Introduction
Chronic obstructive pulmonary disease (COPD) is a
common illness with increasing incidence in elderly people
(1,2). Smoking is the most significant risk
factor contributing to COPD (3,4), as
the cigarette smoke-induced alveolar epithelial cell apoptosis is
an important cause of COPD emphysema (5–7).
Numerous studies have also demonstrated that cigarette smoke
induces endoplasmic reticulum stress (ERS) and bronchial epithelial
cell apoptosis (8,9). However, the influence of cigarette
smoke on alveolar epithelial cells remains poorly understood.
Glucose-regulated protein 78 (GRP78), a chaperone
protein of endoplasmic reticulum, is termed ERS protein marker. In
the early stage of ERS, the upregulated GRP78 expression (10) is associated with protein folding
and maintenance of endoplasmic reticulum calcium homeostasis, which
reduces the ERS (11). An
increasing number of studies have shown the anti-apoptotic nature
of GRP78 (12–14). However, it remains to be
demonstrated whether GRP78 is protective in the process of
cigarette smoke extract (CSE)-induced apoptosis of alveolar
epithelial cells.
Furthermore, the signal transduction pathway to
upregulate GRP78 expression remains unknown, regardless of the fact
that studies have confirmed its protective nature.
Mitogen-activated protein kinase (MAPK) is an important cell
response-mediated signaling system. MAPK consists of three
predominant families, the extracellular signal-regulated kinase
(ERK), c-Jun N-terminal kinase (JNK) and p38/MAPK families. The
p38/MAPK pathway is important in a variety of physiological and
pathological processes, including cell growth, cell cycle,
inflammation, cell stress and apoptosis (15). It has been demonstrated that the
activation of the p38/MAPK pathway may be involved in the
upregulation of GRP78. Shear stress related blood damage
upregulates GRP78 levels in vascular endothelial cells through the
p38/MAPK pathway, leading to reduced apoptosis of vascular
endothelial cells (16). Moreover,
lipid-lowering drugs, such as atorvastatin, reduce macrophage
apoptosis by increasing GRP78 expression through the p38/MAPK
pathway (17). Studies have also
shown that cigarette smoke activates the p38/MAPK pathway in COPD
(18–20), suggesting that the activation of
the p38/MAPK pathway may be associated with the upregulation of
GRP78 in COPD.
In conclusion, it was hypothesized that cigarette
smoke induces GRP78 upregulation in alveolar epithelial cells
through the p38/MAPK pathway and GRP78 upregulation exerts
anti-apoptotic effects on alveolar epithelial cells. In the present
study, using A549 cells, the CSE-induced GRP78 expression was
investigated at mRNA and protein levels with RT-PCR and western
blot analysis. The apoptosis of A549 transfected by GRP78 siRNA was
analyzed to investigate the anti-apoptotic properties of GRP78. p38
inhibitor, SB203580, was used to investigated the involvement of
the p38/MAPK pathway in CSE-induced GRP78 expression.
Materials and methods
Cell lines and reagents
A549 cells, a cell line widely used in vitro
for type II pulmonary epithelial cell-related studies, were
purchased from the cell center of Xiangya Medical College
(Changsha, Hunan, China). Cigarettes (Lotus brand) were provided by
China Tobacco Hunan Industrial Co., Ltd. (Changsha, Hunan, China)
and Dulbecco’s modified Eagle’s medium (DMEM) was purchased from
Gibco-BRL (Grand Island, NY, USA). The p38 inhibitor SB203580, was
purchased from Alexis Corporation (Lausen, Switzerland). Goat
polyclonal antibody against GRP78, GRP78 siRNA and control siRNA
(fluorescein conjugated) were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA). Rabbit polyclonal active caspase-3 antibody was obtained
from Abcam (Cambridge, UK). The secondary antibodies were obtained
from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.,
(Beijing, China), and an In Situ Cell Death Detection kit was
purchased from Roche (Basel, Switzerland). The study was approved
by the ethics committee of Xiangya Hospital, Central South
University (Changsha, China).
Cell culture
A549 cells were cultured in DMEM containing 4.5
mg/ml glucose and supplemented with 10% fetal bovine serum (FBS;
Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.,
Hangzhou, China) in a humidified incubator with 5% CO2
at 37°C. The cells were passaged into a 6-well culture dish at an
optimal density of 2×104 cells/ml and starved for 24 h
in high glucose DMEM containing 1% FBS, when the cells had reached
~80% confluence. The cells were then subjected to the designed
experiments in this study. The cell number was counted using a
trypan blue exclusion assay (Beyotime Institute of Biotechnology,
Jiangsu, China).
