Introduction
Open fractures, particularly those that are severely
contaminated, frequently result in infection-related bone diseases,
such as osteomyelitis, nonunion and infectious bone defects. It is
difficult to effectively repair bone and control infection at the
same time. As infection is favored by the devitalization of bone,
soft tissue and loss of skeletal stability, systemic administration
of antibiotics is unable to achieve a sufficient local drug
concentration. However, long-term administration of antibiotics may
give rise to side-effects, including myelosuppression,
nephrotoxicity and drug-induced hepatitis (1). Conventional treatments, such as
surgical debridement and suction irrigation, are only able to
control local infection. For patients with nonunion or bone
defects, these treatments require two stages; control of infection
followed by bone grafting (2–4).
Therefore, the high cost and length of treatment cause suffering of
the patients. Another conventional clinical procedure for treating
severely contaminated open fractures is the application of
antibiotic-loaded poly(methyl methacrylate) (PMMA) cement beads,
which are generally used as a local antibiotic release system
(LARS) and have been shown to decrease infection in a number of
clinical studies (5). However,
PMMA is non-biodegradable and cannot be allowed to remain in the
wound bed during definitive closure, thus it requires removal
during a second surgical step. At the time of definitive closure,
any remaining defect or fracture is repaired by using other methods
(6).
To overcome the limitations of non-biodegradable
PMMA and the other two-stage treatment methods, several
biodegradable materials, whether they are solid or injectable, have
been widely investigated as scaffolds for the release of various
drugs in LARS (1,6). In contrast to solid and prefabricated
scaffolds, injectable scaffolds hold great promise for the
treatment of infectious bone disease. Firstly, injectable scaffolds
may take the shape of the irregular cavities or bone defects after
routine debridement. Furthermore, antibiotics or growth factors may
be easily incorporated into the solution by mixing prior to the
injection. Calcium phosphate cement (CPC) is one type of injectable
scaffold, which is obtained by mixing tricalcium phosphate or
so-called amorphous calcium phosphate powders with an aqueous
solution to form a paste that hardens within a restricted period of
time (15–30 min) under low-processing temperatures (7,8). Due
to its good osteoconductivity, CPC has been used as a scaffold of
LARS for the controlled release of antibiotics and has been shown
to release more antibiotics over a longer period compared with PMMA
(9). Furthermore, for the
treatment of complicated fractures, bone defects and nonunion, the
improvement of osteoinductive properties is also required in these
scaffolds.
To improve the bone repair capability of scaffolds
(e.g., CPC), osteoinductive agents, such as growth factors (GFs)
and biological molecules, are usually administered in the LARS.
However, the high cost and rapid degradation of such expensive GFs
limit their widespread use, particularly in clinics (10). Therefore, there is an urgent need
to develop alternative osteogenic products or drugs with higher
efficacies and lower costs than GFs (11). Certain herbal medicines have been
widely used in the treatment of fractures and bone disorders for
thousands of years in Asia (12,13).
Herba epimedii is one of the most frequently used herbs in
formulas that are prescribed for the treatment of osteoporosis in
China, Japan and Korea (13).
Icariin (IC; molecular formula,
C33H40O15; molecular weight,
676.67 g/mol) is a flavonoid isolated from Herba epimedii
and is considered to be the major (77%) bioactive component of this
herb (14). IC was shown to have
markedly positive effects on the proliferation of osteoblasts in
numerous studies (12,14). It also showed a marked effect on
the ossific differentiation of bone marrow-derived stromal cells
(BMSCs), which was demonstrated to occur in a BMP and core binding
factor α-1 (Cbfa-1)-dependent manner (15).
In the present study, we developed a dual-drug
release system that comprised of IC, vancomycin (VA) and CPC. In
the evaluation of this new system, we hypothesized that IC-VA/CPC,
with its controlled antibiotic release kinetics and osteoinductive
capabilities, may concomitantly control infection and improve bone
healing when applied in infected bone segmental defects. To test
this hypothesis, we examined the ultrastructure, biocompatibility
and drug release profiles of IC-VA/CPC. Subsequently, this system
was implanted into an infected segmental radial defect in a rabbit
model. We specifically evaluated the ability of IC-VA/CPC to treat
infected bone defects.
Materials and methods
Preparation of IC-VA/CPC system
The system was prepared as described previously
(16). Briefly, IC (National
Institute for the Control of Pharmaceuticals and Biological
Products, Beijing, China) was dissolved into ethanol to prepare an
IC solution at a concentration of 8 mg/ml. A solution of vancomycin
hydrochloride (VA; molecular formula,
C66H75Cl2N9O24·HCl;
molecular weight, 1485.71 g/mol; Sigma, Shanghai, China) was also
prepared by dissolving VA in phosphate-buffered solution (PBS, pH
8.0) at a concentration of 80 mg/ml. The two solutions were then
mixed at a ratio of 1:1 (v/v). The IC-VA mixture was then stirred
for 2 h, followed by the slow addition of CPC powder (Shanghai
Rebone Biomaterials Co., Ltd., Shanghai, China) to prepare an
IC-VA/CPC paste precursor at a liquid/powder ratio of 1:1 (ml/g).
