Introduction
As the most common form of arthritis, osteoarthritis
(OA) is one of the most significant causes of disability in older
adults (1), yet its exact etiology
remains unknown (2). The diagnosis
and evaluation of joint damage are predominantly based on clinical
and radiological findings. With medical advances, molecular markers
are likely to become promising indicators for evaluating local
inflammation, joint alterations and cartilage damage (3).
Fibroblast-like synoviocytes (FLS) have been
accepted to be key in OA inflammation and joint destruction,
primarily through their secretion of a wide range of
proinflammatory mediators (4,5). In
response to the proinflammatory cytokines, including interleukin-1β
(IL-1β), the OAFLS produce chemokines that promote inflammation,
neovascularization and cartilage degradation via activation of
matrix-degrading enzymes, including matrix metalloproteinases
(MMPs) (5). IL-1β is a
multifunctional cytokine that contributes to the pathogenesis of OA
(6). Investigations into the IL-1β
signaling pathway led to the identification of novel potential
drugs for the treatment of OA (7).
However, current treatment with these drugs remains unsatisfactory,
and further research is required to achieve the desired therapeutic
goals (7).
The inflammasome is a multi-protein complex that
mediates the activation of caspase-1, which in turn produces the
proinflammatory cytokines, IL-1β and IL-18 (8). The human NLRP1 inflammasome was the
first caspase-1-activating protein complex to be identified
(9). Recent studies have indicated
that NLRP3 is involved in the genetic predisposition and
pathogenesis of crystal arthritis, including gouty and rheumatoid
arthritis (10,11). Recently, a study has revealed that
the expression of the P2X4 receptor is required for IL-1β and IL-18
release in mouse bone marrow-derived dendritic cells (12). However, it is unknown whether the
P2X4 receptor modulates the NLRP1-mediated release of IL-1β. In the
present study, the correlation between the P2X4 receptor and NLRP1
was investigated in FLS.
Materials and methods
Patients and synovial samples
Human synovial samples were collected with informed
consent in line with the Declaration of Helsinki (2000 revision).
The study protocol was approved by the local Ethics Committee of
Shandong University (Jinan, China). The diagnosis of OA was based
on clinical and radiological evidence of degenerative changes
during surgery. Synovial tissues were obtained under aseptic
conditions from 30 OA patients undergoing total knee replacement
surgery, and samples of non-arthritic synovial tissues were
obtained at arthroscopy following trauma/joint derangement.
All the patients (n=30; F/M, 19/11; age, 62.9±4.6
years) in this study were enrolled from the Department of
Orthopedic Surgery, The General Hospital of Jinan Military Command
(Jinan, China) and from the Department of Orthopedic Surgery, The
Third Hospital of Jinan (Jinan, China). Data from a medical
history, physical examination, electrocardiogram and routine blood
test were compiled for each patient. The mean (± SD) disease
duration was 7.2±1.6 years. A total of 75% of the enrolled patients
were receiving non-steroidal anti-inflammatory drugs and 25% were
not on medication.
Cell isolation and culture
Synovial tissues were homogenized in Dulbecco’s
modified Eagle’s medium and then incubated overnight at 37°C with 1
mg/ml type I collagenase (Sigma-Aldrich, St. Louis, MO, USA).
Following cell dissociation, the samples were filtered through a
cell strainer. The cell suspensions and cultures were conducted as
described previously (13). The
cell confluence and morphology were assessed throughout the
experiments by phase-contrast microscopy (DMI3000B; Leica, Wetzlar,
Germany) (14). All the functional
experiments were conducted using primary synovial cultured cells
from passages 3 to 6.
Small interfering RNA (siRNA)
To inhibit the NLRP1 expression in the FLS,
commercially available NLRP1 siRNA (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) was used. The cells were transfected using
transfection reagent (Qiagen, Hilden, Germany) according to the
manufacturer’s instructions. siRNA was diluted in transfection
reagent and culture medium, and the cells were incubated with 20 nM
siRNA for 12 h.