Preparation of CSE
CSE was prepared using a device made by our
laboratory (21) according to the
method described previously (22).
CSE was freshly produced from three cigarettes by blowing smoke,
generated using a vacuum syringe system on a smoking machine,
through 3 ml of phosphate buffered saline (PBS) in a siliconised
glass tonometer (Vitalograph, Buckingham, UK). The solution was
filtered through 0.22-μm cellulose membranes to achieve sterile
smoke extract solutions. Absorbance values were measured by a UV
spectrophotometer (DU-640 UV-VIS Biotech Spectrophotometer; Beckman
Coulter, Miami, FL, USA) at 270–280 nm. The concentration of the
prepared CSE was set as 100%. This solution was applied immediately
to the cell cultures when the cells had been cultured for 24 h. CSE
with 10% FBS at different concentrations was added to the culture
media. The cells were treated with CSE at 37°C in a humidified
atmosphere containing 5% CO2 for various time periods
according to the experimental design.
siRNA experiments
GRP78 inhibition was performed using commercially
available siRNA kits (human, sc-29338; Santa Cruz Biotechnology,
Inc.), in which the siRNA is target-specific, designed to knockdown
the gene expression. The minimal levels of GRP78 was assessed by
Western blotting. For siRNA experiment, negative controls were
performed using the irrelevant siRNA provided in the siRNA kits and
using the siRNA buffers. The irrelevant siRNA was a scrambled
sequence which did not lead to the specific degradation of any
cellular mRNA (Santa Cruz Biotechnology). The transfection was
performed according to the manufacturer’s instructions. A549 cells
were transfected in 6-well plates according to the manufacturer’s
instructions. The cells were divided into four groups the control,
CSE, CSE+GRP78 siRNA and CSE+control siRNA groups.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from the cells using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according
to the manufacturer’s instructions. cDNA was synthesized using the
Superscript II reverse transcription system (Invitrogen Life
Technologies). Primer sequences were as follows: Forward:
5′-TCTGCTTGATGTGTGTCCTCTT-3′ and reverse: 5′-GTC
GTTCACCTTCGTAGACCT-3′ for GRP78, PCR product 156 bp; and forward:
5′-GGAAGGTGAAGGTCGGAGT-3′ and reverse: 5′-GCTCCTGGAAGATGGTGATGG-3′
for GAPDH, PCR product 234 bp (synthesized by Shanghai Sangon
Biological Engineering Technology & Service Co., Ltd.,
Shanghai, China). Amplification conditions were as follows:
pre-denaturation at 94°C for 5 min; denaturation at 94°C for 30
sec, annealing at 59°C for 30 sec and elongation at 72°C for 1 min
for a total of 30 cycles, and finally an elongation step at 72°C
for 10 min. The PCR products were separated by electrophoresis
using 1.5% agarose gel. The absorbance values of product bands were
analyzed with an automatic image analysis system (FluorChem 8900
software system; Alpha Innotech, Witec, Littau, Switzerland).
Western blot analysis
Total protein was extracted and then separated by
sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis.
The proteins were transferred to polyvinylidene fluoride membranes
for western blot analysis. Primary antibodies against GRP78
(1:1,000 dilution), p38 (1:500), P-p38 (1:500), active caspase-3
(1:200) and β-actin (1:2,000) were purchased from Santa Cruz
Biotechnology, Inc. The dilution of secondary antibody was 1:2,000.
ECL exposure images were captured. Absorbance analysis was
conducted using an automatic image analysis system (FluorChem 8900
software system). The ratio of phosphorylated p38 (P-p38) to p38
was taken as the relative expression level of proteins. The ratio
of remaining indicators to β-actin bands was taken as the relative
expression levels of proteins.
Deoxynucleotidyl transferase dUTP
nick-end labeling (TUNEL)
The TUNEL analysis was performed using the In Situ
Cell Death Detection kit (POD, Roche, Basel, Switzerland),
according to the manufacturer’s instructions. The labeled solution
served as the negative control. The cell apoptosis index (AI) was
obtained by counting the TUNEL-positive cells and total cells in
the selected areas on the slides. AI = TUNEL - positive cells/total
cells × 100%.
Statistical analysis
Statistical analyses were performed using SPSS 15.0
software (SPSS Inc., Chicago, IL, USA). The data were presented as
the mean ± SD. Multiple sets of measurement data were compared
using single factor analysis of variance. All statistical tests
were two-sided using a significance level of α=0.05. P<0.05 was
considered to indicate a statistically significant difference.