Subsequently, the precursor was homogenized by 4 h vigorous
stirring. The resulting solution was cast by a glass mold (4 mm in
diameter, 15 mm in length) and placed at 4°C for 6 h, −10°C for 3
h, then freeze-dried to obtain IC-VA/CPC cylinders. Each cylinder
contained ~2 mg of IC and 20 mg of VA. We also prepared a cylinder
of IC/CPC or VA/CPC at the same concentration of VA or IC for the
animal experiments. A pure CPC cylinder was used as a control in
the animal experiment and biocompatibility tests. All cylinders
were sterilized with 20 kGy 60Co and stored in vacuum
packages at room temperature prior to subsequent use.
Ultrastructure examination
The microstructure of the cylinder samples was
examined using a scanning electron microscope (SEM, S-3000N;
Hitachi, Japan) after the samples were sputter-coated with gold
under a vacuum.
Biocompatibility analysis
Biocompatibility was determined by evaluating the
toxicity of sample extracts (according to ISO10993-5) and the
survival of cells seeded directly onto the sample surfaces
(17). Briefly, sample extracts of
IC-VA/CPC and CPC were created in Dulbecco’s modified Eagle’s
medium (DMEM; Sigma) without fetal calf serum (FCS) at a
concentration of 100 mg/ml (extract/DMEM culture medium) for 24 h
at 37°C in a 5% CO2 atmosphere, following the guidelines
of ISO standard 10993-12. Balb/c 3T3 cells (American Type Culture
Collection, Manassas, VA, USA) were cultured in DMEM containing
ampicillin (0.025 g/l) and streptomycin (0.1 g/l) supplemented with
10% FCS (Sigma) at 37°C in a 5% CO2 atmosphere. The
tests were performed using 24-well plates, by plating 3T3 cells at
a density of 3×104 cells/well. Cells plated at the same
density in wells without any extracts were used as blank controls.
The total cell number of the four groups at days 1, 3, 5 and 7 was
estimated by quantifying the double-stranded DNA (dsDNA) content of
each sample using a PicoGreen dsDNA Quantification kit (Molecular
Probes, Eugene, OR, USA). The total dsDNA was extracted by
enzymatic digestion and assayed according to the manufacturer’s
instructions. The proliferation of the cells was interpreted by
changes in dsDNA quantity. In order to observe the morphology of
cells cultivated on the surface of granules, 3T3 cells were plated
onto the samples at a density of 5×104 cells/sample. At
24 and 72 h after plating, the cells were fixed with modified
Karnovsky’s solution and post-fixed with 1% osmium tetroxide in
0.05 M cacodylate buffer (pH 7.2) for 1 h at room temperature. The
samples were washed with distilled water, dehydrated using
increasing solutions of ethanol and critical point drying,
metallized with gold and analyzed under the SEM.
Release kinetics of IC or VA from
IC-VA/CPC
The release behaviors of IC and VA from the
IC-VA/CPC system were measured by high-performance liquid
chromatography (HPLC) with UV detection at 230 nm (Agilent1200;
Agilent Technologies, Santa Clara, CA, USA) according a previous
method with modifications (18).
Briefly, samples of IC-VA/CPC were soaked in 5 ml PBS (pH 7.4,
37°C) and agitated gently at 10 rpm. At 0, 1, 3, 5, 7, 10, 15, 20,
25 and 30 days, 5 ml PBS was collected (stored at 4°C for HPLC
examination) and replaced by adding the same amount of fresh PBS.
For analysis of IC concentration, the samples were centrifuged
(1,000 × g) for 10 min and 0.5 ml supernatants were isolated. After
the addition of 0.5 ml acetonitrile, the mixed solution was
re-centrifuged (4,000 × g) for 10 min and 20 μl supernatants were
applied for HPLC analysis. Peak area data was calculated by
integration using Empower 2 software (Waters Co., Milford, MA,
USA), which identifies the absorbance peaks of different proteins
and calculates the area of the peak, therefore determining the
concentration of the proteins. IC or VA released from the sample
was calculated according to the standard IC level of VA solutions
and the percentage of IC or VA released from IC-VA/CPC was
assessed. Each test was replicated three times (n=5).
IC-VA/CPC repairs osseous defects in a
rabbit model
Forty-eight male New Zealand white rabbits weighing
2.2–2.6 kg (male and 6 months old) were randomly divided into 4
groups, and each group contained 12 animals. Under general
anesthesia (i.v. injection of 15 mg/kg pentobarbital) an
osteoperiosteal defect of 15 mm was created through the whole
thickness of the shaft of the right radius by removing the
segmental bone. Staphylococcus aureus (ATCC28923) suspension
(0.2 ml) at a concentration of 5×106 colony forming
units (CFU)/ml was injected into each defect. After 60 min, the
defects were washed with sterile saline. In group A, the defects
were filled with one IC-VA/CPC containing 2 mg of IC and 20 mg of
VA. In groups B and C, the defects were filled with one IC/CPC
(containing 2 mg of IC) and VA/CPC (containing 20 mg of VA),
respectively. The defects of group D were left empty without any
filling material as a blank control. The muscles held the graft in
place without the need for internal fixation or external splints.