Quantitative PCR (qPCR)
For qPCR, the primers were designed as follows:
P2X4, (forward) 5′-CTACCAGGAAACTGACTCCGT-3′ and (reverse)
5′-GGTATCACATAATCCGCCACAT-3′; IL-1β, (forward)
5′-ATGATGGCTTATTACAGTGGCAA-3′ and (reverse)
5′-GTCGGAGATTCGTAGCTGGA-3′; NLRP1, (forward)
5′-GCAGTGCTAATGCCCTGGAT-3′ and (reverse)
5′-GAGCTTGGTAGAGGAGTGAGG-3′; MMP-3, (forward)
5′-CTGGACTCCGACACTCTGGA-3′ and (reverse)
5′-CAGGAAAGGTTCTGAAGTGACC-3′; MMP-9, (forward)
5′-GGGACGCAGACATCGTCATC-3′ and (reverse)
5′-TCGTCATCGTCGAAATGGGC-3′; GAPDH, (forward)
5′-ACAACTTTGGTATCGTGGAAGG-3′ and (reverse)
5′-GCCATCACGCCACAGTTTC-3′. The total RNA was isolated using the
total RNA isolation kit (Qiagen). The total RNA (20 ng) was reverse
transcribed for all targets. The temperature profile for PCR
included reverse transcription at 50°C for 30 min, hot start Taq
(1.25 units/sample; Thermo Fisher Scientific, Waltham, MA, USA)
activation for 15 min at 95°C, 28 cycles of denaturation for 15 sec
at 94°C, annealing for 30 sec at 56°C and extension for 30 sec at
72°C. SYBR-Green fluorescence was acquired at the end of the
extension cycle or at 79°C. A melting curve analysis was performed
at the end of each run to verify the single product formation for
each reaction. The relative expression of the target genes was
determined by comparison with GAPDH using the CT method.
Western blotting
Human FLS were washed and then lysed in cell lysis
buffer, and the protein concentration was determined using a
bicinchoninic acid assay kit (Pierce Biotechnology, Inc., Rockford,
IL, USA). Aliquots of 60 μg total protein sample were analyzed
using monoclonal rabbit antibodies specific for human P2X4 and
NLRP1 (Cell Signaling Technology, Inc., Danvers, MA, USA). The
filters were washed and incubated overnight at 4°C with secondary
antibody (1:2,000; Cell Signaling Technology, Inc.). Western
blotting assays were revealed with enhanced chemiluminescence
(Pierce, Rockford, IL, USA) and normalized against the internal
control, GAPDH.
Measurements of IL-1β and MMPs in the
medium
Human FLS were cultured in 24-well plates.
Subsequent to reaching 80% confluence, the cells were treated with
NLRP1 and then incubated in a humidified incubator at 37°C for 12
h. The cells were pretreated with NLRP1 siRNA. Following
incubation, the medium was removed and stored at −80°C until the
assay was performed. The IL-1β and MMPs in the medium were assayed
using an ELISA (eBioscience, San Diego, CA, USA) according to the
manufacturer’s instructions.
Statistical analysis
The data are expressed as the mean ± SD. The
statistical analysis was performed with Graphpad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). An analysis of
variance and unpaired two-tailed Student’s t-test were used to
determine the significant differences between the means. P<0.05
was used to indicate a statistically significant difference.