Results
CSE induces GRP78 expression in A549
cells
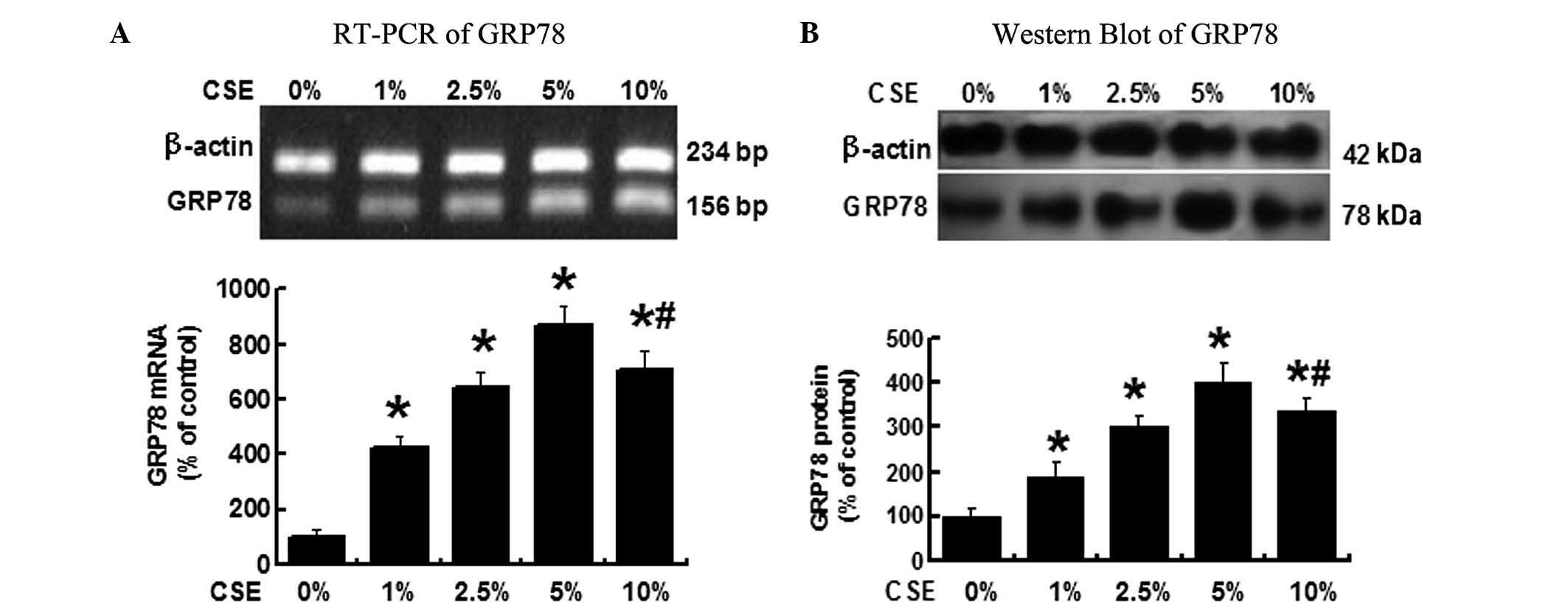
As shown in Fig.
1A, when the A549 cells were treated with different
concentrations of CSE (0, 1, 2.5, 5 and 10%) for 12 h, RT-PCR
demonstrated that the GRP78 mRNA levels were significantly
increased in the cells treated by 1, 2.5, 5 and 10% CSE compared
with the cells treated with 0% CSE, P<0.05. Among the various
CSE concentrations, 5% CSE induced the highest GRP78 expression.
The GRP78 mRNA level, however, declined when CSE concentration was
increased to 10%, but the level remained greater than that when the
cells treated with 2.5% CSE.
The GRP78 protein levels were determined by western
blot analysis (Fig. 1B) and
demonstrated a similar change in the GRP78 mRNA. GRP78 protein was
significantly increased following the CSE treatment, cells treated
with 5% CSE exhibited the highest GRP78 protein level, P<0.05
vs. all other groups. These results suggested that CSE induces
GRP78 expression in A549 cells and 5% CSE is the concentration
which induces the greatest level of GRP78 expression in the
cells.