At 4, 8 and 12 weeks, four animals from each group were sacrificed
for radiological and histological evaluation. All animal
experiments were conducted according to the Chinese Regulations of
Animal Welfare and permission was granted by the Ethics Committee
of the Ningxia Medical University
Results
Ultrastructure examination
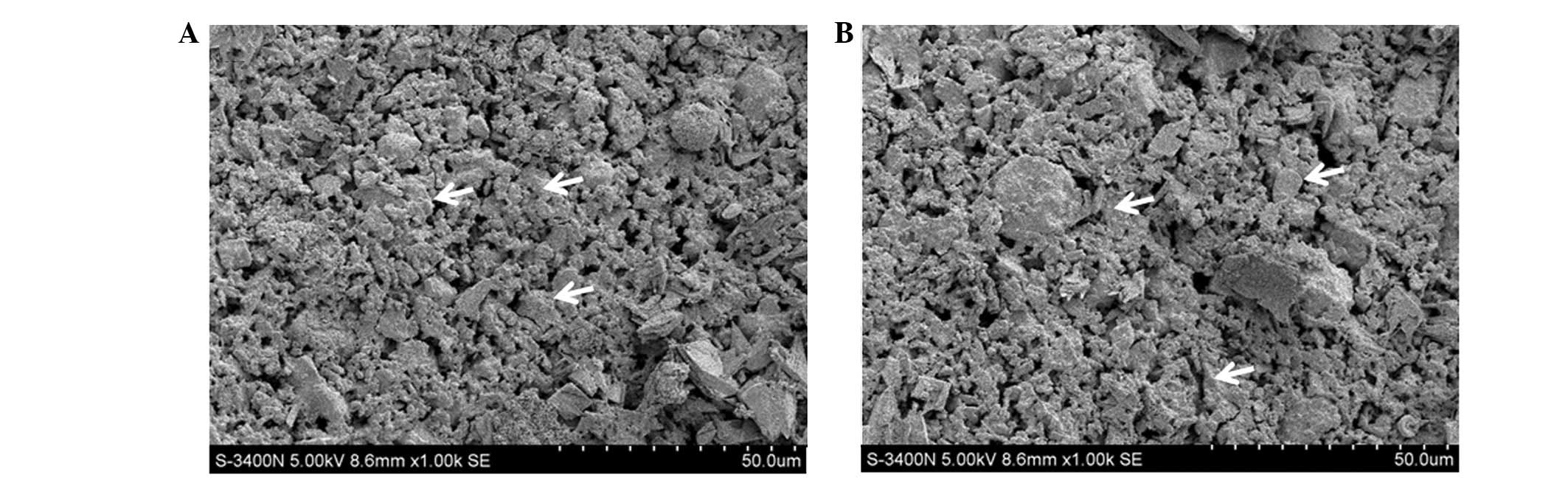
The samples of CPC (Fig. 1A) and IC-VA/CPC (Fig. 1B) both exhibited three-dimensional
structures with numerous granules. The SEM images demonstrated that
the loading of IC and VA caused no clear changes on the
ultrastructure of CPC. Using SEM, it was also observed that the
granules contained in the samples were ~5–10 μm and ball-like or
irregular, and were formed on the surface and in the middle of the
samples. Furthermore, among the granules, numerous spongy pores
were formed, which is hypothesized to be helpful for the
degradation of the scaffold as well as the release of drugs.
Biocompatibility analysis
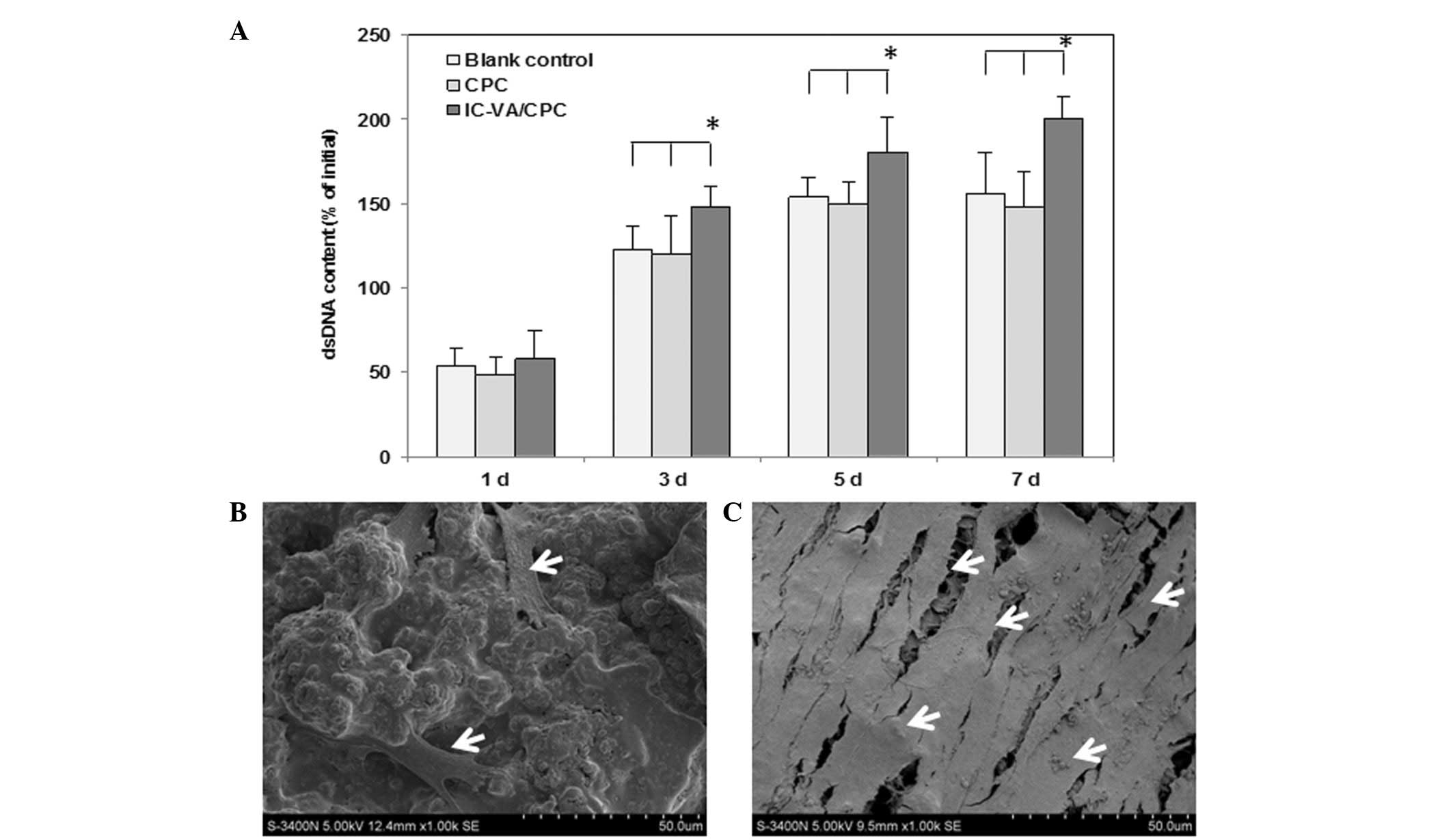
The proliferation of the cells, which were
co-cultured with CPC and IC-VA/CPC, was interpreted by changes in
dsDNA quantity. In the CPC and blank control groups, cells
proliferated faster during the first 3 days and plateaued at day 5,
as indicated by the change in dsDNA content. By contrast, the dsDNA
contents of IC-VA/CPC increased rapidly from 1 to 7 days. Between
days 3 and 7, the dsDNA contents of the IC-VA/CPC group were