Results
Expression of P2X4 and NLRP1 is
upregulated in OAFLS
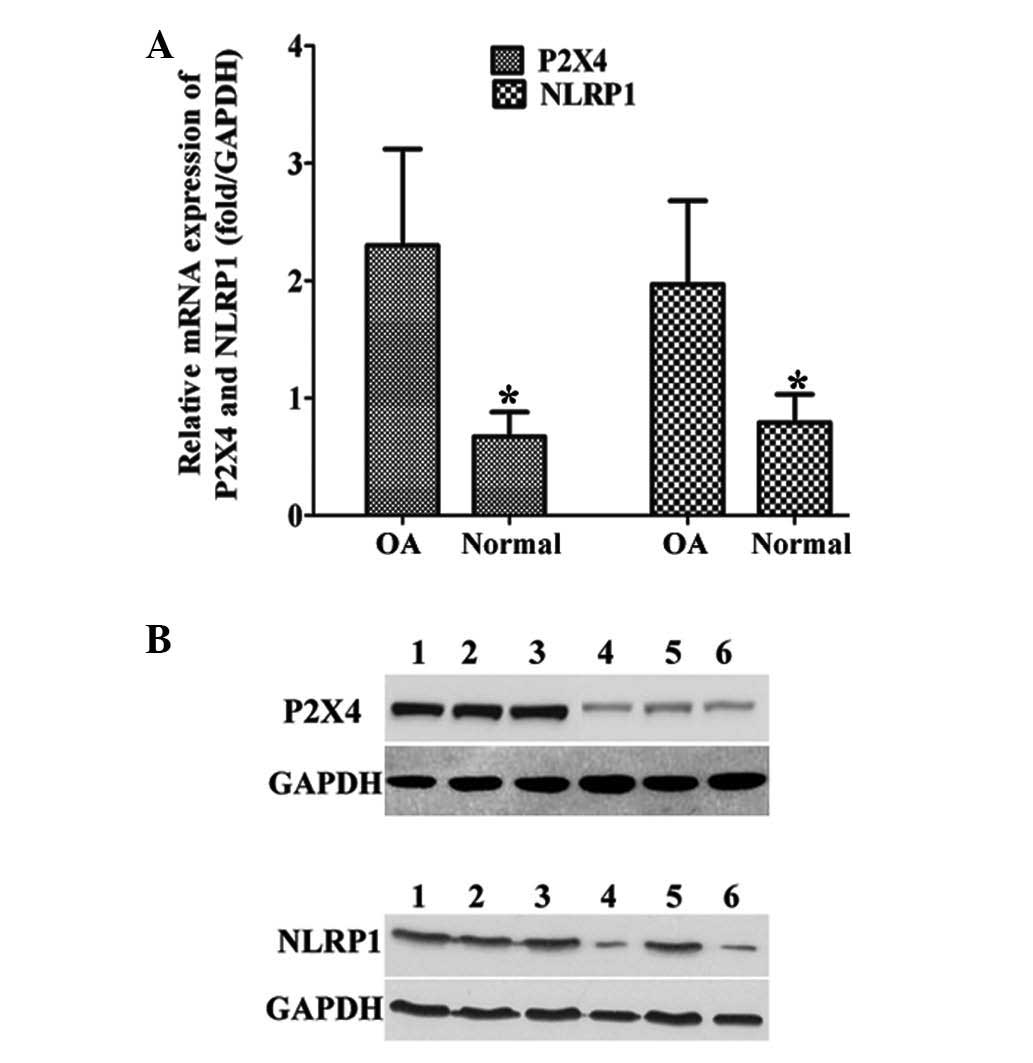
The expression levels of P2X4 and NLRP1 were
examined in the samples from the patients with OA. The expression
of P2X4 and NLRP1 in the human OAFLS was demonstrated to be
significantly higher than in the normal FLS at the mRNA and protein
levels (Fig. 1A–B), indicating the
potential roles of P2X4 and NLRP1 in OA.