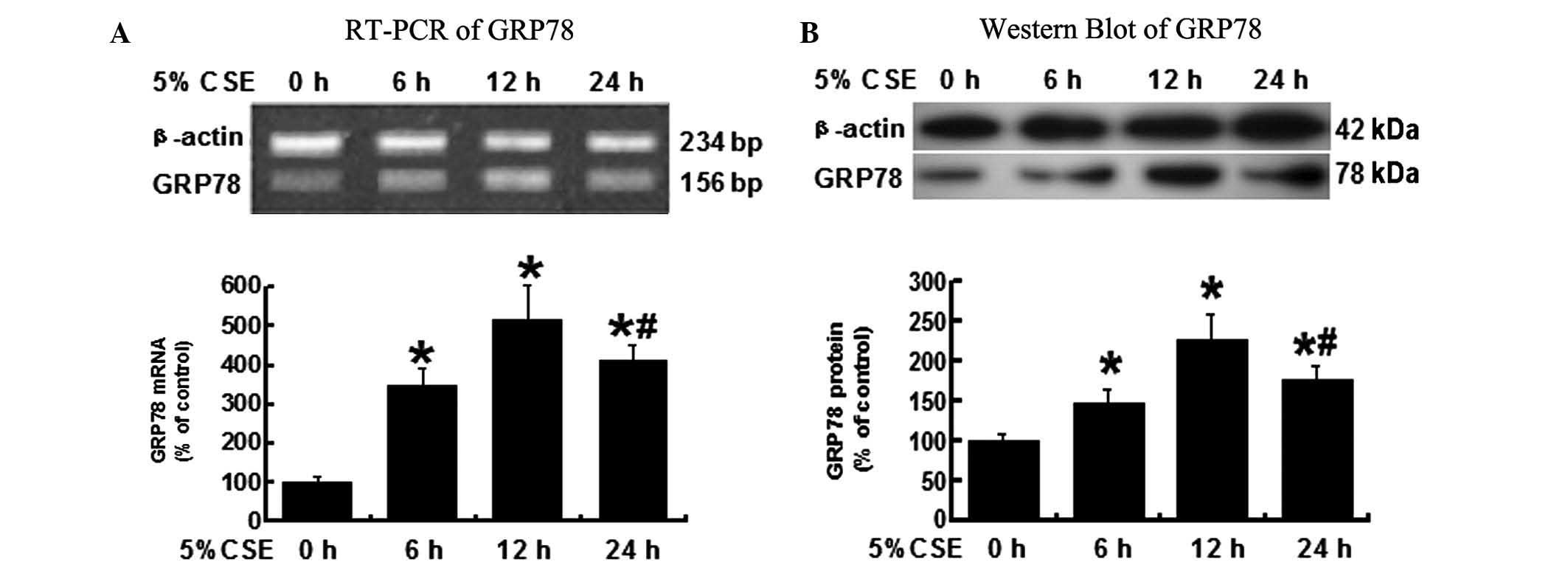
12-h 5% CSE treatment is the most
efficient to induce GRP78 expression
To determine the time duration of CSE
administration, which induces the greatest GRP78 expression, the
A549 cells were treated with 5% CSE for 0, 6, 12 or 24 h. Cells
were collected for RNA isolation and protein extraction. As shown
in Fig. 2A, the GRP78 mRNA levels
measured by RT-PCR were significantly higher in the cells treated
by 5% CSE for 6, 12 and 24 h, when compared with the untreated
cells (0 h), P<0.05.
Similar to the RT-PCR results, the protein levels of
GRP78 that were tested by western blot analysis were significantly
increased in all the groups except 0 h group (Fig. 2B). The 12 h treatment of 5% CSE
induced the most significant expression of GRP78 protein, P<0.05
vs. all the other groups. These results suggest that the 12 h
treatment of 5% CSE is the most efficient to induce GRP78
expression in A549 cells.