significantly higher than those of the CPC and blank control groups
(P<0.05). There were no significant differences in dsDNA content
between the CPC and blank groups (P>0.05; Fig. 2A). These findings were further
corroborated by SEM analysis. One day after the seeding of 3T3
cells on the CPC and IC-VA/CPC, cells appeared flattened, polygonal
and spindle-shaped and were distributed evenly. At 7 days, the
number of cells on the surface of IC-VA/CPC (Fig. 2B) was increased compared with that
on the surface of CPC (Fig. 2C). A
number of cells on the IC-VA/CPC had spread and colonized patches
of the CPC surface. The spread cells maintained physical contact
with each other through filopodia or lamellipodia.
Release kinetics of IC and VA from
IC-VA/CPC
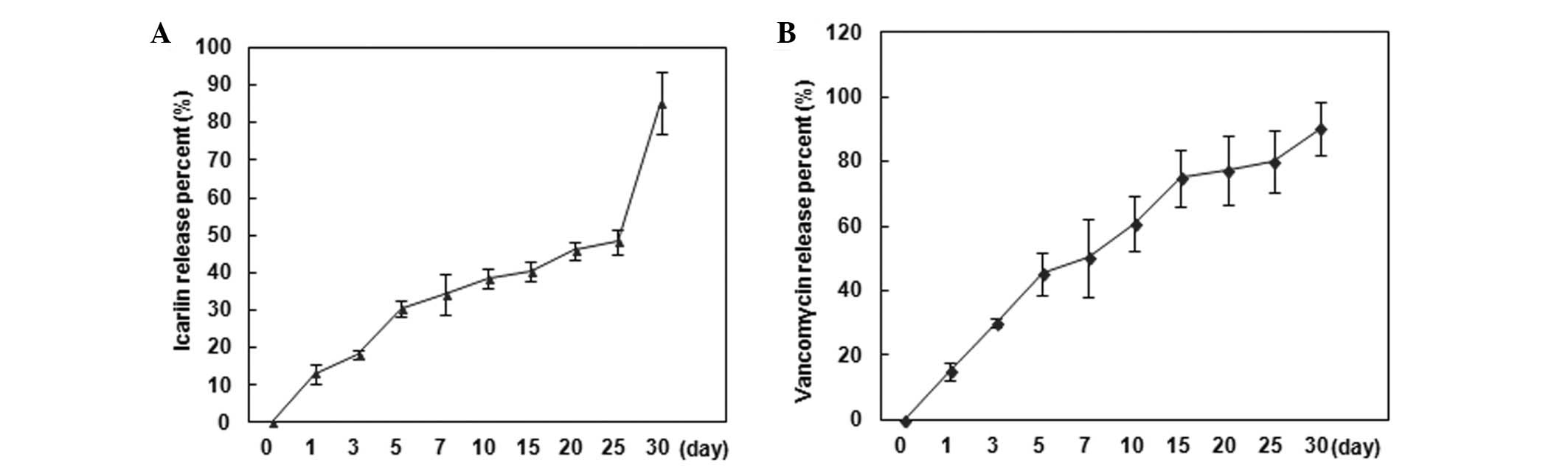
The drug release behavior of the IC-VA/CPC system
in vitro was investigated by HPLC examination and then
calculated based on a standard curve, and was demonstrated as the
accumulated percentage of IC and VA release, respectively (Fig. 3). The IC-release profile exhibited
a low burst of rapid drug release. From 0 to 7 days, ~35% IC was
released and then the speed decreased and <50% of IC was
released by 25 days. From 25 days, there was a second rapid release
and 30 days later there was ~15% of IC remaining in the system
(Fig. 3A). The VA-release profile
showed a modest burst of rapid drug release. From 0 to 5 days, ~45%
VA was released and then the speed decreased and ~90% VA was
released by 30 days (Fig. 3A).