P2X4 induces expression of IL-1β and MMPs
in OAFLS
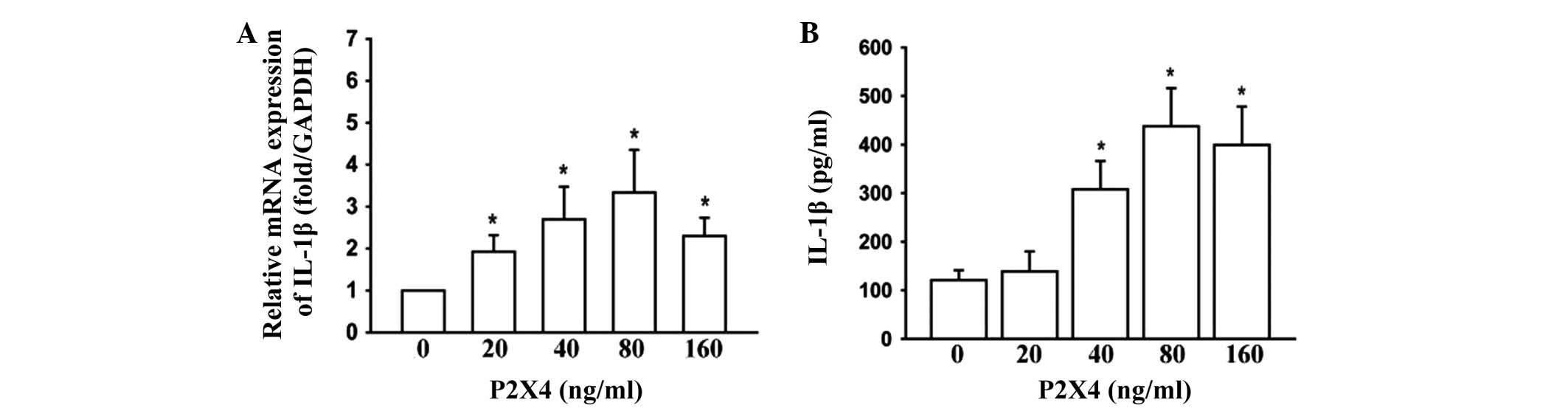
The effects of P2X4 stimulation on IL-1β and the
MMPs were examined in the OAFLS. P2X4 (Abnova, Walnut, CA, USA) was
applied directly to the OAFLS. The treatment of the OAFLS with P2X4
at concentrations of 0, 20, 40, 80 and160 ng/ml for 12 h was
demonstrated to induce IL-1β mRNA (Fig. 2A) and protein expression (Fig. 2B) in a dose-dependent manner.
According to these results, P2X4 at 80 ng/ml was most effective,
and this concentration was selected in all the subsequent
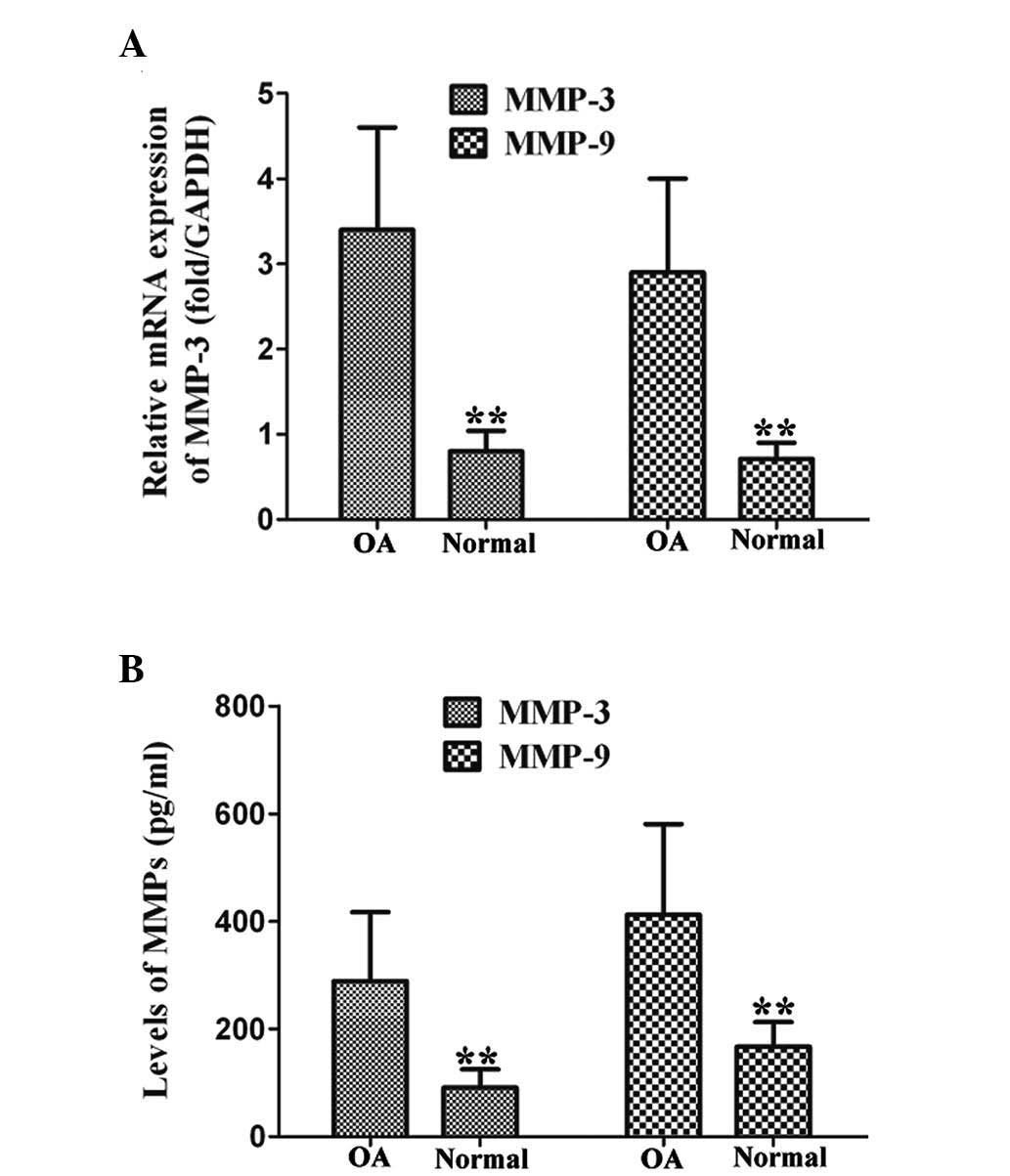
experiments. In addition, the levels of MMP-3 and MMP-9 in the
OAFLS were significantly higher than in the normal FLS, as observed
by qPCR and ELISA, following 12 h stimulation of P2X4 at 80 ng/ml
