therefore, a more
precise analysis needs to be performed upon availability of data
from additional investigations with an improved design.
In conclusion, the present meta-analysis supports
the hypothesis that the MTHFR 677TT polymorphism is associated with
an increased risk of cervical cancer in Asian females, while an
inverse association applies to Caucasian females. Meanwhile, no
association was detected between the MTHFR C677T polymorphism and
susceptibility to CIN II/III overall or in ethnically defined
populations. Similarly, MTHFR A1298C and MS A2756G polymorphisms
did not appear to be associated with overall cervical cancer risk
or in ethnically defined populations. Larger population-based
surveys concerning gene-gene, gene-nutrients and gene-behavioral
risk factors interactions in a specific population are needed to
further determine the role of MTHFR and MS gene polymorphisms in
the risk of cervical cancer.
References
|
1
|
Piyathilake CJ, Macaluso M, Johanning GL,
Whiteside M, Heimburger DC and Giuliano A:
Methylenetetrahydrofolate reductase (MTHFR) polymorphism increases
the risk of cervical intraepithelial neoplasia. Anticancer Res.
20:1751–1757. 2000.PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Kohaar I, Kumar J, Thakur N, et al:
Homocysteine levels are associated with cervical cancer independent
of methylene tetrahydrofolate reductase gene (MTHFR) polymorphisms
in Indian population. Biomarkers. 15:61–68. 2010. View Article : Google Scholar
|
|
4
|
Kohaar I, Thakur N, Salhan S, et al:
TNFα-308G/A polymorphism as a risk factor for HPV-associated
cervical cancer in Indian population. Cell Oncol. 29:249–256.
2007.
|
|
5
|
García-Closas R, Castellsagué X, Bosch X
and González CA: The role of diet and nutrition in cervical
carcinogenesis: a review of recent evidence. Int J Cancer.
117:629–637. 2005.PubMed/NCBI
|
|
6
|
Tong SY, Kim MK, Lee JK, et al: Common
polymorphisms in methylenetetrahydrofolate reductase gene are
associated with risks of cervical intraepithelial neoplasia and
cervical cancer in women with low serum folate and vitamin B12.
Cancer Causes Control. 22:63–72. 2011. View Article : Google Scholar
|
|
7
|
Kjellberg L, Hallmans G, Ahren AM, et al:
Smoking, diet, pregnancy and oral contraceptive use as risk factors
for cervical intra-epithelial neoplasia in relation to human
papillomavirus infection. Br J Cancer. 82:1332–1338.
2000.PubMed/NCBI
|
|
8
|
Josefsson AM, Magnusson PK, Ylitalo N, et
al: Viral load of human papilloma virus 16 as a determinant for
development of cervical carcinoma in situ: a nested case-control
study. Lancet. 355:2189–2193. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gerhard DS, Nguyen LT, Zhang ZY, Borecki
IB, Coleman BI and Rader JS: A relationship between
methylenetetrahydrofolate reductase variants and the development of
invasive cervical cancer. Gynecol Oncol. 90:560–565. 2003.
View Article : Google Scholar
|
|
10
|
Yamada K, Chen Z, Rozen R and Matthews RG:
Effects of common polymorphisms on the properties of recombinant
human methylenetetrahydrofolate reductase. Proc Natl Acad Sci USA.
98:14853–14858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tong SY, Lee JM, Song ES, et al: The
effects of polymorphisms in methylenetetrahydrofolate reductase
(MTHFR), methionine synthase (MTR), and methionine synthase
reductase (MTRR) on the risk of cervical intraepithelial neoplasia
and cervical cancer in Korean women. Cancer Causes Control.
21:23–30. 2010. View Article : Google Scholar
|
|
12
|
Prasad VV and Wilkhoo H: Association of
the functional polymorphism C677T in the methylenetetrahydrofolate
reductase gene with colorectal, thyroid, breast, ovarian, and
cervical cancers. Onkologie. 34:422–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goodman MT, McDuffie K, Hernandez B, et
al: Association of methylenetetrahydrofolate reductase polymorphism
C677T and dietary folate with the risk of cervical dysplasia.
Cancer Epidemiol Biomarkers Prev. 10:1275–1280. 2001.PubMed/NCBI
|
|
14
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
15
|
Kang S, Kim JW, Kang GH, Park NH, Song YS,
Kang SB and Lee HP: Polymorphism in folate- and
methionine-metabolizing enzyme and aberrant CpG island
hypermethylation in uterine cervical cancer. Gynecol Oncol.
96:173–180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Powers HJ: Interaction among folate,
riboflavin, genotype, and cancer, with reference to colorectal and
cervical cancer. J Nutr. 135(Suppl 12): S2960–S2966.
2005.PubMed/NCBI
|
|
17
|
Zoodsma M, Nolte IM, Schipper M, et al:
Methylenetetrahydrofolate reductase (MTHFR) and susceptibility for
(pre)neoplastic cervical disease. Hum Genet. 116:247–254. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hazra A, Wu K, Kraft P, Fuchs CS,
Giovannucci EL and Hunter DJ: Twenty-four non-synonymous
polymorphisms in the one-carbon metabolic pathway and risk of
colorectal adenoma in the Nurses’ Health Study. Carcinogenesis.