Osseous defect repaired by IC-VA/CPC
All wounds in the rabbits of group A (IC-VA/CPC) and
group C (VA/CPC) healed completely within 10 days. The wounds of
group D (blank control) showed delayed healing of 3–5 weeks, while
the wounds in group B (IC/CPC) failed to heal after >5 weeks.
The wounds of certain rabbits in groups B and D appeared red and
swollen for >7 days, and there was pus exuding from the wounds.
Upon bacteriological examination, S. aureus ATCC28923 was
detected in the secreted pus. Pus secretion in the wound area
stopped and was absorbed without any treatment in >3 weeks. Of
the total 48 rabbits used in this study, three animals died; on the
50th day (one animal in group D), the 10th day (one animal of group
B) and the 14th day (one animal of group B), respectively. Blood
samples from the dead rabbits, collected from the heart under
sterile conditions, were cultured for 48 h and S. aureus was
detected. Twelve weeks after surgery, S. aureus could still
be detected in the bone defects of rabbits from groups B (9/10) and
D (2/11) by tissue culture, but not in any animals of groups A and
C.
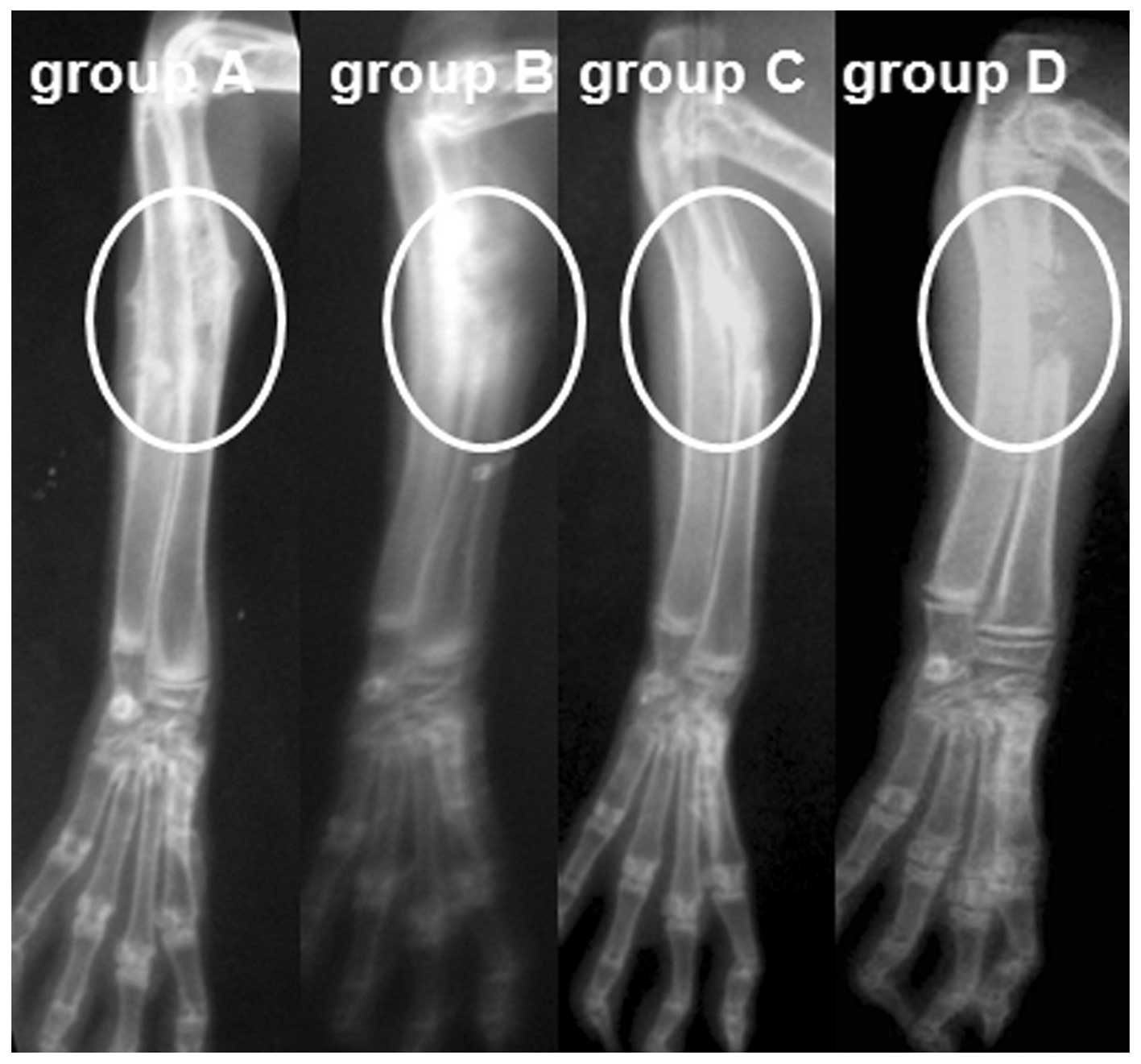
Through radiological observation, a standard defect
shadow was observed at the radius of rabbits in group D, while in
groups A, B and C, the defects were filled with high-density
material, as revealed by the postoperative radiograph. In animals
of group A, a bone callus formed by the 4th week, defects started
repairing by the 8th week and the medullary cavity was recanalized