(Fig. 3). These data indicated
that P2X4 is a critical factor in the production of IL-1β and MMPs
in OAFLS.
P2X4-induced IL-1β in OAFLS is mediated
via NLRP1
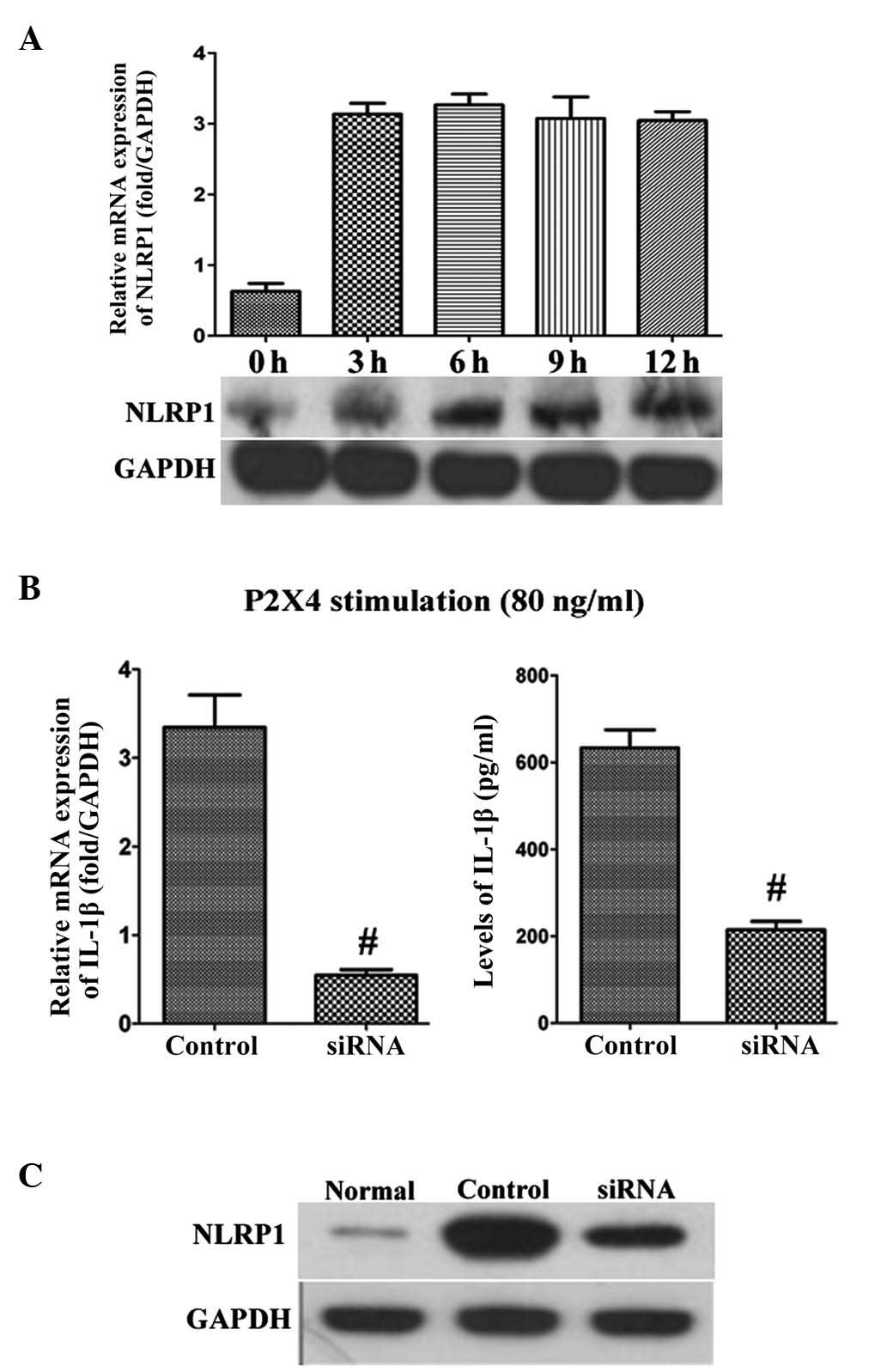
NLRP1 is involved in the production of IL-1β
(12). P2X4 at 80 ng/ml was
identified to significantly upregulate the expression of NLRP1 at
the mRNA and protein levels in the OAFLS (Fig. 4A). Furthermore, the NLRP1 siRNA at
20 nM was added in combination with P2X4 at 80 ng/ml into the
culture system. The NLRP1 siRNA was observed to block the
production of IL-1β induced by P2X4 at the mRNA and protein levels
compared with the control scrambled siRNA (Fig. 4B). The silencing effect of the
NLRP1 siRNA was also confirmed by western blotting (Fig. 4C). The normal FLS were used as a
normal control. These data reveal that NLRP1 is a crucial
determinant in P2X4-mediated IL-1β release.
Discussion
The P2X4 receptor is expressed in human
osteoblast-like cells (15). P2X4
has only recently been revealed to have a functional role in the
induction of brain-derived neurotrophic factor expression from
OAFLS (16). In the present study,
the expression of P2X4 and NLRP1 was confirmed to be significantly
higher in patients with OAFLS compared with those with normal FLS.
P2X4 induced the expression of IL-1β and MMPs in the OAFLS.
Furthermore, IL-1β was identified as a target protein for the P2X4
signaling pathway that regulates the inflammatory response. In
addition, secretion of IL-1β by the OAFLS was shown to require
NLRP1. These findings indicate that P2X4 acts as an important
inducer of inflammatory cytokines, including IL-1β and MMPs, in
OA.