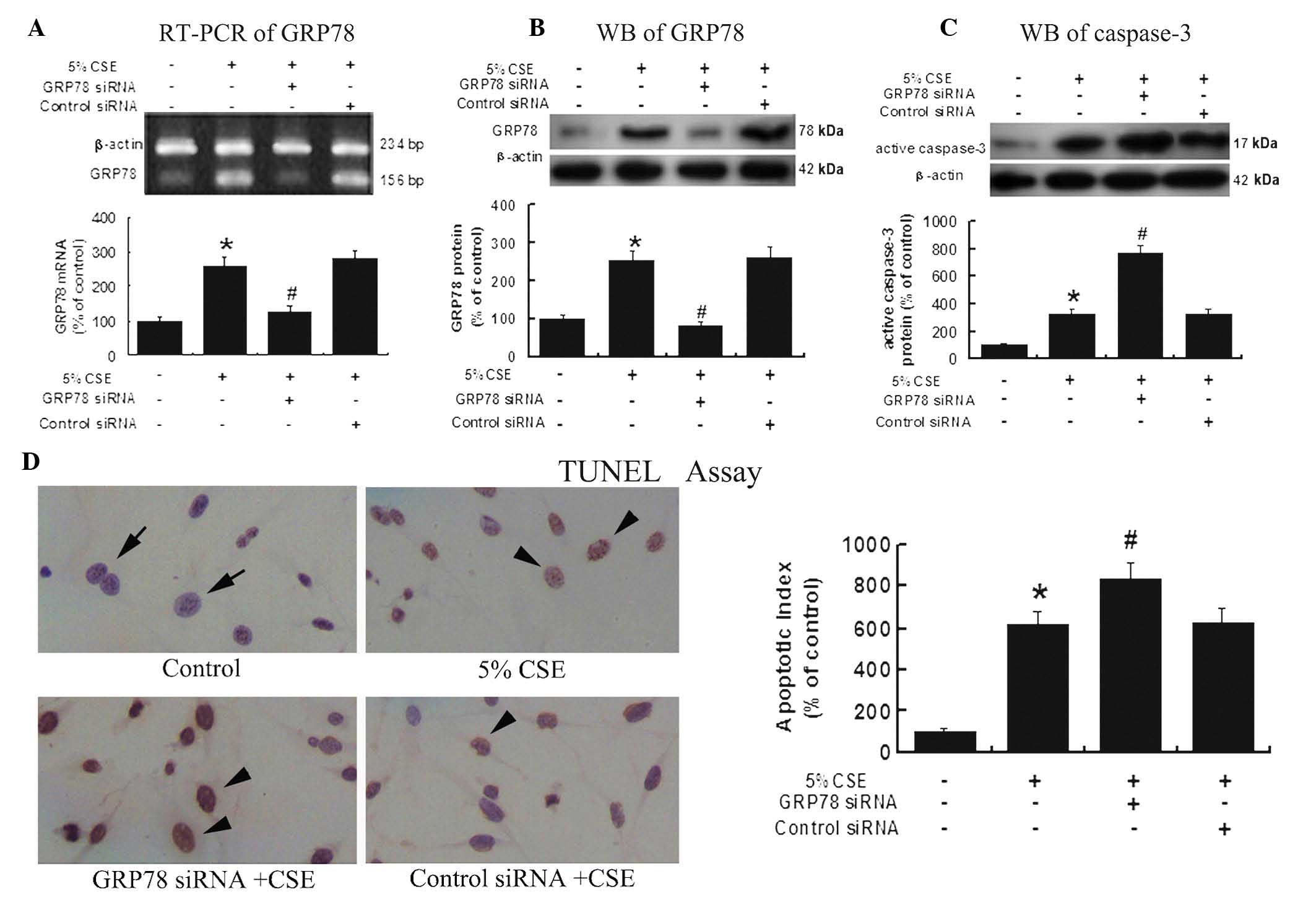
Upregulated GRP78 expression under CSE
insult is protective against CSE-induced apoptosis
To further investigate the anti-apoptotic effects of
GRP78, A549 cells were transfected with GRP78 siRNA or control
siRNA, then subjected to 5% CSE treatment for 12 h. RT-PCR and
western blot analysis results (Fig. 3A
and B) showed that the GRP78 expression in the cells from the
GRP78 siRNA group was significantly decreased compared with the
cells from the control siRNA group, P<0.05.
Notably, western blot analysis (Fig. 3C) also demonstrated a significant
increase in the active caspase-3 protein expression in the group
pretreated with GRP78 siRNA compared with the group treated with
the control siRNA (P<0.05), suggesting that GRP78 exhibits
inhibitive effects on caspase-3, a predominant apoptosis activator.
Thus suppressing GRP78 may enhance caspase-3 activity.
TUNEL results (Fig.
3D) further confirmed the protective effect of GRP78 against
CSE-induced apoptosis. TUNEL showed that the knockdown of GRP78 by
GRP78 specific siRNA resulted in a significant increase of
apoptosis in the A549 cells following CSE exposure.