by the 12th week. By comparison, the defects appeared to be delayed
union or nonunion in group C (delayed union, 7; nonunion, 5) and
group D (nonunion, 11), and osteomyelitis was observed in group B
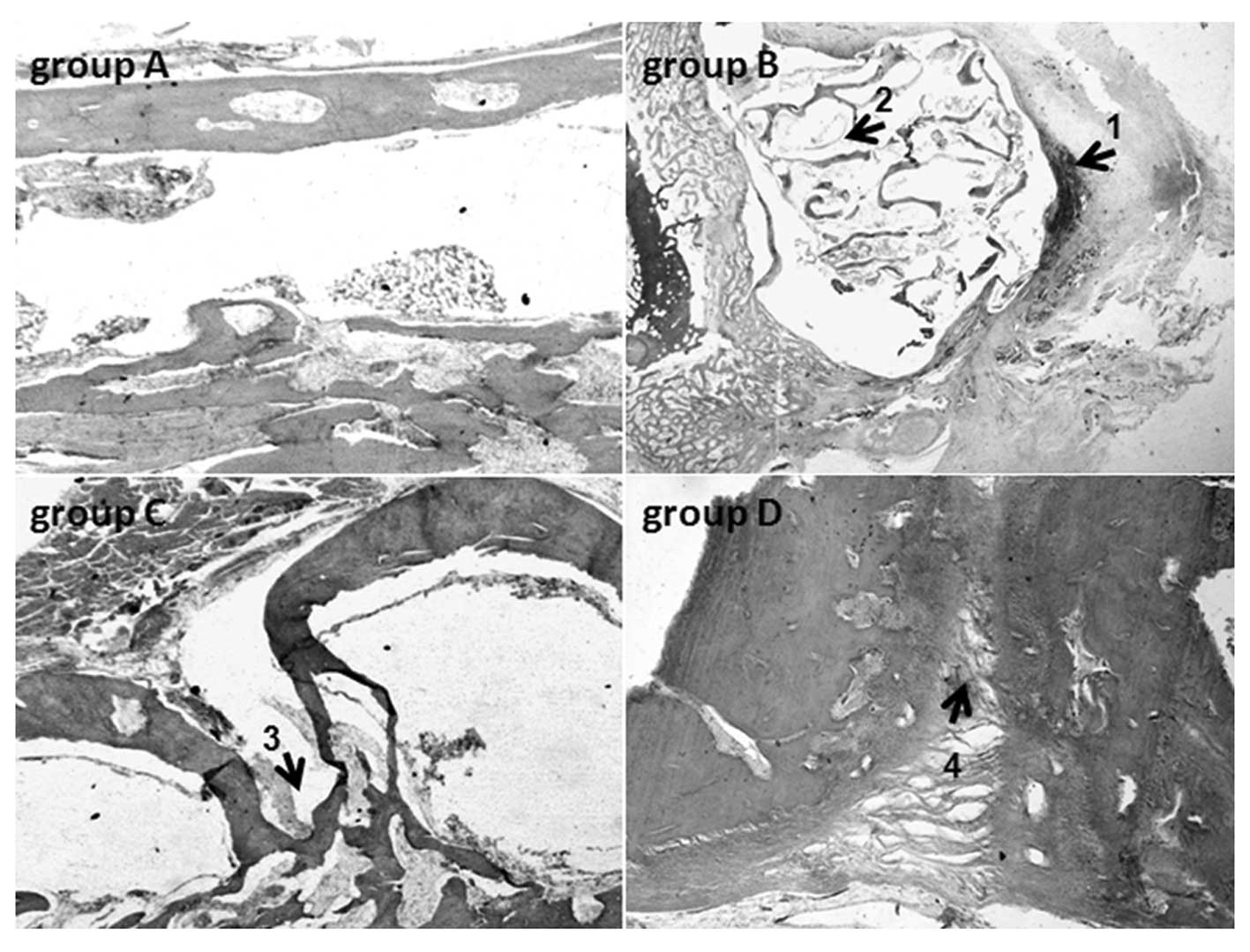
(10/10) at week 12 (Fig. 4).
In accordance with the radiological examination,
different changes were observed in the four groups by histological
evaluation. In group A, chondrogenesis and osteogenesis were
observed, while no indications of inflammatory infiltration were
found 4 weeks postoperatively. On the 8th week, copious new bone
tissue was observed within and around the material. There was clear
creeping substitution in some areas and the graft was disorganized,
largely resorbed and combined with the new bone. The woven bone,
lamellar bone and recanalization of the marrow cavity were
identified in the majority of the specimens, and the defects were
found to have been completely repaired by the 12th week. In group
B, animals showed signs of infiltration of chronic inflammatory
cells, while the remaining material degraded into small fragments
and was embedded in the connective tissue. In group C, a number of
animals (7/12) had bone defects that were filled with scar tissue,
while the other animals (5/12) had repaired defects. In group D,
the bone defects of the animals (11/11) were filled with scar
tissue (Fig. 5).
Discussion
In the present study, we firstly prepared the
solution of IC and VA, respectively, at various concentrations and
then mixed them with PCP powder to create a precursor paste.