A previous study revealed that P2X4R-deficient mice
exhibit reduced inflammatory pain behaviors and an impaired
production of prostaglandin E2 (PGE2) in the peripheral tissue in
response to inflammatory challenges (17). Ulmann et al(17) identified paw-resident macrophages
as the predominant cell type responsible for P2X4R-evoked PGE2
production. In the present study, it was revealed that in the
OAFLS, P2X4R activation triggered the necessary intracellular
signals leading to the activation of IL-1β synthesis, indicating
P2X4 to be the key receptor mediating the inflammatory response in
OA inflammation. In addition, P2X4 induced the expression of MMP-3
and MMP-9. MMPs have been demonstrated to be involved in joint
destruction via degradation of the articular cartilage (18). The data have revealed significantly
increased concentrations of plasma MMP-3 and MMP-9 in early OA, and
a positive correlation of plasma MMP-3 and MMP-9 with the severity
of clinical symptoms in early OA has also been reported (19). In the present study, P2X4
stimulation was observed to result in increased levels of MMPs,
indicating that P2X4 may be a potential therapeutic target for
healing joint damage.
Another significant finding in the present study is
that IL-1β induced by P2X4 is mediated by NLRP1. NLRP1 is a
regulator of the innate immune response and is expressed in a
number of immunocompetent cell types (20). The assembly of the NLRP1
inflammasome and the subsequent activation of caspase-1 cleaves the
inactive IL-1β precursor to the mature bioactive IL-1β, thereby
stimulating downstream inflammatory responses (21). A dominant activating mutation in
mouse NLRP1 has recently been demonstrated to result in a severe
systemic inflammatory phenotype associated with a greatly elevated
release of active IL-1β (22). The
NLRP1/IL-1β axis has been reported to be associated with
autoimmunity (20). NLRP1
polymorphisms are involved in the predisposition to systemic lupus
erythematosus and rheumatoid arthritis (23,24).
The results of the present study are concordant with previous
studies and add novel findings to the current understanding of
NLRP1 in arthritis. In conclusion, the results of the present study
demonstrated that P2X4 mediates the inflammatory response of OAFLS
by inducing the NLRP1/IL-1β axis. These results also indicate that
the P2X4/NLRP1 pathway may be a promising treatment target in human
OA.
References
|
1
|
Peat G, McCarney R and Croft P: Knee pain
and osteoarthritis in older adults: a review of community burden
and current use of primary health care. Ann Rheum Dis. 60:91–97.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clouet J, Vinatier C, Merceron C,
Pot-vaucel M, Maugars Y, Weiss P, Grimandi G and Guicheux J: From
osteoarthritis treatments to future regenerative therapies for
cartilage. Drug Discov Today. 14:913–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wright AA, Cook C and Abbott JH: Variables
associated with the progression of hip osteoarthritis: a systematic
review. Arthritis Rheum. 61:925–936. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pap T, Müller-Ladner U, Gay RE and Gay S:
Fibroblast biology. Role of synovial fibroblasts in the
pathogenesis of rheumatoid arthritis. Arthritis Res. 2:361–367.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mor A, Abramson SB and Pillinger MH: The
fibroblast-like synovial cell in rheumatoid arthritis: a key player
in inflammation and joint destruction. Clin Immunol. 115:118–128.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dinarello CA: A clinical perspective of
IL-1β as the gatekeeper of inflammation. Eur J Immunol.
41:1203–1217. 2011.
|
|
7
|
Jotanovic Z, Mihelic R, Sestan B and
Dembic Z: Role of interleukin-1 inhibitors in osteoarthritis: an
evidence-based review. Drugs Aging. 29:343–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bauernfeind F, Ablasser A, Bartok E, Kim
S, Schmid-Burgk J, Cavlar T and Hornung V: Inflammasomes: current
understanding and open questions. Cell Mol Life Sci. 68:765–783.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martinon F, Burns K and Tschopp J: The
inflammasome: a molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ray K: Crystal arthritis: NLRP3
inflammasome mediates crystal-induced joint inflammation and
dysfunction. Nat Rev Rheumatol. 7:6842011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mathews RJ, Robinson JI, Battellino M,
Wong C and Taylor JC; Biologics in Rheumatoid Arthritis Genetics
and Genomics Study Syndicate (BRAGGSS). Eyre S, Churchman SM,
Wilson AG, Isaacs JD, et al: Evidence of NLRP3-inflammasome
activation in rheumatoid arthritis (RA); genetic variants within
the NLRP3-inflammasome complex in relation to susceptibility to RA
and response to anti-TNF treatment. Ann Rheum Dis. Aug
16–2013.(Epub ahead of print).
|
|
12
|
Sakaki H, Fujiwaki T, Tsukimoto M, Kawano
A, Harada H and Kojima S: P2X4 receptor regulates P2X7
receptor-dependent IL-1β and IL-18 release in mouse bone
marrow-derived dendritic cells. Biochem Biophys Res Commun.