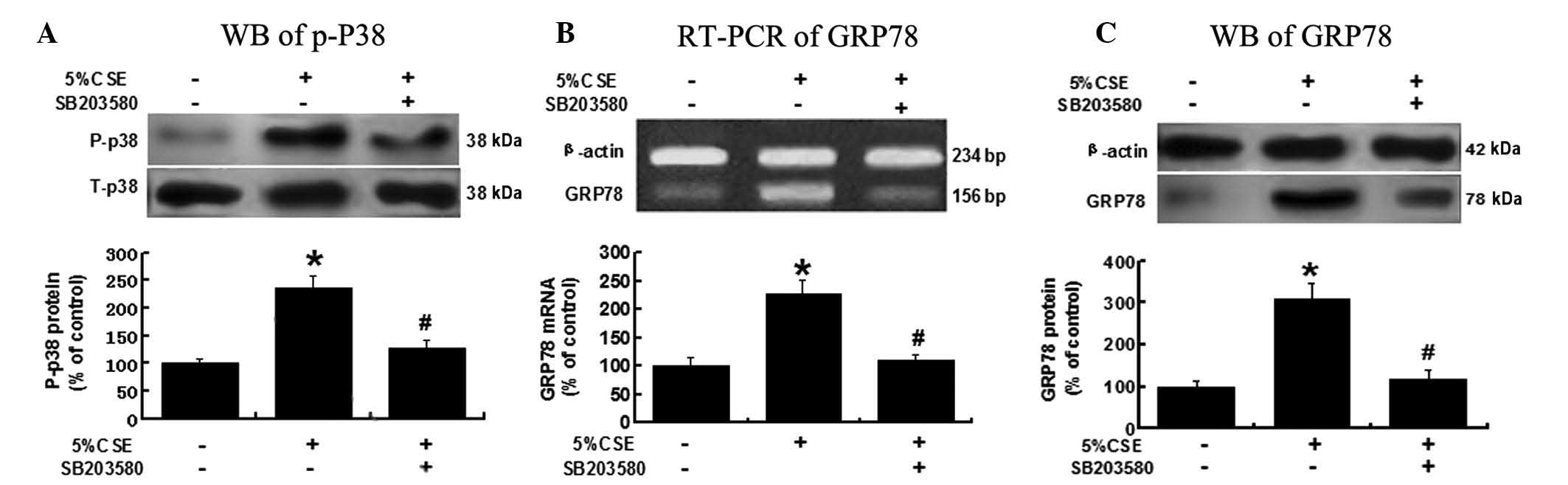
CSE induces GRP78 expression via the
p38/MAPK pathway
To investigate the possible mechanisms underlying
CSE’s effects on GRP78 expression, A549 cells were pretreated with
SB203580 (3 μM), a p38/MAPK pathway inhibitor, and then exposed to
5% CSE. As shown in Fig. 4, the
activity of the p38/MAPK pathway was significantly reduced, as
indicated by a decreased P-p38 level, due to the pretreatment with
SB203580, P<0.05 vs. the 5% CSE group (Fig. 4A). The CSE-induced GRP78 expression
at the mRNA and protein levels was significantly attenuated in the
presence of SB203580, P<0.05 vs. the 5% CSE group (Fig. 4B and C). These results suggested
that CSE regulates GRP78 expression through a p38/MAPK-related
pathway.
Discussion
Stress signals are transmitted from the endoplasmic
reticulum into the nucleus through the unfolded protein response
(UPR) signaling pathway (10,23).
The ERS, to a certain extent activates protection mechanisms, such
as molecular chaperone GRP78 expression that resists stress,
exerting cytoprotective effects (16,24).
When stress levels are too strong, this compromises or overcomes
the protection mechanism, leading to cell damage. Consequently, the
stress-induced upregulation of GRP78 expression becomes
unsustainable; activating caspase-12 and -3, and eventually leading
to apoptosis (25–27).
The involvement of GRP78 in COPD pathogenesis
remains unknown. In 2008, Kelsen et al(8) demonstrated that smoking initiates the
ERS and activates the UPR, leading to overexpression of GRP78 in
vitro in bronchial epithelial cells. Another study demonstrated
that diesel exhaust chemicals were able to induce upregulated GRP78
expression in bronchial epithelial cells (9). In the present study, A549 cells were
treated with CSE and GRP78 expression was observed to increase
within a certain time frame (≤12 h) and concentration (≤5%) of the
CSE treatment. However, when CSE concentration was increased to 10%
and the exposure time to 24 h, GRP78 expression started to decline.
This indicated that when the CSE, as a stressor, is too strong
either in its dose or duration, it results in dysfunction of the
endoplasmic reticulum, leading to a decreased GRP78 expression.