Subsequent homogeneous distribution of the drugs was obtained by
stirring, and spongy pores were also formed at that time. After
freeze-drying, the two drugs were completely absorbed by CPC, which
had the lowest effect on the porosity of the composite. Moreover,
in situ composition CPC paste may contain homogeneous
dispersed granules, which are easily aggregated and hard scattered.
Using water as a pore-forming agent, the freeze-drying technique
contributed greatly to the production of satisfactory micro-pores
(11).
The requirements of injectable scaffolds are
numerous, but the most important factor is that they must be
biocompatible. CPC has been shown to have good biocompatibility and
possess excellent osteoconductivity and bone replacement
capability. Due to its highly promising potential for use in a
number of restorative dental and craniofacial procedures, CPC was
approved by the Food and Drug Administration (FDA) for craniofacial
indications a decade ago (19). IC
has been shown to have a number of positive effects on the
proliferation of osteoblasts and BMSCs in several studies (14–15).
In the present study, we used 3T3 cells for the biocompatibility
test according to the ISO standard (10993-12)(20) and found that IC did not have any
negative effects on the biocompatibility of CPC. Notably, the
introduction of IC to CPC appeared to have much improved effects on
the proliferation of 3T3 cells; the number of surviving cells
cultured in the IC-VA/CPC extracts was higher than that cultured in
the pure CPC extracts and blank control at the seventh day of
culture. We hypothesize that the mechanism may be related to the
mitogen-activated protein kinase (MAPK) signaling pathways
(21), and further, more detailed
studies are required. VA has been reported to have a less negative
effect on new bone formation than other antibiotics and has also
been demonstrated not to compromise the healing effect of BMP-2 in
a non-infected segmental defect model (6). Based on the results of the
biocompatibility test, we hypothesize that VA at the concentration
used in this study also has no negative effect on the proliferation
of 3T3 cells.
For the regeneration of bone tissue, an ideal local
antibiotic release system should serve two primary roles; as a
delivery carrier to provide maximal antibiotics for infection
control without causing side-effects, such as myelosuppression,
nephrotoxicity and drug-induced hepatitis, and as an
osteoconductive scaffold with suitable pore structure for
osteoinductive agent loading and bone formation (16). The optimal release kinetics of the
high dose of osteoinductive agents and the co-delivery of
antibiotics should overcome the detrimental effects of bacteria on
bone healing. In the present study, we observed that CPC allowed
the dual-delivery of VA and IC in the local area of injury for more
than 30 days, which is hypothesized to be helpful for the control
of infection and improvement of bone healing at the same time. In
particular, the burst release of VA during the first five days
should contribute greatly to the inhibition of bacteria.
It is well-known that Staphylococcus strains
cause the majority of infectious complications in orthopedics and
traumatology (22).
Methicillin-resistant S. aureus (MRSA) strains and
coagulase-negative staphylococci (MRCoNS) impose a serious
limitation on the selection of effective antibiotics in both
prophylaxis and therapy. Glycopeptide antibiotics, such as
vancomycin and teicoplanin, still maintain their high efficacy
against Staphylococcus strains. Resistance to this group of
antibiotics remains rare and has only been observed in strains with
lower sensitivity to VA (VA intermediate S. aureus) and a
minimum inhibitory concentration (MIC) of 4–8 mg/l (20,23).
In this study, VA was loaded in CPC for the control of infection
and was shown to be effective for the treatment of S.
aureus-infected bone defects (VA/CPC and IC-VA/CPC groups). In
contrast to the groups with VA, the groups without VA (IC/CPC and
blank control groups) exhibited serious signs of chronic
inflammation. Although this model, with an inoculum of
5×106 of this strain of S. aureus and 1 h
treatment delay, is not fully susceptible to local antibiotics, it
has previously been shown to yield significant levels of bacterial
infections (1). Moreover, all
animal models are contrived and it is difficult to mimic the
clinical scenario. We chose to use an extremely challenging model
to ensure that a substantial bio-burden would be present to test
this dual-purpose graft. Due to a lack of osteoinductive agents,
the defects in the VA/CPC group were not completely repaired,
although no indication of infection was found in this group. By
contrast, ossification and medullary cavity recanalization were
observed in defects of the IC-VA/CPC group. Based on these results,
we hypothesize that IC may be used as an effective osteoinductive
agent for the treatment of fractures and bone defects.
In conclusion, these results demonstrate that
IC-VA/CPC as a local antibiotic release system for treating
infection-related bone disease in patients with open fractures
displays several advantages. The CPC bone graft is injectable,
provides a more sustained release of icariin or other
osteoinductive molecules and is able to release antibiotics for
more than 30 days. Thus, the dual-delivery approach may improve
patient outcomes by providing osteoinductive agents and antibiotics
until bone healing occurs. In addition, the slow, sustained release
of antibiotics at the site of infection yields elevated local
concentrations, while minimizing any risk of systemic toxicity. A
variety of antibiotics may be selected to cover both Gram-negative
and Gram-positive bacteria, thereby facilitating individualized
chemotherapy. Furthermore, in contrast to expensive growth factors,
icariin is low-cost. Finally, the biodegradability of the scaffold
materials eliminates the requirement for a second surgical
procedure for their removal.
Acknowledgements
This study was supported by the Key Projects of
Science and Technology Pillar Program of Ningxia Province,
China.