432:406–411. 2013.PubMed/NCBI
|
|
13
|
Uzan B, Ea HK, Launay JM, Garel JM, Champy
R, Cressent M and Lioté F: A critical role for
adrenomedullin-calcitonin receptor-like receptor in regulating
rheumatoid fibroblast-like synoviocyte apoptosis. J Immunol.
176:5548–5558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lioté F, Champy R, Moenner M,
Boval-Boizard B and Badet J: Elevated angiogenin levels in synovial
fluid from patients with inflammatory arthritis and secretion of
angiogenin by cultured synovial fibroblasts. Clin Exp Immunol.
132:163–168. 2003.PubMed/NCBI
|
|
15
|
Alqallaf SM, Evans BA and Kidd EJ:
Atypical P2X receptor pharmacology in two human osteoblast-like
cell lines. Br J Pharmacol. 156:1124–1135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klein K, Aeschlimann A, Jordan S, Gay R,
Gay S and Sprott H: ATP induced brain-derived neurotrophic factor
expression and release from osteoarthritis synovial fibroblasts is
mediated by purinergic receptor P2X4. PLoS One. 7:e366932012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ulmann L, Hirbec H and Rassendren F: P2X4
receptors mediate PGE2 release by tissue-resident macrophages and
initiate inflammatory pain. EMBO J. 29:2290–2300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Troeberg L and Nagase H: Proteases
involved in cartilage matrix degradation in osteoarthritis. Biochim
Biophys Acta. 1824:133–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Li L, Min J, Yang H, Xu X, Yuan Y
and Wang D: Levels of metalloproteinase (MMP-3, MMP-9), NF-kappaB
ligand (RANKL), and nitric oxide (NO) in peripheral blood of
osteoarthritis (OA) patients. Clin Lab. 58:755–762. 2012.PubMed/NCBI
|
|
20
|
Levandowski CB, Mailloux CM, Ferrara TM,
Gowan K, Ben S, Jin Y, McFann KK, Holland PJ, Fain PR, Dinarello CA
and Spritz RA: NLRP1 haplotypes associated with vitiligo and
autoimmunity increase interleukin-1β processing via the NLRP1
inflammasome. Proc Natl Acad Sci USA. 110:2952–2956.
2013.PubMed/NCBI
|
|
21
|
van de Veerdonk FL, Netea MG, Dinarello CA
and Joosten LA: Inflammasome activation and IL-1β and IL-18
processing during infection. Trends Immunol. 32:110–116. 2011.
|
|
22
|
Masters SL, Gerlic M, Metcalf D, Preston
S, Pellegrini M, O’Donnell JA, McArthur K, Baldwin TM, Chevrier S,
Nowell CJ, et al: NLRP1 inflammasome activation induces pyroptosis
of hematopoietic progenitor cells. Immunity. 37:1009–1023. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pontillo A, Girardelli M, Kamada AJ,
Pancotto JA, Donadi EA, Crovella S and Sandrin-Garcia P:
Polimorphisms in inflammasome genes are involved in the
predisposition to systemic lupus erythematosus. Autoimmunity.
45:271–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sui J, Li H, Fang Y, Liu Y, Li M, Zhong B,
Yang F, Zou Q and Wu Y: NLRP1 gene polymorphism influences gene
transcription and is a risk factor for rheumatoid arthritis in han
chinese. Arthritis Rheum. 64:647–654. 2012. View Article : Google Scholar : PubMed/NCBI
|