Although GRP78 is considered to be anti-apoptotic
(12–14), its involvement in alveolar
epithelial cells remains unclear. In the present study, the
anti-apoptotic nature of GRP78 was confirmed by genetic knockdown
of GRP78 in the A549 cells. Following GRP78 knockdown, the
decreased GRP78 expression was demonstrated to be correlated with
increased caspase-3 activity and a higher apoptotic index (AI).
The p38/MAPK signaling pathway is predominantly
involved in the inflammatory response of cells and apoptosis
regulation under stress conditions. This pathway is demonstrated to
be activated by reactive oxygen species in cigarette smoke, such as
peroxynitrite, H2O2 and
O2−, in COPD related studies, and the
activated p38/MAPK pathway is important in mediating COPD
inflammation (19,28,29).
Studies have also shown that p38/MAPK pathway is involved in the
regulation of GRP78 expression (16,17).
In the present study, the expression of P-p38 protein, the
activated form of p38, was significantly increased, which was
correlated with an increased GRP78 expression when the cells were
treated by CSE. Moreover, when SB203580, a p38/MAPK inhibitor, was
used prior to CSE exposure, the expression levels of GRP78 and
P-p38 significantly decreased. This result suggested that CSE
induces GRP78 overexpression predominantly through p38/MAPK
pathway.
In conclusion, this study shows that cigarette smoke
induces GRP78 expression in A549 cells, and the upregulated GRP78
expression in the cells may exhibit an anti-apoptotic effect. The
p38/MAPK pathway is involved in the regulation of GRP78 expression.
The A549 cell line originates from alveolar epithelial cells, and
therefore, these cells are commonly used in studies related to
alveolar epithelial cells. The results obtained in the present
study in A549 cells, may therefore be useful in further studies of
COPD and alveolar epithelial cells.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 30971324).
References
|
1
|
Rycroft CE, Heyes A, Lanza L and Becker K:
Epidemiology of chronic obstructive pulmonary disease: a literature
review. Int J Chron Obstruct Pulmon Dis. 7:457–494. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Afonso AS, Verhamme KM, Sturkenboom MC and
Brusselle GG: COPD in the general population: prevalence, incidence
and survival. Respir Med. 105:1872–1884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tamimi A, Serdarevic D and Hanania NA: The
effects of cigarette smoke on airway inflammation in asthma and
COPD: therapeutic implications. Respir Med. 106:319–328. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartal M: COPD and tobacco smoke. Monaldi
Arch Chest Dis. 63:213–225. 2005.
|
|
5
|
Ruwanpura SM, McLeod L, Miller A, Jones J,
Bozinovski S, Vlahos R, Ernst M, Armes J, Bardin PG, Anderson GP
and Jenkins BJ: Interleukin-6 promotes pulmonary emphysema
associated with apoptosis in mice. Am J Respir Cell Mol Biol.
45:720–730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Imai K, Mercer BA, Schulman LL, Sonett JR
and D’Armiento JM: Correlation of lung surface area to apoptosis
and proliferation in human emphysema. Eur Respir J. 25:250–258.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aoshiba K, Yokohori N and Nagai A:
Alveolar wall apoptosis induces lung destruction and emphysematous
changes. Am J Respir Cell Mol Biol. 28:555–562. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelsen SG, Duan X, Ji R, Perez O, Liu C
and Merali S: Cigarette smoke induces an unfolded protein response
in the human lung: a proteomic approach. Am J Respir Cell Mol Biol.
38:541–550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jung EJ, Avliyakulov NK, Boontheung P, Loo
JA and Nel AE: Pro-oxidative DEP chemicals induce heat shock
proteins and an unfolding protein response in a bronchial
epithelial cell line as determined by DIGE analysis. Proteomics.
7:3906–3918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S and Kaufman RJ: The impact of the
unfolded protein response on human disease. J Cell Biol.
197:857–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jäger R, Bertrand MJ, Gorman AM,
Vandenabeele P and Samali A: The unfolded protein response at the
crossroads of cellular life and death during endoplasmic reticulum
stress. Biol Cell. 104:259–270. 2012.PubMed/NCBI
|
|
12
|
Sato Y, Hatta M, Karim MF, Sawa T, Wei FY,
Sato S, Magnuson MA, Gonzalez FJ, Tomizawa K, Akaike T, Yoshizawa T
and Yamagata K: Anks4b, a novel target of HNF4α protein, interacts
with GRP78 protein and regulates endoplasmic reticulum
stress-induced apoptosis in pancreatic β-cells. J Biol Chem.