References
|
1
|
Bi L, Hu Y, Fan H, Meng G, Liu J, Li D and
Lv R: Treatment of contaminated bone defects with
clindamycin-reconstituted bone xenograft-composites. J Biomed Mater
Res B Appl Biomater. 82:418–427. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korkusuz F, Korkusuz P, Eksioglu F, Gursel
I and Hasirci V: In vivo response to biodegradable controlled
antibiotic release systems. J Biomed Mater Res. 55:217–228. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shinto Y, Uchida A, Korkusuz F, Araki N
and Ono K: Calcium hydroxyapatite ceramic used as a delivery system
for antibiotics. J Bone Joint Surg Br. 74:600–604. 1992.PubMed/NCBI
|
|
4
|
Cornell CN, Tyndall D, Waller S, Lane JM
and Brause BD: Treatment of experimental osteomyelitis with
antibioticim-pregnated bone graft substitute. J Orthop Res.
11:619–626. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moehring HD, Gravel C, Chapman MW and
Olson SA: Comparison of antibiotic beads and intravenous
antibiotics in open fractures. Clin Orthop Relat Res. 372:254–261.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guelcher SA, Brown KV, Li B, Guda T, Lee
BH and Wenke JC: Dual-purpose bone grafts improve healing and
reduce infection. J Orthop Trauma. 25:477–482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bi L, Cheng W, Fan H and Pei G:
Reconstruction of goat tibial defects using an injectable
tricalcium phosphate/chitosan in combination with autologous
platelet-rich plasma. Biomaterials. 31:3201–3211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cui G, Li J, Lei W, Bi L, Tang P, Liang Y,
Tao S and Wang Y: The mechanical and biological properties of an
injectable calcium phosphate cement-fibrin glue composite for bone
regeneration. J Biomed Mater Res B Appl Biomater. 92:377–385.
2010.PubMed/NCBI
|
|
9
|
Urabe K, Naruse K, Hattori H, Hirano M,
Uchida K, Onuma K, Park HJ and Itoman M: In vitro comparison of
elution characteristics of vancomycin from calcium phosphate cement
and polymethylmethacrylate. J Orthop Sci. 14:784–793. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okada M, Sangadala S, Liu Y, Yoshida M,
Reddy BV, Titus L and Boden SD: Development and optimization of a
cell-based assay for the selection of synthetic compounds that
potentiate bone morphogenetic protein-2 activity. Cell Biochem
Funct. 27:526–534. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan JJ, Cao LG, Wu T, Wang DX, Jin D,
Jiang S, Zhang ZY, Bi L and Pei GX: The dose-effect of icariin on
the proliferation and osteogenic differentiation of human bone
mesenchymal stem cells. Molecule. 16:10123–10133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao J, Ohba S, Komiyama Y, Shinkai M,
Chung UI and Nagamune T: Icariin: a potential osteoinductive
compound for bone tissue engineering. Tissue Eng Part A.
16:233–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang F, Wang XL, Wang NL and Yao XS: Two
new flavonol glycosides from Epimedium koreanum Nakai. J
Asian Nat Prod Res. 11:401–409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nian H, Ma MH, Nian SS and Xu LL:
Antiosteoporotic activity of icariin in ovariectomized rats.
Phytomedicine. 16:320–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao J, Ohba S, Shinkai M, Chung UI and
Nangamue T: Icariin induces osteogenic differentiation in vitro in
a BMP-and Runx2-dependent manner. Biochem Biophys Res Commun.
369:444–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan J, Bi L, Wu T, Cao L, Wang D, Nan K,
Chen J, Jin D, Jiang S and Pei G: A combined chitosan/nano-size
hydroxyapatite system for the controlled release of icariin. J
Mater Sci Mater Med. 23:399–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bitar M, Friederici V, Imgrund P, Brose C
and Bruinink A: In vitro bioactivity of micro metal injection
moulded stainless steel with defined surface features. Eur Cell
Mater. 23:333–347. 2012.PubMed/NCBI
|
|
18
|
Li HB and Chen F: Separation and
purification of epimedin A, B, C, and icariin from the medicinal
herb Epimedium brevicornum maxim by dual-mode HSCCC. J
Chromatogr Sci. 47:337–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Larsson S and Bauer TW: Use of injectable
calcium phosphate cement for fracture fixation: a review. Clin
Orthop Relat Res. 395:23–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
International Organization for
Standardization. ISO 10993-12:2007: Biological evaluation of
medical devices. Part 12: Sample preparation and reference
materials. ISO; Geneva, Switzerland: 2007
|
|
21
|
Yang L, Wang NL and Cai GP: Maohuoside A
promotes osteogenesis of rat mesenchymal stem cells via BMP and
MAPK signaling pathways. Mol Cell Biochem. 358:37–44. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Melicherčík P, Jahoda D, Nyč O, Klapková
E, Barták V, Landor I, Pokorný D, Judl T and Sosna A: Bone grafts
as vancomycin carriers in local therapy of resistant infections.
Folia Microbiol (Praha). 57:459–462. 2012.PubMed/NCBI
|
|
23
|
Bert F, Leflon-Guibout V, Le GJ, Bourdon N
and Nicolas MH: Emergence of vancomycin-dependent enterococci
following glycopeptide therapy: case report and review. Pathol Biol
(Paris). 57:56–60. 2009.(In French).
|