28:1510–1519. 2007.PubMed/NCBI
|
|
19
|
Stroup DF, Berlin JA, Morton SC, et al:
Meta-analysis of observational studies in epidemiology: a proposal
for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar
|
|
20
|
Qi X, Ma X, Yang X, et al:
Methylenetetrahydrofolate reductase polymorphisms and breast cancer
risk: a meta-analysis from 41 studies with 16,480 cases and 22,388
controls. Breast Cancer Res Treat. 123:499–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sull JW, Jee SH, Yi S, et al: The effect
of methylenetetrahydrofolate reductase polymorphism C677T on
cervical cancer in Korean women. Gynecol Oncol. 95:557–563. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shekari M, Sobti RC, Kordi Tamandani DM,
et al: Impact of methylenetetrahydrofolate reductase (MTHFR) codon
(677) and methionine synthase (MS) codon (2756) on risk of cervical
carcinogenesis in North Indian population. Arch Gynecol Obstet.
278:517–524. 2008. View Article : Google Scholar
|
|
23
|
Mostowska A, Myka M, Lianeri M, Roszak A
and Jagodziński PP: Folate and choline metabolism gene variants and
development of uterine cervical carcinoma. Clin Biochem.
44:596–600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lambropoulos AF, Agorastos T, Foka ZJ,
Chrisafi S, Constantinidis TC, Bontis J and Kotsis A:
Methylenetetrahydrofolate reductase polymorphism C677T is not
associated to the risk of cervical dysplasia. Cancer Lett.
191:187–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Delgado-Enciso I, Martínez-Garza SG,
Rojas-Martínez A, et al: The effect of MTHFR polymorphisms,
pregnancy and first intercourse on cervical cancer in a population
from the Northeastern Mexico. Rev Invest Clin. 58:462–469. 2006.(In
Spanish).
|
|
26
|
Piyathilake CJ, Azrad M, Macaluso M,
Johanning GL, Cornwell PE, Partridge EE and Heimburger DC:
Protective association of MTHFR polymorphism on cervical
intraepithelial neoplasia is modified by riboflavin status.
Nutrition. 23:229–235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang JT, Ma XC, Cheng YY, Ding L and Zhou
Q: A case-control study on the association between folate and
cervical cancer. Zhonghua Liu Xing Bing Xue Za Zhi. 27:424–427.
2006.(In Chinese).
|
|
28
|
Agodi A, Barchitta M, Cipresso R, et al:
Distribution of p53, GST, and MTHFR polymorphisms and risk of
cervical intraepithelial lesions in sicily. Int J Gynecol Cancer.
20:141–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muñoz N, Bosch FX, de Sanjosé S, et al;
International Agency for Research on Cancer Multicenter Cervical
Cancer Study Group. Epidemiologic classification of human
papillomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003.
|
|
30
|
Hemminki K, Dong C and Vaittinen P:
Familial risks in cervical cancer: is there a hereditary component?
Int J Cancer. 82:775–781. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Blount BC, Mack MM, Wehr CM, et al: Folate
deficiency causes uracil misincorporation into human DNA and
chromosome breakage: implications for cancer and neuronal damage.
Proc Natl Acad Sci USA. 94:3290–3295. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Esteller M and Herman JG: Cancer as an
epigenetic disease: DNA methylation and chromatin alterations in
human tumours. J Pathol. 196:1–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fowler BM, Giuliano AR, Piyathilake C,
Nour M and Hatch K: Hypomethylation in cervical tissue: is there a
correlation with folate status? Cancer Epidemiol Biomarkers Prev.
7:901–906. 1998.PubMed/NCBI
|
|
34
|
Weisberg I, Tran P, Christensen B, Sibani
S and Rozen R: A second genetic polymorphism in
methylenetetrahydrofolate reductase (MTHFR) associated with
decreased enzyme activity. Mol Genet Metab. 64:169–172. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rao GG, Kurien A, Gossett D, Griffith WF,
Coleman RL and Muller CY: A case-control study of
methylenetetrahydrofolate reductase polymorphisms in cervical
carcinogenesis. Gynecol Oncol. 101:250–254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hubner RA and Houlston RS: MTHFR C677T and
colorectal cancer risk: a meta-analysis of 25 populations. Int J
Cancer. 120:1027–1035. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen J, Giovannucci E, Kelsey K, et al: A
methylenetetrahydrofolate reductase polymorphism and the risk of
colorectal cancer. Cancer Res. 56:4862–4864. 1996.PubMed/NCBI
|
|
38
|
Hankey GJ and Eikelboom JW: Homocysteine
and stroke. Lancet. 365:194–196. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Magnusson PK, Sparén P and Gyllensten UB:
Genetic link to cervical tumours. Nature. 400:29–30. 1999.
View Article : Google Scholar : PubMed/NCBI
|