287:23236–23245. 2012.PubMed/NCBI
|
|
13
|
Suyama K, Watanabe M, Sakabe K, Okada Y,
Matsuyama D, Kuroiwa M and Mochida J: Overexpression of GRP78
protects glial cells from endoplasmic reticulum stress. Neurosci
Lett. 504:271–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kishi S, Shimoke K, Nakatani Y, Shimada T,
Okumura N, Nagai K, Shin-Ya K and Ikeuchi T: Nerve growth factor
attenuates 2-deoxy-d-glucose-triggered endoplasmic reticulum
stress-mediated apoptosis via enhanced expression of GRP78.
Neurosci Res. 66:14–21. 2010. View Article : Google Scholar
|
|
15
|
Kyriakis JM and Avruch J: Mammalian MAPK
signal transduction pathways activated by stress and inflammation:
a 10-year update. Physiol Rev. 92:689–737. 2012.PubMed/NCBI
|
|
16
|
Feaver RE, Hastings NE, Pryor A and
Blackman BR: GRP78 upregulation by atheroprone shear stress via
p38-, alpha2beta1-dependent mechanism in endothelial cells.
Arterioscler Thromb Vasc Biol. 28:1534–1541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen JC, Wu ML, Huang KC and Lin WW:
HMG-CoA reductase inhibitors activate the unfolded protein response
and induce cytoprotective GRP78 expression. Cardiovasc Res.
80:138–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chung KF: p38 mitogen-activated protein
kinase pathways in asthma and COPD. Chest. 139:1470–1479. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lemire BB, Debigaré R, Dubé A, Thériault
ME, Côté CH and Maltais F: MAPK signaling in the quadriceps of
patients with chronic obstructive pulmonary disease. J Appl
Physiol. 113:159–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Renda T, Baraldo S, Pelaia G, Bazzan E,
Turato G, Papi A, Maestrelli P, Maselli R, Vatrella A, Fabbri LM,
et al: Increased activation of p38 MAPK in COPD. Eur Respir J.
31:62–69. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan T, Luo BL, Wei TH, Zhang L, He BM and
Niu RC: Salubrinal protects against cigarette smoke extract-induced
HBEpC apoptosis likely via regulating the activity of PERK-eIF2α
signaling pathway. Arch Med Res. 43:522–529. 2012.PubMed/NCBI
|
|
22
|
Moodie FM, Marwick JA, Anderson CS,
Szulakowski P, Biswas SK, Bauter MR, Kilty I and Rahman I:
Oxidative stress and cigarette smoke alter chromatin remodeling but
differentially regulate NF-kappaB activation and proinflammatory
cytokine release in alveolar epithelial cells. FASEB J.
18:1897–1899. 2004.
|
|
23
|
Zhang K and Kaufman RJ: The unfolded
protein response: a stress signaling pathway critical for health
and disease. Neurology. 66(Suppl 1): S102–S109. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guerriero CJ and Brodsky JL: The delicate
balance between secreted protein folding and endoplasmic
reticulum-associated degradation in human physiology. Physiol Rev.
92:537–576. 2012. View Article : Google Scholar
|
|
25
|
Momoi T: Caspases involved in ER
stress-mediated cell death. J Chem Neuroanat. 28:101–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hitomi J, Katayama T, Taniguchi M, Honda
A, Imaizumi K and Tohyama M: Apoptosis induced by endoplasmic
reticulum stress depends on activation of caspase-3 via caspase-12.
Neurosci Lett. 357:127–130. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weng S, Zhu X, Jin Y, Wang T and Huang H:
Protective effect of erythropoietin on myocardial infarction in
rats by inhibition of caspase-12 expression. Exp Ther Med.
2:833–836. 2011.PubMed/NCBI
|
|
28
|
Moretto N, Bertolini S, Iadicicco C,
Marchini G, Kaur M, Volpi G, Patacchini R, Singh D and Facchinetti
F: Cigarette smoke and its component acrolein augment IL-8/CXCL8
mRNA stability via p38 MAPK/MK2 signaling in human pulmonary cells.
Am J Physiol Lung Cell Mol Physiol. 303:L929–L938. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jope RS, Zhang L and Song L: Peroxynitrite
modulates the activation of p38 and extracellular regulated kinases
in PC12 cells. Arch Biochem Biophys. 376:365–370. 2000. View Article : Google Scholar : PubMed/NCBI
|